User login
Compression therapy prevents recurrence of cellulitis
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Dr. Fauci: HIV advances ‘breathtaking,’ but steadfast focus on disparities needed
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.
Proper Use and Compliance of Facial Masks During the COVID-19 Pandemic: An Observational Study of Hospitals in New York City
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
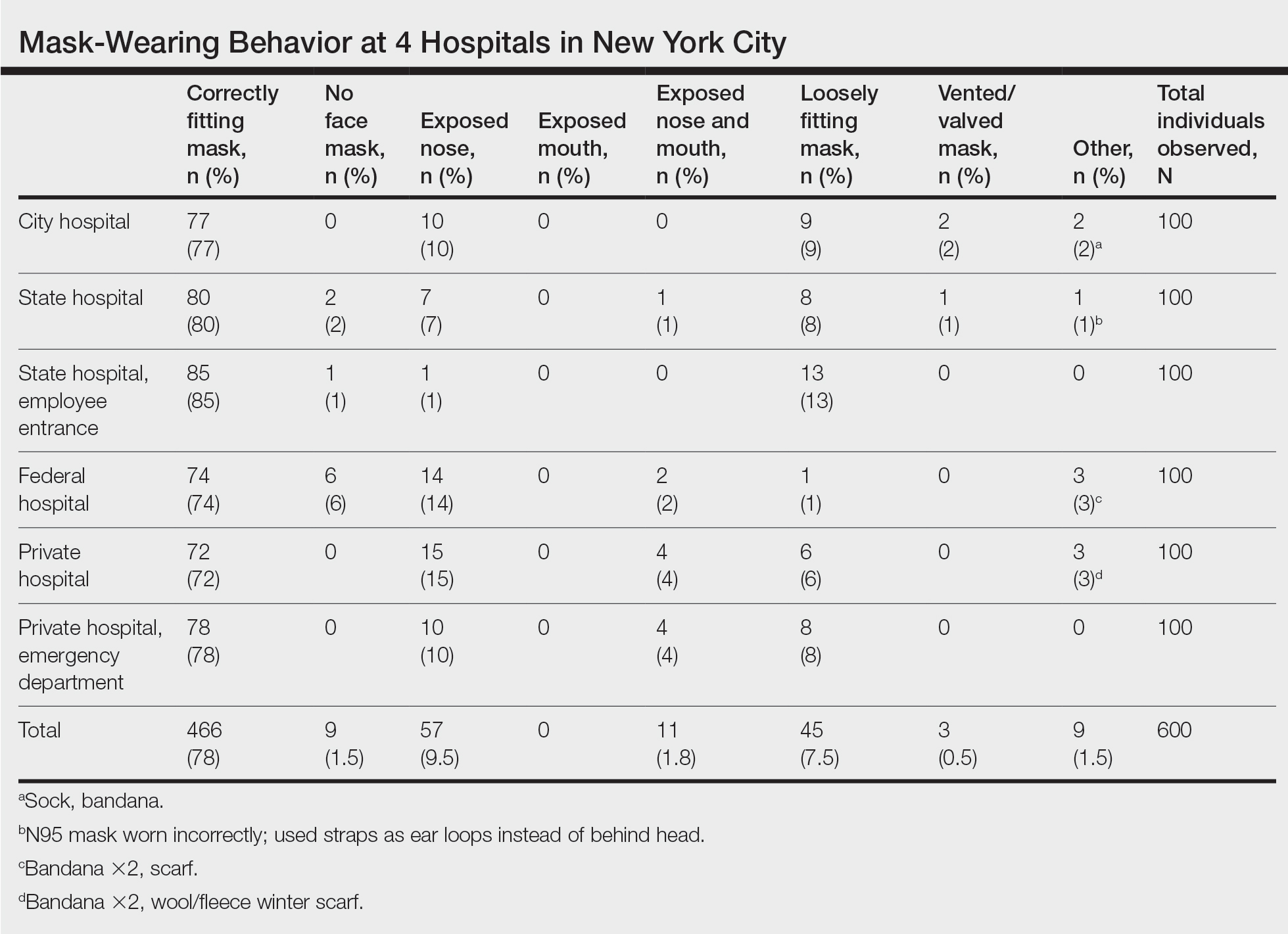
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.

Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.

Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Practice Points
- Enormous financial and human resources have been utilized by health care systems to prevent the spread of COVID-19 in health care settings, including universal temperature checks, clinical symptom triage, and masking policies. Despite these mitigation practices, mask noncompliance continues to be a major problem in hospitals.
- Mask compliance among 600 individuals entering 4 New York City hospitals was observed to be 78%, despite months of policies for universal masking and the city’s high mortality rates during the first COVID-19 wave.
- Masks have been shown to reduce the spread of COVID-19, and proper mask compliance is an important issue that must be addressed by health care administrations and governmental agencies.
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.

Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.

Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Practice Points
- Clinicians should be aware of the association between COVID-19 vaccination and the development of pityriasis rosea.
- Pityriasis rosea has been linked to reactivation of human herpesvirus 6 and human herpesvirus 7 and has been reported following administration of the influenza and human papillomavirus vaccines.
- Pityriasis rosea is a self-limited, cutaneous eruption that resolves within 6 to 8 weeks, and patients should be educated on the benign nature of this condition.
Seven legal risks of promoting unproven COVID-19 treatments
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
Successful COVID-19 Surge Management With Monoclonal Antibody Infusion in Emergency Department Patients
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
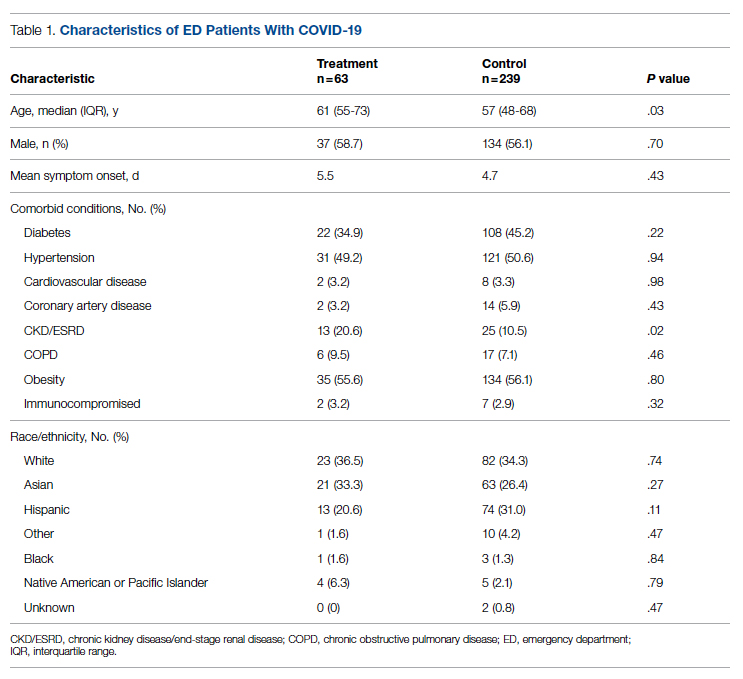
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
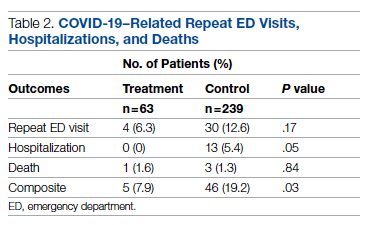
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
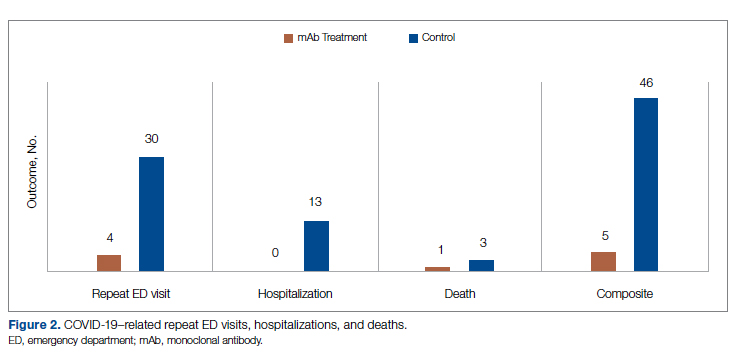
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
Antibiotics vs. placebo in acute uncomplicated diverticulitis
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Study shows wider gaps, broader inequities in U.S. sex education than 25 years ago
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
FROM THE JOURNAL OF ADOLESCENT HEALTH
HIV: Syringe services fill the gap when clinicians refuse to prescribe PrEP to people who inject drugs
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Single-dose HPV vaccination highly effective
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.