User login
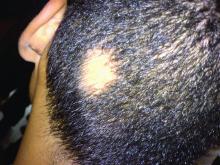
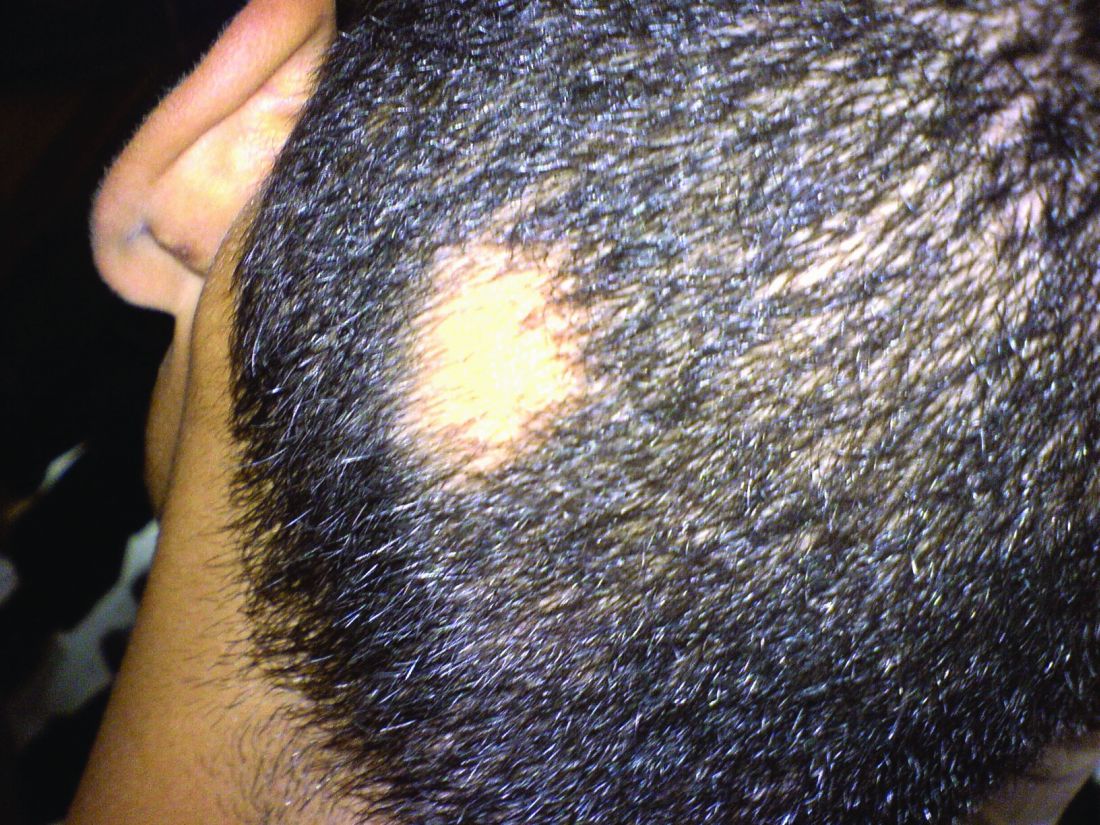
Consider hydroxychloroquine in treating pediatric alopecia areata
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
FROM PEDIATRIC DERMATOLOGY
Alopecia areata has female predominance, more severe types common in boys
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The predominant presentation is total hair loss and nail involvement is common.
Major finding: The female to male ratio was 1.5:1; the boys were significantly more likely to have severe types of AA (P = .009).
Study details: National Alopecia Areata Registry registrants under age 18 years were asked to complete a survey.
Disclosures: There were no conflicts of interest or funding information reported.
Source: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13387.
Clinical pattern may help distinguish pediatric NMN from subungal melanoma
When longitudinal melanonychia appears as a sharply demarcated pigment band of even width against normal nail in a child, Hutchinson’s sign with longitudinal brushy pigmentation may be a useful clinical pattern suggesting a diagnosis of nail matrix nevus rather than subungual melanoma, said Jae Ho Lee, MD, of Sungkyunkwan University, Seoul, South Korea, and associates.
with 14 children having melanonychia greater than 20% the width of the nail, compared with 2 adults. Total melanonychia occurred just twice, in two children. A total of 12 children had nail dystrophy, while none of the adults did; nail dystrophy was more frequent in wider lesions.
Hutchinson’s sign was seen in seven pediatric patients, but no adult patients. In most cases, Hutchinson’s sign had hyponychial pigmentation, and on dermoscopy showed a pigment pattern presenting longitudinally and resembling a brush mark (longitudinal brushy pigmentation or LBP). LBP of nail matrix nevi is different from the Hutchinson’s sign that occurs in subungual melanoma (SUM), where it is typically a “haphazard pigmentation pattern involving periungual skin.
“We propose that Hutchinson’s sign occurs more commonly in pediatric NMN than in adult NMN, and that the presence of the LBP pattern can help distinguish pediatric NMN from SUM,” the investigators said.
Histologically, the biopsies of the NMN in this study showed some important differences from known SUM histology. All the study biopsies “showed a melanocytic proliferation exhibiting a predominantly nested growth pattern, with the nests mostly located at the dermoepithelial junction and with retraction artifact surrounding the nests. There were variable nuclear hyperchromatism, nuclear sizes, and cytologic atypia within the NMN biopsy specimens,” the researchers said. “In contrast, the histology of SUM demonstrates a predominance of atypical single melanocytes over nests, retraction artifacts around individual melanocytes, and uniform atypia of melanocytes throughout the biopsy specimen.”
SOURCE: Lee JH et al. J Am Acad Dermatol. 2018 Mar;78(3):479-89.
When longitudinal melanonychia appears as a sharply demarcated pigment band of even width against normal nail in a child, Hutchinson’s sign with longitudinal brushy pigmentation may be a useful clinical pattern suggesting a diagnosis of nail matrix nevus rather than subungual melanoma, said Jae Ho Lee, MD, of Sungkyunkwan University, Seoul, South Korea, and associates.
with 14 children having melanonychia greater than 20% the width of the nail, compared with 2 adults. Total melanonychia occurred just twice, in two children. A total of 12 children had nail dystrophy, while none of the adults did; nail dystrophy was more frequent in wider lesions.
Hutchinson’s sign was seen in seven pediatric patients, but no adult patients. In most cases, Hutchinson’s sign had hyponychial pigmentation, and on dermoscopy showed a pigment pattern presenting longitudinally and resembling a brush mark (longitudinal brushy pigmentation or LBP). LBP of nail matrix nevi is different from the Hutchinson’s sign that occurs in subungual melanoma (SUM), where it is typically a “haphazard pigmentation pattern involving periungual skin.
“We propose that Hutchinson’s sign occurs more commonly in pediatric NMN than in adult NMN, and that the presence of the LBP pattern can help distinguish pediatric NMN from SUM,” the investigators said.
Histologically, the biopsies of the NMN in this study showed some important differences from known SUM histology. All the study biopsies “showed a melanocytic proliferation exhibiting a predominantly nested growth pattern, with the nests mostly located at the dermoepithelial junction and with retraction artifact surrounding the nests. There were variable nuclear hyperchromatism, nuclear sizes, and cytologic atypia within the NMN biopsy specimens,” the researchers said. “In contrast, the histology of SUM demonstrates a predominance of atypical single melanocytes over nests, retraction artifacts around individual melanocytes, and uniform atypia of melanocytes throughout the biopsy specimen.”
SOURCE: Lee JH et al. J Am Acad Dermatol. 2018 Mar;78(3):479-89.
When longitudinal melanonychia appears as a sharply demarcated pigment band of even width against normal nail in a child, Hutchinson’s sign with longitudinal brushy pigmentation may be a useful clinical pattern suggesting a diagnosis of nail matrix nevus rather than subungual melanoma, said Jae Ho Lee, MD, of Sungkyunkwan University, Seoul, South Korea, and associates.
with 14 children having melanonychia greater than 20% the width of the nail, compared with 2 adults. Total melanonychia occurred just twice, in two children. A total of 12 children had nail dystrophy, while none of the adults did; nail dystrophy was more frequent in wider lesions.
Hutchinson’s sign was seen in seven pediatric patients, but no adult patients. In most cases, Hutchinson’s sign had hyponychial pigmentation, and on dermoscopy showed a pigment pattern presenting longitudinally and resembling a brush mark (longitudinal brushy pigmentation or LBP). LBP of nail matrix nevi is different from the Hutchinson’s sign that occurs in subungual melanoma (SUM), where it is typically a “haphazard pigmentation pattern involving periungual skin.
“We propose that Hutchinson’s sign occurs more commonly in pediatric NMN than in adult NMN, and that the presence of the LBP pattern can help distinguish pediatric NMN from SUM,” the investigators said.
Histologically, the biopsies of the NMN in this study showed some important differences from known SUM histology. All the study biopsies “showed a melanocytic proliferation exhibiting a predominantly nested growth pattern, with the nests mostly located at the dermoepithelial junction and with retraction artifact surrounding the nests. There were variable nuclear hyperchromatism, nuclear sizes, and cytologic atypia within the NMN biopsy specimens,” the researchers said. “In contrast, the histology of SUM demonstrates a predominance of atypical single melanocytes over nests, retraction artifacts around individual melanocytes, and uniform atypia of melanocytes throughout the biopsy specimen.”
SOURCE: Lee JH et al. J Am Acad Dermatol. 2018 Mar;78(3):479-89.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
VIDEO: Device-based therapy for onychomycosis
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
Onychomycosis Diagnosis and Long-term Treatment
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
Phase 3 trials show halobetasol/tazarotene lotion works for psoriasis
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Refractory alopecia patients could consider a JAK inhibitor
SAN DIEGO – Treatment with a Janus kinase inhibitor such as tofacitinib is a reasonable option for patients with refractory alopecia who seek resolution of their hair loss and understand the adverse event risk from using this drug class, John E. Harris, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Using a Janus kinase (JAK) inhibitor to treat alopecia areata or totalis is a “highly successful, emerging therapy,” with efficacy rates for good responses of about a third or higher in a handful of published reports with experiences in more than 150 patients, said Dr. Harris, an associate professor of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts Medical School in Worcester.

“Some clinicians may say ‘I’d never prescribe a JAK inhibitor for hair loss, it’s too dangerous,’ but several hundred alopecia patients have now received this with no serious adverse effects,” said Dr. Harris. He acknowledged, however, that eventually some patients treated this way will have serious adverse effects. He said that he is treating with tofacitinib a “handful” of alopecia patients who have been unresponsive to other treatments, are eager for an intervention that might regrow their hair, and who understand and accept the risk for developing an infection, shingles, or myelosuppression (with ruxolitinib treatment).
Roughly half of the alopecia patients prescribed tofacitinib (Xeljanz) by Dr. Harris have had their drug cost covered by health insurance, with the others paying for it themselves. Arranging for insurance coverage has usually involved filing an appeal, Dr. Harris said in an interview. The reported successful dosages have been 5 mg bid, with a boost to 10 mg bid for patients who don’t initially respond.
Insurance companies have not yet paid for treatment with ruxolitinib (Jakafi), which has been described in case reports in far fewer patients and which costs about $120,000 a year, he noted.
Dr. Harris said that JAK inhibitors also have great potential for treating vitiligo, a disorder he thinks shares many features in common with alopecia ( J Allergy Clin Immunol. 2017 Sept;140[3]:654-62).
“I think [JAK inhibitors] will have the same efficacy in vitiligo,” he predicted. He is particularly enthused about the possibility of administering ruxolitinib topically to patients with alopecia or vitiligo. Ruxolitinib is well suited to topical administration because of its good skin penetration, Dr. Harris said. Several trials now in progress are further studying JAK inhibitors for alopecia using oral or topical formulations.
Dr. Harris has been a consultant to and has received research funding from Pfizer, the company that markets tofacitinib (Xeljanz). He has also been a consultant to a dozen other companies, and has also received research support from Aclaris Therapeutics, Celgene, Dermavant, Genzyme, Sanofi, and Stiefel/GlaxoSmithKline.
SAN DIEGO – Treatment with a Janus kinase inhibitor such as tofacitinib is a reasonable option for patients with refractory alopecia who seek resolution of their hair loss and understand the adverse event risk from using this drug class, John E. Harris, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Using a Janus kinase (JAK) inhibitor to treat alopecia areata or totalis is a “highly successful, emerging therapy,” with efficacy rates for good responses of about a third or higher in a handful of published reports with experiences in more than 150 patients, said Dr. Harris, an associate professor of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts Medical School in Worcester.

“Some clinicians may say ‘I’d never prescribe a JAK inhibitor for hair loss, it’s too dangerous,’ but several hundred alopecia patients have now received this with no serious adverse effects,” said Dr. Harris. He acknowledged, however, that eventually some patients treated this way will have serious adverse effects. He said that he is treating with tofacitinib a “handful” of alopecia patients who have been unresponsive to other treatments, are eager for an intervention that might regrow their hair, and who understand and accept the risk for developing an infection, shingles, or myelosuppression (with ruxolitinib treatment).
Roughly half of the alopecia patients prescribed tofacitinib (Xeljanz) by Dr. Harris have had their drug cost covered by health insurance, with the others paying for it themselves. Arranging for insurance coverage has usually involved filing an appeal, Dr. Harris said in an interview. The reported successful dosages have been 5 mg bid, with a boost to 10 mg bid for patients who don’t initially respond.
Insurance companies have not yet paid for treatment with ruxolitinib (Jakafi), which has been described in case reports in far fewer patients and which costs about $120,000 a year, he noted.
Dr. Harris said that JAK inhibitors also have great potential for treating vitiligo, a disorder he thinks shares many features in common with alopecia ( J Allergy Clin Immunol. 2017 Sept;140[3]:654-62).
“I think [JAK inhibitors] will have the same efficacy in vitiligo,” he predicted. He is particularly enthused about the possibility of administering ruxolitinib topically to patients with alopecia or vitiligo. Ruxolitinib is well suited to topical administration because of its good skin penetration, Dr. Harris said. Several trials now in progress are further studying JAK inhibitors for alopecia using oral or topical formulations.
Dr. Harris has been a consultant to and has received research funding from Pfizer, the company that markets tofacitinib (Xeljanz). He has also been a consultant to a dozen other companies, and has also received research support from Aclaris Therapeutics, Celgene, Dermavant, Genzyme, Sanofi, and Stiefel/GlaxoSmithKline.
SAN DIEGO – Treatment with a Janus kinase inhibitor such as tofacitinib is a reasonable option for patients with refractory alopecia who seek resolution of their hair loss and understand the adverse event risk from using this drug class, John E. Harris, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Using a Janus kinase (JAK) inhibitor to treat alopecia areata or totalis is a “highly successful, emerging therapy,” with efficacy rates for good responses of about a third or higher in a handful of published reports with experiences in more than 150 patients, said Dr. Harris, an associate professor of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts Medical School in Worcester.

“Some clinicians may say ‘I’d never prescribe a JAK inhibitor for hair loss, it’s too dangerous,’ but several hundred alopecia patients have now received this with no serious adverse effects,” said Dr. Harris. He acknowledged, however, that eventually some patients treated this way will have serious adverse effects. He said that he is treating with tofacitinib a “handful” of alopecia patients who have been unresponsive to other treatments, are eager for an intervention that might regrow their hair, and who understand and accept the risk for developing an infection, shingles, or myelosuppression (with ruxolitinib treatment).
Roughly half of the alopecia patients prescribed tofacitinib (Xeljanz) by Dr. Harris have had their drug cost covered by health insurance, with the others paying for it themselves. Arranging for insurance coverage has usually involved filing an appeal, Dr. Harris said in an interview. The reported successful dosages have been 5 mg bid, with a boost to 10 mg bid for patients who don’t initially respond.
Insurance companies have not yet paid for treatment with ruxolitinib (Jakafi), which has been described in case reports in far fewer patients and which costs about $120,000 a year, he noted.
Dr. Harris said that JAK inhibitors also have great potential for treating vitiligo, a disorder he thinks shares many features in common with alopecia ( J Allergy Clin Immunol. 2017 Sept;140[3]:654-62).
“I think [JAK inhibitors] will have the same efficacy in vitiligo,” he predicted. He is particularly enthused about the possibility of administering ruxolitinib topically to patients with alopecia or vitiligo. Ruxolitinib is well suited to topical administration because of its good skin penetration, Dr. Harris said. Several trials now in progress are further studying JAK inhibitors for alopecia using oral or topical formulations.
Dr. Harris has been a consultant to and has received research funding from Pfizer, the company that markets tofacitinib (Xeljanz). He has also been a consultant to a dozen other companies, and has also received research support from Aclaris Therapeutics, Celgene, Dermavant, Genzyme, Sanofi, and Stiefel/GlaxoSmithKline.
EXPERT ANALYSIS AT AAD 18
Nail-Patella Syndrome: Clinical Clues for Making the Diagnosis
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
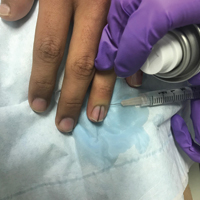
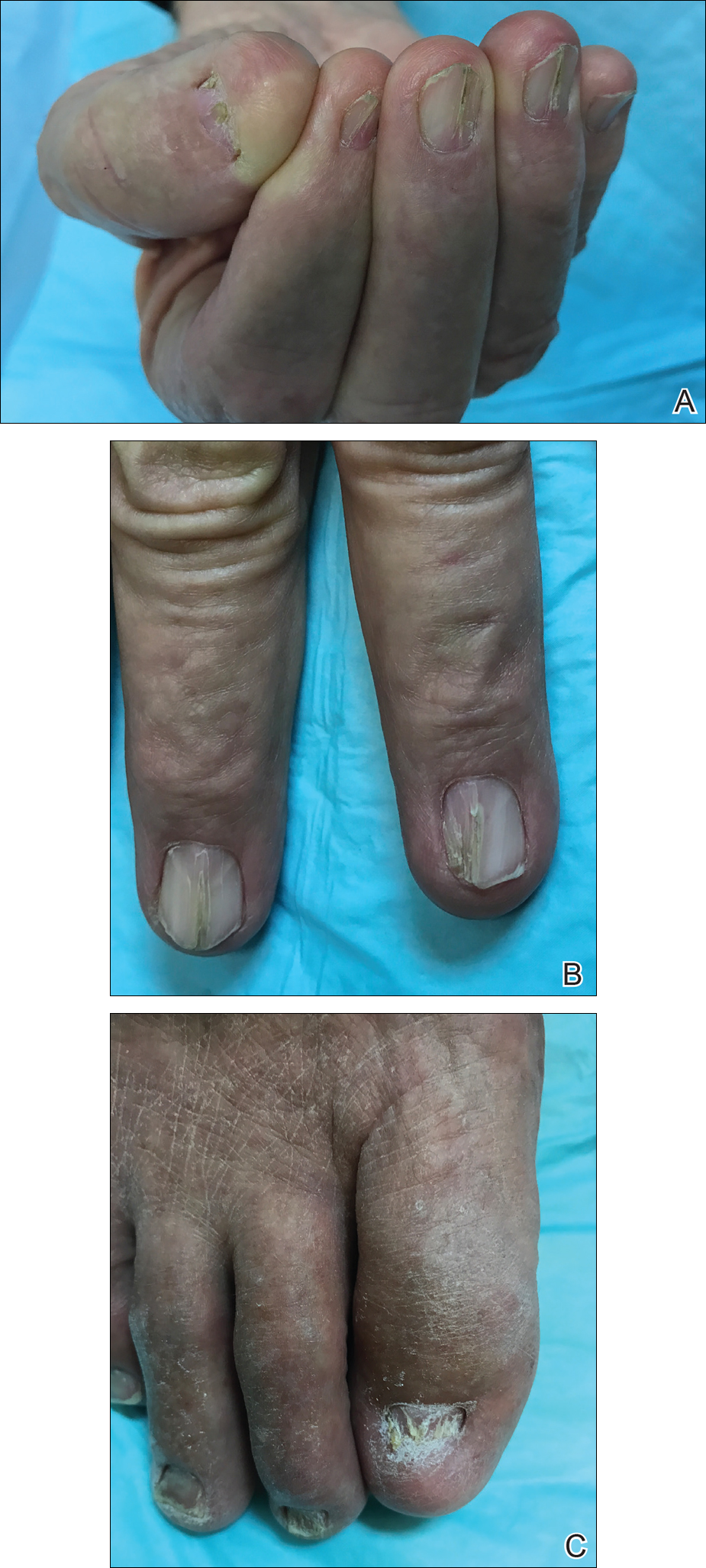
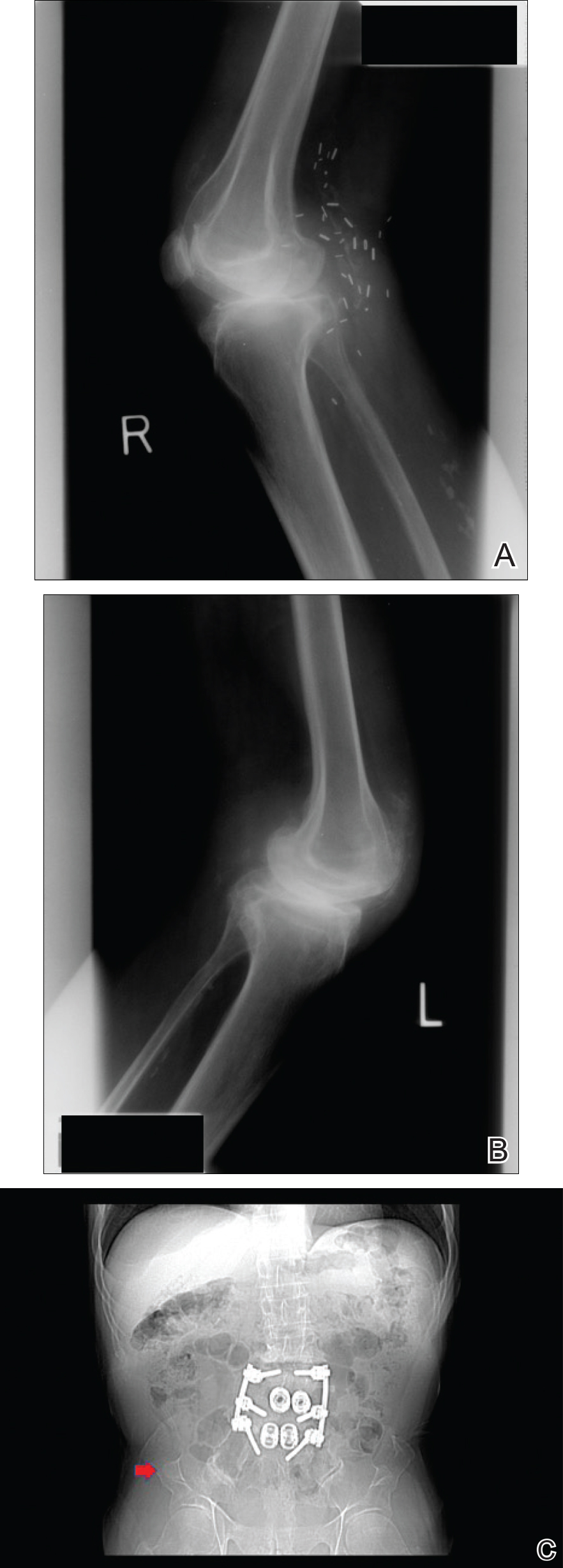
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.
Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.


Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.


Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
Practice Points
- Nail-patella syndrome (NPS) is a multisystem disease.
- Nail findings (eg, triangular lunulae) may be the first clue to NPS and should prompt investigation of associated renal, ocular, neurologic, skeletal, and orthopedic abnormalities.
- Early intervention and a multidisciplinary approach to care can improve morbidity and mortality in patients with NPS.
Maximizing topical toenail fungus therapy
KAUAI, HAWAII – Two keys to effective topical treatment of onychomycosis are treat it early and address coexisting tinea pedis, according to Theodore Rosen, MD.
A third element in achieving treatment success is to use one of the newer topical agents: efinaconazole (Jublia) or tavaborole (Kerydin). The efficacy of efinaconazole approaches that of terbinafine, the most effective and widely prescribed oral agent, which has a 59% rate of almost complete cure, defined as less than 10% residual abnormal nail with no requirement for mycologic cure.
And while tavaborole isn’t quite as effective, it’s definitely better than previous topical agents, Dr. Rosen said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Both of these topicals are well tolerated and feature good nail permeation. They also allow for spread to the lateral nail folds and hyponychium. They even penetrate nail polish, although efinaconazole often causes the polish to lose its spiffy gloss, said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
To underscore the importance of early treatment and addressing concomitant tinea pedis, he cited published secondary analyses of two identical double-blind, multicenter, 48-week clinical trials totaling 1,655 adults with onychomycosis who were randomized 3:1 to once-daily efinaconazole 10% topical solution or its vehicle.
Treat early
Phoebe Rich, MD, of the Oregon Dermatology and Research Center, Portland, broke down the outcomes according to disease duration, in a study of more than 1,500 patients with onychomycosis. She found that the complete cure rate at 52 weeks dropped off markedly in patients with a history of onychomycosis for 1 year or longer at baseline.
Complete cure – defined as no clinical involvement of the target toenail along with both a negative potassium hydroxide examination and a negative fungal culture at 52 weeks – was achieved in 43% of efinaconazole-treated patients with onychomycosis for less than 1 year. The rate then plunged to 17% in those with a disease duration of 1-5 years and 16% in patients with onychomycosis for more than 5 years. Nevertheless, the topical antifungal was significantly more effective than was the vehicle, across the board, with complete cure rates in the vehicle group of roughly 18%, 5%, and 2%, respectively, in patients with onychomycosis for less than 1 year, 1-5 years, and more than 5 years (J Drugs Dermatol. 2015 Jan;14[1]:58-62).
Tackle coexisting tinea pedis
Podiatrists analyzed the combined efinaconazole outcome data based on whether participants had no coexisting tinea pedis, baseline tinea pedis treated concomitantly with an investigator-approved topical antifungal, or tinea pedis left untreated. They concluded that treatment of coexisting tinea pedis decisively enhanced the efficacy of efinaconazole for onychomycosis.
A total of 21% of study participants had concomitant tinea pedis, and 61% of them were treated for it. At week 52, the onychomycosis complete cure rate was 29% in the efinaconazole group concurrently treated for tinea pedis, compared with just 16% if their tinea pedis was untreated (J Am Podiatr Med Assoc. 2015 Sep;105[5]:407-11).
“If you see tinea pedis, don’t blow it off. Treat it. Otherwise, you’re not getting rid of the fungal reservoir,” Dr. Rosen emphasized.
He noted that two topical agents approved for tinea pedis – naftifine 2% cream or gel and luliconazole 1% cream – are effective as once-daily therapy for 2 weeks, a considerably briefer regimen than with other approved topicals. And short-course therapy spells improved adherence, he added.
In the pivotal trials, naftifine had an effective treatment rate – a clinically useful endpoint defined as a small amount of residual scaling and/or redness but no itching – of 57%, while for luliconazole the rates were 33%-48%.
These two agents also are approved for treatment of tinea corporis and tinea cruris. Naftifine is approved as a once-daily treatment for 2 weeks, while luliconazole is, notably, a 7-day treatment. Luliconazole, in particular, is a relatively expensive drug, Dr. Rosen added, so insurers may require prior failure on clotrimazole.
When to treat onychomycosis topically
The pivotal trials of tavaborole and efinaconazole were conducted in patients with 20%-60% nail involvement. The infection didn’t extend to the matrix, and nail thickness and crumbly subungual debris were modest at baseline.
“There are always potential safety issues – drug interactions, GI disturbance, taste loss, headache, teratogenicity, cardiotoxicity, hepatotoxicity – anytime you put a pill in your mouth. So if you have a patient who’s dedicated enough to use a topical for 48 weeks and it’s a modestly affected nail, think about it,” Dr. Rosen advised.
He reported serving on scientific advisory boards for Aclaris, Anacor, Cipla, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Two keys to effective topical treatment of onychomycosis are treat it early and address coexisting tinea pedis, according to Theodore Rosen, MD.
A third element in achieving treatment success is to use one of the newer topical agents: efinaconazole (Jublia) or tavaborole (Kerydin). The efficacy of efinaconazole approaches that of terbinafine, the most effective and widely prescribed oral agent, which has a 59% rate of almost complete cure, defined as less than 10% residual abnormal nail with no requirement for mycologic cure.
And while tavaborole isn’t quite as effective, it’s definitely better than previous topical agents, Dr. Rosen said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Both of these topicals are well tolerated and feature good nail permeation. They also allow for spread to the lateral nail folds and hyponychium. They even penetrate nail polish, although efinaconazole often causes the polish to lose its spiffy gloss, said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
To underscore the importance of early treatment and addressing concomitant tinea pedis, he cited published secondary analyses of two identical double-blind, multicenter, 48-week clinical trials totaling 1,655 adults with onychomycosis who were randomized 3:1 to once-daily efinaconazole 10% topical solution or its vehicle.
Treat early
Phoebe Rich, MD, of the Oregon Dermatology and Research Center, Portland, broke down the outcomes according to disease duration, in a study of more than 1,500 patients with onychomycosis. She found that the complete cure rate at 52 weeks dropped off markedly in patients with a history of onychomycosis for 1 year or longer at baseline.
Complete cure – defined as no clinical involvement of the target toenail along with both a negative potassium hydroxide examination and a negative fungal culture at 52 weeks – was achieved in 43% of efinaconazole-treated patients with onychomycosis for less than 1 year. The rate then plunged to 17% in those with a disease duration of 1-5 years and 16% in patients with onychomycosis for more than 5 years. Nevertheless, the topical antifungal was significantly more effective than was the vehicle, across the board, with complete cure rates in the vehicle group of roughly 18%, 5%, and 2%, respectively, in patients with onychomycosis for less than 1 year, 1-5 years, and more than 5 years (J Drugs Dermatol. 2015 Jan;14[1]:58-62).
Tackle coexisting tinea pedis
Podiatrists analyzed the combined efinaconazole outcome data based on whether participants had no coexisting tinea pedis, baseline tinea pedis treated concomitantly with an investigator-approved topical antifungal, or tinea pedis left untreated. They concluded that treatment of coexisting tinea pedis decisively enhanced the efficacy of efinaconazole for onychomycosis.
A total of 21% of study participants had concomitant tinea pedis, and 61% of them were treated for it. At week 52, the onychomycosis complete cure rate was 29% in the efinaconazole group concurrently treated for tinea pedis, compared with just 16% if their tinea pedis was untreated (J Am Podiatr Med Assoc. 2015 Sep;105[5]:407-11).
“If you see tinea pedis, don’t blow it off. Treat it. Otherwise, you’re not getting rid of the fungal reservoir,” Dr. Rosen emphasized.
He noted that two topical agents approved for tinea pedis – naftifine 2% cream or gel and luliconazole 1% cream – are effective as once-daily therapy for 2 weeks, a considerably briefer regimen than with other approved topicals. And short-course therapy spells improved adherence, he added.
In the pivotal trials, naftifine had an effective treatment rate – a clinically useful endpoint defined as a small amount of residual scaling and/or redness but no itching – of 57%, while for luliconazole the rates were 33%-48%.
These two agents also are approved for treatment of tinea corporis and tinea cruris. Naftifine is approved as a once-daily treatment for 2 weeks, while luliconazole is, notably, a 7-day treatment. Luliconazole, in particular, is a relatively expensive drug, Dr. Rosen added, so insurers may require prior failure on clotrimazole.
When to treat onychomycosis topically
The pivotal trials of tavaborole and efinaconazole were conducted in patients with 20%-60% nail involvement. The infection didn’t extend to the matrix, and nail thickness and crumbly subungual debris were modest at baseline.
“There are always potential safety issues – drug interactions, GI disturbance, taste loss, headache, teratogenicity, cardiotoxicity, hepatotoxicity – anytime you put a pill in your mouth. So if you have a patient who’s dedicated enough to use a topical for 48 weeks and it’s a modestly affected nail, think about it,” Dr. Rosen advised.
He reported serving on scientific advisory boards for Aclaris, Anacor, Cipla, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Two keys to effective topical treatment of onychomycosis are treat it early and address coexisting tinea pedis, according to Theodore Rosen, MD.
A third element in achieving treatment success is to use one of the newer topical agents: efinaconazole (Jublia) or tavaborole (Kerydin). The efficacy of efinaconazole approaches that of terbinafine, the most effective and widely prescribed oral agent, which has a 59% rate of almost complete cure, defined as less than 10% residual abnormal nail with no requirement for mycologic cure.
And while tavaborole isn’t quite as effective, it’s definitely better than previous topical agents, Dr. Rosen said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Both of these topicals are well tolerated and feature good nail permeation. They also allow for spread to the lateral nail folds and hyponychium. They even penetrate nail polish, although efinaconazole often causes the polish to lose its spiffy gloss, said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
To underscore the importance of early treatment and addressing concomitant tinea pedis, he cited published secondary analyses of two identical double-blind, multicenter, 48-week clinical trials totaling 1,655 adults with onychomycosis who were randomized 3:1 to once-daily efinaconazole 10% topical solution or its vehicle.
Treat early
Phoebe Rich, MD, of the Oregon Dermatology and Research Center, Portland, broke down the outcomes according to disease duration, in a study of more than 1,500 patients with onychomycosis. She found that the complete cure rate at 52 weeks dropped off markedly in patients with a history of onychomycosis for 1 year or longer at baseline.
Complete cure – defined as no clinical involvement of the target toenail along with both a negative potassium hydroxide examination and a negative fungal culture at 52 weeks – was achieved in 43% of efinaconazole-treated patients with onychomycosis for less than 1 year. The rate then plunged to 17% in those with a disease duration of 1-5 years and 16% in patients with onychomycosis for more than 5 years. Nevertheless, the topical antifungal was significantly more effective than was the vehicle, across the board, with complete cure rates in the vehicle group of roughly 18%, 5%, and 2%, respectively, in patients with onychomycosis for less than 1 year, 1-5 years, and more than 5 years (J Drugs Dermatol. 2015 Jan;14[1]:58-62).
Tackle coexisting tinea pedis
Podiatrists analyzed the combined efinaconazole outcome data based on whether participants had no coexisting tinea pedis, baseline tinea pedis treated concomitantly with an investigator-approved topical antifungal, or tinea pedis left untreated. They concluded that treatment of coexisting tinea pedis decisively enhanced the efficacy of efinaconazole for onychomycosis.
A total of 21% of study participants had concomitant tinea pedis, and 61% of them were treated for it. At week 52, the onychomycosis complete cure rate was 29% in the efinaconazole group concurrently treated for tinea pedis, compared with just 16% if their tinea pedis was untreated (J Am Podiatr Med Assoc. 2015 Sep;105[5]:407-11).
“If you see tinea pedis, don’t blow it off. Treat it. Otherwise, you’re not getting rid of the fungal reservoir,” Dr. Rosen emphasized.
He noted that two topical agents approved for tinea pedis – naftifine 2% cream or gel and luliconazole 1% cream – are effective as once-daily therapy for 2 weeks, a considerably briefer regimen than with other approved topicals. And short-course therapy spells improved adherence, he added.
In the pivotal trials, naftifine had an effective treatment rate – a clinically useful endpoint defined as a small amount of residual scaling and/or redness but no itching – of 57%, while for luliconazole the rates were 33%-48%.
These two agents also are approved for treatment of tinea corporis and tinea cruris. Naftifine is approved as a once-daily treatment for 2 weeks, while luliconazole is, notably, a 7-day treatment. Luliconazole, in particular, is a relatively expensive drug, Dr. Rosen added, so insurers may require prior failure on clotrimazole.
When to treat onychomycosis topically
The pivotal trials of tavaborole and efinaconazole were conducted in patients with 20%-60% nail involvement. The infection didn’t extend to the matrix, and nail thickness and crumbly subungual debris were modest at baseline.
“There are always potential safety issues – drug interactions, GI disturbance, taste loss, headache, teratogenicity, cardiotoxicity, hepatotoxicity – anytime you put a pill in your mouth. So if you have a patient who’s dedicated enough to use a topical for 48 weeks and it’s a modestly affected nail, think about it,” Dr. Rosen advised.
He reported serving on scientific advisory boards for Aclaris, Anacor, Cipla, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Pain-Minimizing Strategies for Nail Surgery
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11
Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.