User login
Evidence still lacking that vitamins prevent CVD, cancer: USPSTF
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Collagen ‘tile’ delivers postsurgical radiation in glioblastoma
and spares healthy tissue, new research suggests.
The results showed inserting a collagen matrix containing radioactive seeds into the brain postsurgery did not impede wound healing. It also showed a favorable safety profile, researchers note.
Benefits for patients undergoing this GammaTile (GT) intervention include not having to wait weeks to receive radiation treatment, which in turn improves their quality of life, said study investigator Clark C. Chen, MD, PhD, chair, department of neurosurgery, University of Minnesota Medical School, Minneapolis.
“These initial results are highly promising and offer hope for patients afflicted with an otherwise devastating disease,” Dr. Chen said in an interview.
If replicated in larger trials, GT therapy “could define a new standard of care, and there would really be no reason why patients shouldn’t get this therapy,” he added.
This is the first clinical series describing GT use since its approval by the U.S. Food and Drug Administration (FDA) for recurrent brain cancer.
The findings were presented at the annual meeting of the American Association of Neurological Surgeons (AANS) and were published recently in Neuro-Oncology Advances.
Radioactive seeds
GT therapy is a version of brachytherapy where radioactive sources are placed adjacent to cancerous tissue. It consists of radioactive seeds embedded with a collagen tile.
The neurosurgeon inserts these “tiles” immediately after tumor removal to cover the entire resection cavity, Dr. Chen said. The tiles maintain the cavity architecture to prevent radiation “hot spots” associated with cavity collapse.
Dr. Chen noted the therapy is “short range,” with most of the radiation delivered within 8 millimeters of the radioactive seeds.
The radiation lasts for about a month and the collagen tiles are eventually absorbed within the body. “You put in the tiles and you don’t need to do anything more,” Dr. Chen said.
GT has a number of advantages. Unlike with traditional brachytherapy, the collagen tile provides a buffer around the radiation sources, allowing delivery of the optimal radiation dose while preserving healthy tissue.
It also avoids the up-to-6-weeks patients have to wait postsurgery to get external beam radiation therapy. “If you start radiation too early, it actually compromises wound healing, and in the meantime the tumor is growing,” said Dr. Chen.
“I have several patients where I removed a large tumor and within that 6-week period, the tumor came back entirely,” he added.
With the gamma-tile, however, radiation from the seeds kills the tumor while the body heals.
Safety profile
The study included 22 patients (mean age, 57.7 years; 15 men, 7 women) with wild-type isocitrate dehydrogenase glioblastoma. They were all having surgery for recurrent tumors.
“One of the most challenging aspects of glioblastomas is that not only do the tumors come back, they come back immediately adjacent to where you have done the surgery, and for many patients this is demoralizing,” Dr. Chen said.
Six participants had 0 6 -Methylguanine-DNA methyltranferase (MGMT) methylated glioblastoma, while the others had unmethylated MGMT.
The mean follow-up from initial diagnosis was 733 days (2 years).
Results showed one patient had to be readmitted to the hospital for hydrocephalus, but there were no re-admissions within 30 days attributable to GT.
Despite participants having undergone a second and third resection through the same surgical incision, there were no wound infections. “One of the concerns of giving radiation right after surgery is it can compromise wound healing, and this is why you wait 6 weeks,” Dr. Chen noted.
He stressed that no patient in the study suffered from adverse radiation effects that required medical or surgical intervention.
As the radiation is so short-range, hair loss and skin irritation are not side effects of GT, he added.
“The radiation is inside the brain and highly targeted, so it doesn’t hit hair follicles,” said Dr. Chen. “As best as I can observe in these patients, I did not see toxicity associated with radiation.”
One and done
Among the 22 participants, 18 had neurologic symptoms at baseline. There were no new neurologic deficits that developed after GT placement.
In addition, GT therapy improved “local control” — preventing the tumor from growing back at the site of the surgery. The local control was 86% at 6 months and 81% at 12 months.
The median progression-free survival was about 8 months. The median overall survival was 20 months (about 600 days) for the unmethylated MGMT group and 37.4 months (about 1120 days) for the methylated group.
Outcomes compared favorably to an independent glioblastoma cohort of similar patients who did not receive GT treatment during the study period, Dr. Chen noted.
“This therapy can potentially redefine how we treat glioblastoma patients whose cancer came back,” he said.
A study limitation was that it did not include quality-of-life data, which makes it challenging to assess the therapy’s overall impact, Dr. Chen said. However, he added that from his experience, patients very much appreciate not having to repeatedly take time off work for clinic or hospital visits to receive radiation treatments.
“One of the beauties of this therapy is it’s a one-and-done deal,” he said.
Interesting, timely
Commenting for this news organization, William T. Curry Jr, MD, co-director at MassGeneral Neuroscience and director of neurosurgical oncology at Mass General Cancer Center, Boston, called the study “interesting and timely.”
These new data “underscore that GT is safe in patients that have undergone gross total resection of recurrent glioblastoma and that rates of progression free survival may exceed those treated with resection alone,” said Dr. Curry, who was not involved with the research.
“Surgeons are excited about anything that has the potential to improve outcomes for patients with this very challenging disease, and it is wonderful to be able to offer hope and survival tools to patients,” he added.
However, Dr. Curry noted there are challenges and potential biases when studying survival in cancer patients without conducting a randomization process. The investigators “admit to methodological flaws inherent in the single-arm design in a patient population with recurrent glioblastoma not treated uniformly,” he said.
In addition, he noted overall survival may not have been related to the GT intervention. “Multicenter randomization is probably required to get to the bottom of the survival advantage in different subsets of glioblastoma patients,” Dr. Curry said.
Further research is needed to confirm the efficacy, appropriate indications, and timing of the intervention, but “I would support a randomized multicenter study in patients undergoing near gross total resection of recurrent glioblastoma,” he concluded.
The study received no outside funding. Dr. Chen and Dr. Curry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and spares healthy tissue, new research suggests.
The results showed inserting a collagen matrix containing radioactive seeds into the brain postsurgery did not impede wound healing. It also showed a favorable safety profile, researchers note.
Benefits for patients undergoing this GammaTile (GT) intervention include not having to wait weeks to receive radiation treatment, which in turn improves their quality of life, said study investigator Clark C. Chen, MD, PhD, chair, department of neurosurgery, University of Minnesota Medical School, Minneapolis.
“These initial results are highly promising and offer hope for patients afflicted with an otherwise devastating disease,” Dr. Chen said in an interview.
If replicated in larger trials, GT therapy “could define a new standard of care, and there would really be no reason why patients shouldn’t get this therapy,” he added.
This is the first clinical series describing GT use since its approval by the U.S. Food and Drug Administration (FDA) for recurrent brain cancer.
The findings were presented at the annual meeting of the American Association of Neurological Surgeons (AANS) and were published recently in Neuro-Oncology Advances.
Radioactive seeds
GT therapy is a version of brachytherapy where radioactive sources are placed adjacent to cancerous tissue. It consists of radioactive seeds embedded with a collagen tile.
The neurosurgeon inserts these “tiles” immediately after tumor removal to cover the entire resection cavity, Dr. Chen said. The tiles maintain the cavity architecture to prevent radiation “hot spots” associated with cavity collapse.
Dr. Chen noted the therapy is “short range,” with most of the radiation delivered within 8 millimeters of the radioactive seeds.
The radiation lasts for about a month and the collagen tiles are eventually absorbed within the body. “You put in the tiles and you don’t need to do anything more,” Dr. Chen said.
GT has a number of advantages. Unlike with traditional brachytherapy, the collagen tile provides a buffer around the radiation sources, allowing delivery of the optimal radiation dose while preserving healthy tissue.
It also avoids the up-to-6-weeks patients have to wait postsurgery to get external beam radiation therapy. “If you start radiation too early, it actually compromises wound healing, and in the meantime the tumor is growing,” said Dr. Chen.
“I have several patients where I removed a large tumor and within that 6-week period, the tumor came back entirely,” he added.
With the gamma-tile, however, radiation from the seeds kills the tumor while the body heals.
Safety profile
The study included 22 patients (mean age, 57.7 years; 15 men, 7 women) with wild-type isocitrate dehydrogenase glioblastoma. They were all having surgery for recurrent tumors.
“One of the most challenging aspects of glioblastomas is that not only do the tumors come back, they come back immediately adjacent to where you have done the surgery, and for many patients this is demoralizing,” Dr. Chen said.
Six participants had 0 6 -Methylguanine-DNA methyltranferase (MGMT) methylated glioblastoma, while the others had unmethylated MGMT.
The mean follow-up from initial diagnosis was 733 days (2 years).
Results showed one patient had to be readmitted to the hospital for hydrocephalus, but there were no re-admissions within 30 days attributable to GT.
Despite participants having undergone a second and third resection through the same surgical incision, there were no wound infections. “One of the concerns of giving radiation right after surgery is it can compromise wound healing, and this is why you wait 6 weeks,” Dr. Chen noted.
He stressed that no patient in the study suffered from adverse radiation effects that required medical or surgical intervention.
As the radiation is so short-range, hair loss and skin irritation are not side effects of GT, he added.
“The radiation is inside the brain and highly targeted, so it doesn’t hit hair follicles,” said Dr. Chen. “As best as I can observe in these patients, I did not see toxicity associated with radiation.”
One and done
Among the 22 participants, 18 had neurologic symptoms at baseline. There were no new neurologic deficits that developed after GT placement.
In addition, GT therapy improved “local control” — preventing the tumor from growing back at the site of the surgery. The local control was 86% at 6 months and 81% at 12 months.
The median progression-free survival was about 8 months. The median overall survival was 20 months (about 600 days) for the unmethylated MGMT group and 37.4 months (about 1120 days) for the methylated group.
Outcomes compared favorably to an independent glioblastoma cohort of similar patients who did not receive GT treatment during the study period, Dr. Chen noted.
“This therapy can potentially redefine how we treat glioblastoma patients whose cancer came back,” he said.
A study limitation was that it did not include quality-of-life data, which makes it challenging to assess the therapy’s overall impact, Dr. Chen said. However, he added that from his experience, patients very much appreciate not having to repeatedly take time off work for clinic or hospital visits to receive radiation treatments.
“One of the beauties of this therapy is it’s a one-and-done deal,” he said.
Interesting, timely
Commenting for this news organization, William T. Curry Jr, MD, co-director at MassGeneral Neuroscience and director of neurosurgical oncology at Mass General Cancer Center, Boston, called the study “interesting and timely.”
These new data “underscore that GT is safe in patients that have undergone gross total resection of recurrent glioblastoma and that rates of progression free survival may exceed those treated with resection alone,” said Dr. Curry, who was not involved with the research.
“Surgeons are excited about anything that has the potential to improve outcomes for patients with this very challenging disease, and it is wonderful to be able to offer hope and survival tools to patients,” he added.
However, Dr. Curry noted there are challenges and potential biases when studying survival in cancer patients without conducting a randomization process. The investigators “admit to methodological flaws inherent in the single-arm design in a patient population with recurrent glioblastoma not treated uniformly,” he said.
In addition, he noted overall survival may not have been related to the GT intervention. “Multicenter randomization is probably required to get to the bottom of the survival advantage in different subsets of glioblastoma patients,” Dr. Curry said.
Further research is needed to confirm the efficacy, appropriate indications, and timing of the intervention, but “I would support a randomized multicenter study in patients undergoing near gross total resection of recurrent glioblastoma,” he concluded.
The study received no outside funding. Dr. Chen and Dr. Curry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and spares healthy tissue, new research suggests.
The results showed inserting a collagen matrix containing radioactive seeds into the brain postsurgery did not impede wound healing. It also showed a favorable safety profile, researchers note.
Benefits for patients undergoing this GammaTile (GT) intervention include not having to wait weeks to receive radiation treatment, which in turn improves their quality of life, said study investigator Clark C. Chen, MD, PhD, chair, department of neurosurgery, University of Minnesota Medical School, Minneapolis.
“These initial results are highly promising and offer hope for patients afflicted with an otherwise devastating disease,” Dr. Chen said in an interview.
If replicated in larger trials, GT therapy “could define a new standard of care, and there would really be no reason why patients shouldn’t get this therapy,” he added.
This is the first clinical series describing GT use since its approval by the U.S. Food and Drug Administration (FDA) for recurrent brain cancer.
The findings were presented at the annual meeting of the American Association of Neurological Surgeons (AANS) and were published recently in Neuro-Oncology Advances.
Radioactive seeds
GT therapy is a version of brachytherapy where radioactive sources are placed adjacent to cancerous tissue. It consists of radioactive seeds embedded with a collagen tile.
The neurosurgeon inserts these “tiles” immediately after tumor removal to cover the entire resection cavity, Dr. Chen said. The tiles maintain the cavity architecture to prevent radiation “hot spots” associated with cavity collapse.
Dr. Chen noted the therapy is “short range,” with most of the radiation delivered within 8 millimeters of the radioactive seeds.
The radiation lasts for about a month and the collagen tiles are eventually absorbed within the body. “You put in the tiles and you don’t need to do anything more,” Dr. Chen said.
GT has a number of advantages. Unlike with traditional brachytherapy, the collagen tile provides a buffer around the radiation sources, allowing delivery of the optimal radiation dose while preserving healthy tissue.
It also avoids the up-to-6-weeks patients have to wait postsurgery to get external beam radiation therapy. “If you start radiation too early, it actually compromises wound healing, and in the meantime the tumor is growing,” said Dr. Chen.
“I have several patients where I removed a large tumor and within that 6-week period, the tumor came back entirely,” he added.
With the gamma-tile, however, radiation from the seeds kills the tumor while the body heals.
Safety profile
The study included 22 patients (mean age, 57.7 years; 15 men, 7 women) with wild-type isocitrate dehydrogenase glioblastoma. They were all having surgery for recurrent tumors.
“One of the most challenging aspects of glioblastomas is that not only do the tumors come back, they come back immediately adjacent to where you have done the surgery, and for many patients this is demoralizing,” Dr. Chen said.
Six participants had 0 6 -Methylguanine-DNA methyltranferase (MGMT) methylated glioblastoma, while the others had unmethylated MGMT.
The mean follow-up from initial diagnosis was 733 days (2 years).
Results showed one patient had to be readmitted to the hospital for hydrocephalus, but there were no re-admissions within 30 days attributable to GT.
Despite participants having undergone a second and third resection through the same surgical incision, there were no wound infections. “One of the concerns of giving radiation right after surgery is it can compromise wound healing, and this is why you wait 6 weeks,” Dr. Chen noted.
He stressed that no patient in the study suffered from adverse radiation effects that required medical or surgical intervention.
As the radiation is so short-range, hair loss and skin irritation are not side effects of GT, he added.
“The radiation is inside the brain and highly targeted, so it doesn’t hit hair follicles,” said Dr. Chen. “As best as I can observe in these patients, I did not see toxicity associated with radiation.”
One and done
Among the 22 participants, 18 had neurologic symptoms at baseline. There were no new neurologic deficits that developed after GT placement.
In addition, GT therapy improved “local control” — preventing the tumor from growing back at the site of the surgery. The local control was 86% at 6 months and 81% at 12 months.
The median progression-free survival was about 8 months. The median overall survival was 20 months (about 600 days) for the unmethylated MGMT group and 37.4 months (about 1120 days) for the methylated group.
Outcomes compared favorably to an independent glioblastoma cohort of similar patients who did not receive GT treatment during the study period, Dr. Chen noted.
“This therapy can potentially redefine how we treat glioblastoma patients whose cancer came back,” he said.
A study limitation was that it did not include quality-of-life data, which makes it challenging to assess the therapy’s overall impact, Dr. Chen said. However, he added that from his experience, patients very much appreciate not having to repeatedly take time off work for clinic or hospital visits to receive radiation treatments.
“One of the beauties of this therapy is it’s a one-and-done deal,” he said.
Interesting, timely
Commenting for this news organization, William T. Curry Jr, MD, co-director at MassGeneral Neuroscience and director of neurosurgical oncology at Mass General Cancer Center, Boston, called the study “interesting and timely.”
These new data “underscore that GT is safe in patients that have undergone gross total resection of recurrent glioblastoma and that rates of progression free survival may exceed those treated with resection alone,” said Dr. Curry, who was not involved with the research.
“Surgeons are excited about anything that has the potential to improve outcomes for patients with this very challenging disease, and it is wonderful to be able to offer hope and survival tools to patients,” he added.
However, Dr. Curry noted there are challenges and potential biases when studying survival in cancer patients without conducting a randomization process. The investigators “admit to methodological flaws inherent in the single-arm design in a patient population with recurrent glioblastoma not treated uniformly,” he said.
In addition, he noted overall survival may not have been related to the GT intervention. “Multicenter randomization is probably required to get to the bottom of the survival advantage in different subsets of glioblastoma patients,” Dr. Curry said.
Further research is needed to confirm the efficacy, appropriate indications, and timing of the intervention, but “I would support a randomized multicenter study in patients undergoing near gross total resection of recurrent glioblastoma,” he concluded.
The study received no outside funding. Dr. Chen and Dr. Curry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AANS 2022
Radiotherapy for brain metastases: ASTRO updates guidelines
“In the decade since the previous ASTRO brain metastases guideline, there has been a tremendous evolution in the way we manage patients’ disease,” said Paul D. Brown, MD, chair of the guideline task force and a professor of radiation oncology at the Mayo Clinic in Rochester, Minn.
“The development of stereotactic radiosurgery (SRS) has allowed treatment of limited brain metastases alone, often in a single fraction, while largely sparing the surrounding brain,” he elaborated in a statement. Also, novel techniques such as hippocampal avoidance with whole-brain radiation can greatly improve quality of life, he added.
The guideline was published May 6 in Practical Radiation Oncology.
“With the emergence of novel radiotherapy techniques and technologies, brain-penetrating drug therapies and neurosurgical interventions, modern management of brain metastases has become increasingly personalized, complex and multidisciplinary,” Vinai Gondi, MD, vice chair of the guideline task force and director of research and education at the Northwestern Medicine Cancer Center and Proton Center in Chicago, said in a statement.
“We developed this guideline to help inform and guide clinicians in patient-centered, multidisciplinary care for their patients with brain metastases,” he added.
Key recommendations
Overall, the recommendations address a wide range of topics related to radiation therapy in patients with cancer that has spread to the brain, including delivery techniques for radiation therapy to manage both unresected and resected brain metastases. The guideline also includes treatment algorithms for limited brain metastases and extensive brain metastases.
Key recommendations are as follows:
For patients with intact/unresected brain metastases:
- SRS is recommended for patients with 1-4 brain metastases and reasonable performance status (ECOG performance status 0-2); SRS is conditionally recommended for those with 5-10 brain metastases and reasonable performance status; for patients with tumors exerting mass effect and/or larger size, multidisciplinary discussion with neurosurgery to consider surgical resection is suggested.
- Upfront local therapy (radiation and/or surgery) is strongly recommended for patients with symptomatic brain metastases.
- For patients with asymptomatic brain metastases who are eligible for central nervous system-directed systemic therapy, multidisciplinary and patient-centered decision-making to determine whether local therapy may be safely deferred is conditionally recommended.
- Whole brain radiation therapy (WBRT) is recommended as a primary treatment for patients with favorable prognosis who have brain metastases that are ineligible for surgery and/or SRS. Hippocampal avoidance (HA) is recommended when appropriate to preserve memory function, as is the addition of memantine to delay neurocognitive decline. Adjuvant WBRT added to SRS routinely is not recommended.
- Supportive care only, without WBRT, should be considered for patients with poor prognosis and brain metastases. Reasonable options for this population include palliative care or hospice, or short-course WBRT for symptomatic brain metastases
- Recommendations also include guidance for SRS and WBRT dosing as well as the use of single-fraction vs hypofractionated SRS. Although SRS use is driven by the number of brain metastases, it is critical that other important factors (eg, total tumor volume and location, patient age, and extracranial disease status) should be taken into consideration during patient-centered decision-making by the multidisciplinary team.
For patients with resected brain metastases:
- Radiation therapy is recommended for all patients after resection in order to improve intracranial control.
- For patients with limited brain metastases after resection, postoperative SRS is recommended over WBRT to preserve the patient’s neurocognitive function and quality of life.
- As a potential alternative to SRS postresection, SRS prior to brain metastasis resection is conditionally recommended.
Updating the guidelines
ASTRO emphasizes that the scope of this paper is limited to the radiotherapeutic management of intact and resected brain metastases resulting from nonhematologic solid tumors. It provides guidance on the reasonable use of modern radiation therapy strategies, including single-fraction and fractionated (ie, hypofractionated SRS) SRS and HA-WBRT, and also discusses clinical considerations in selecting the optimal radiation therapy strategy or in deferring it in favor of best supportive care or close neuro-oncologic surveillance.
The authors note, however, that beyond the scope of this guideline, there are many other important questions that may be the subject of other guidance, such as the appropriate role for CNS-active systemic therapies and/or surgical intervention.
A version of this article was first published on Medscape.com.
“In the decade since the previous ASTRO brain metastases guideline, there has been a tremendous evolution in the way we manage patients’ disease,” said Paul D. Brown, MD, chair of the guideline task force and a professor of radiation oncology at the Mayo Clinic in Rochester, Minn.
“The development of stereotactic radiosurgery (SRS) has allowed treatment of limited brain metastases alone, often in a single fraction, while largely sparing the surrounding brain,” he elaborated in a statement. Also, novel techniques such as hippocampal avoidance with whole-brain radiation can greatly improve quality of life, he added.
The guideline was published May 6 in Practical Radiation Oncology.
“With the emergence of novel radiotherapy techniques and technologies, brain-penetrating drug therapies and neurosurgical interventions, modern management of brain metastases has become increasingly personalized, complex and multidisciplinary,” Vinai Gondi, MD, vice chair of the guideline task force and director of research and education at the Northwestern Medicine Cancer Center and Proton Center in Chicago, said in a statement.
“We developed this guideline to help inform and guide clinicians in patient-centered, multidisciplinary care for their patients with brain metastases,” he added.
Key recommendations
Overall, the recommendations address a wide range of topics related to radiation therapy in patients with cancer that has spread to the brain, including delivery techniques for radiation therapy to manage both unresected and resected brain metastases. The guideline also includes treatment algorithms for limited brain metastases and extensive brain metastases.
Key recommendations are as follows:
For patients with intact/unresected brain metastases:
- SRS is recommended for patients with 1-4 brain metastases and reasonable performance status (ECOG performance status 0-2); SRS is conditionally recommended for those with 5-10 brain metastases and reasonable performance status; for patients with tumors exerting mass effect and/or larger size, multidisciplinary discussion with neurosurgery to consider surgical resection is suggested.
- Upfront local therapy (radiation and/or surgery) is strongly recommended for patients with symptomatic brain metastases.
- For patients with asymptomatic brain metastases who are eligible for central nervous system-directed systemic therapy, multidisciplinary and patient-centered decision-making to determine whether local therapy may be safely deferred is conditionally recommended.
- Whole brain radiation therapy (WBRT) is recommended as a primary treatment for patients with favorable prognosis who have brain metastases that are ineligible for surgery and/or SRS. Hippocampal avoidance (HA) is recommended when appropriate to preserve memory function, as is the addition of memantine to delay neurocognitive decline. Adjuvant WBRT added to SRS routinely is not recommended.
- Supportive care only, without WBRT, should be considered for patients with poor prognosis and brain metastases. Reasonable options for this population include palliative care or hospice, or short-course WBRT for symptomatic brain metastases
- Recommendations also include guidance for SRS and WBRT dosing as well as the use of single-fraction vs hypofractionated SRS. Although SRS use is driven by the number of brain metastases, it is critical that other important factors (eg, total tumor volume and location, patient age, and extracranial disease status) should be taken into consideration during patient-centered decision-making by the multidisciplinary team.
For patients with resected brain metastases:
- Radiation therapy is recommended for all patients after resection in order to improve intracranial control.
- For patients with limited brain metastases after resection, postoperative SRS is recommended over WBRT to preserve the patient’s neurocognitive function and quality of life.
- As a potential alternative to SRS postresection, SRS prior to brain metastasis resection is conditionally recommended.
Updating the guidelines
ASTRO emphasizes that the scope of this paper is limited to the radiotherapeutic management of intact and resected brain metastases resulting from nonhematologic solid tumors. It provides guidance on the reasonable use of modern radiation therapy strategies, including single-fraction and fractionated (ie, hypofractionated SRS) SRS and HA-WBRT, and also discusses clinical considerations in selecting the optimal radiation therapy strategy or in deferring it in favor of best supportive care or close neuro-oncologic surveillance.
The authors note, however, that beyond the scope of this guideline, there are many other important questions that may be the subject of other guidance, such as the appropriate role for CNS-active systemic therapies and/or surgical intervention.
A version of this article was first published on Medscape.com.
“In the decade since the previous ASTRO brain metastases guideline, there has been a tremendous evolution in the way we manage patients’ disease,” said Paul D. Brown, MD, chair of the guideline task force and a professor of radiation oncology at the Mayo Clinic in Rochester, Minn.
“The development of stereotactic radiosurgery (SRS) has allowed treatment of limited brain metastases alone, often in a single fraction, while largely sparing the surrounding brain,” he elaborated in a statement. Also, novel techniques such as hippocampal avoidance with whole-brain radiation can greatly improve quality of life, he added.
The guideline was published May 6 in Practical Radiation Oncology.
“With the emergence of novel radiotherapy techniques and technologies, brain-penetrating drug therapies and neurosurgical interventions, modern management of brain metastases has become increasingly personalized, complex and multidisciplinary,” Vinai Gondi, MD, vice chair of the guideline task force and director of research and education at the Northwestern Medicine Cancer Center and Proton Center in Chicago, said in a statement.
“We developed this guideline to help inform and guide clinicians in patient-centered, multidisciplinary care for their patients with brain metastases,” he added.
Key recommendations
Overall, the recommendations address a wide range of topics related to radiation therapy in patients with cancer that has spread to the brain, including delivery techniques for radiation therapy to manage both unresected and resected brain metastases. The guideline also includes treatment algorithms for limited brain metastases and extensive brain metastases.
Key recommendations are as follows:
For patients with intact/unresected brain metastases:
- SRS is recommended for patients with 1-4 brain metastases and reasonable performance status (ECOG performance status 0-2); SRS is conditionally recommended for those with 5-10 brain metastases and reasonable performance status; for patients with tumors exerting mass effect and/or larger size, multidisciplinary discussion with neurosurgery to consider surgical resection is suggested.
- Upfront local therapy (radiation and/or surgery) is strongly recommended for patients with symptomatic brain metastases.
- For patients with asymptomatic brain metastases who are eligible for central nervous system-directed systemic therapy, multidisciplinary and patient-centered decision-making to determine whether local therapy may be safely deferred is conditionally recommended.
- Whole brain radiation therapy (WBRT) is recommended as a primary treatment for patients with favorable prognosis who have brain metastases that are ineligible for surgery and/or SRS. Hippocampal avoidance (HA) is recommended when appropriate to preserve memory function, as is the addition of memantine to delay neurocognitive decline. Adjuvant WBRT added to SRS routinely is not recommended.
- Supportive care only, without WBRT, should be considered for patients with poor prognosis and brain metastases. Reasonable options for this population include palliative care or hospice, or short-course WBRT for symptomatic brain metastases
- Recommendations also include guidance for SRS and WBRT dosing as well as the use of single-fraction vs hypofractionated SRS. Although SRS use is driven by the number of brain metastases, it is critical that other important factors (eg, total tumor volume and location, patient age, and extracranial disease status) should be taken into consideration during patient-centered decision-making by the multidisciplinary team.
For patients with resected brain metastases:
- Radiation therapy is recommended for all patients after resection in order to improve intracranial control.
- For patients with limited brain metastases after resection, postoperative SRS is recommended over WBRT to preserve the patient’s neurocognitive function and quality of life.
- As a potential alternative to SRS postresection, SRS prior to brain metastasis resection is conditionally recommended.
Updating the guidelines
ASTRO emphasizes that the scope of this paper is limited to the radiotherapeutic management of intact and resected brain metastases resulting from nonhematologic solid tumors. It provides guidance on the reasonable use of modern radiation therapy strategies, including single-fraction and fractionated (ie, hypofractionated SRS) SRS and HA-WBRT, and also discusses clinical considerations in selecting the optimal radiation therapy strategy or in deferring it in favor of best supportive care or close neuro-oncologic surveillance.
The authors note, however, that beyond the scope of this guideline, there are many other important questions that may be the subject of other guidance, such as the appropriate role for CNS-active systemic therapies and/or surgical intervention.
A version of this article was first published on Medscape.com.
FROM PRACTICAL RADIATION ONCOLOGY
Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
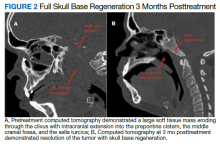
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
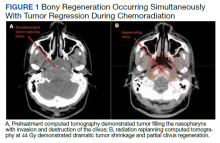
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Head and neck cancer patients recommend 11 needed improvements in health care
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY – HEAD & NECK SURGERY
Dodging potholes from cancer care to hospice transitions
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Mutation testing recommended for advanced and refractory thyroid cancer
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
FROM HEAD & NECK
Poverty-related stress linked to aggressive head and neck cancer
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
FROM HEAD & NECK
No link between cell phones and brain tumors in large U.K. study
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Cancer Data Trends 2022
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.