User login
VA Health Care Facilities Enter a New Smoke-Free Era
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
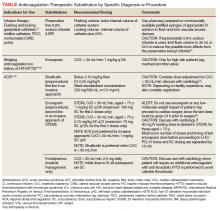
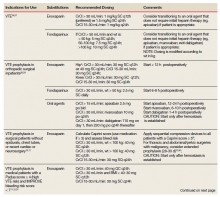
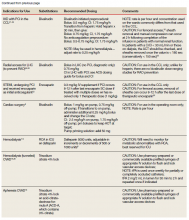
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
Vape lung disease cases exceed 400, 3 dead
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
FROM A CDC TELECONFERENCE AND NEJM
VA Pathologist Indicted for Patient Deaths Due to Misdiagnoses
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Addressing the Shortage of Physician Assistants in Medicine Clerkship Sites
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
Reframing Clinician Distress: Moral Injury Not Burnout
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
ObGyn compensation: Strides in the gender wage gap indicate closure possible
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3
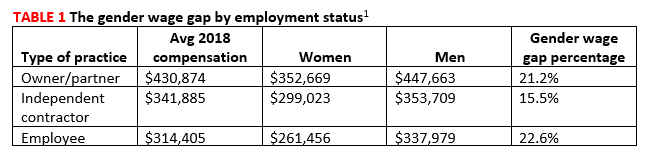
Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6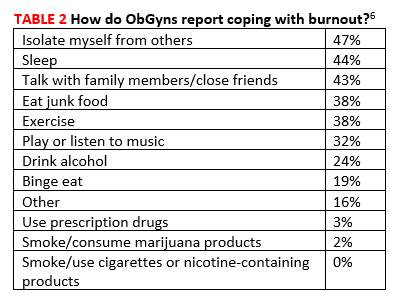
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6
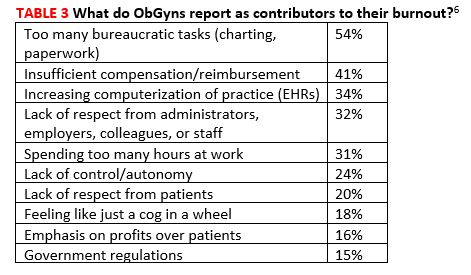
Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3

Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6

Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3

Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6

Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
Opioid Epidemic
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.