User login
New HIV PrEP guidelines call for clinicians to talk to patients about HIV prevention meds
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
Despite ‘getting it wrong’ we must continue to do what’s right
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
Cervical cancer update: The latest on screening & management
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
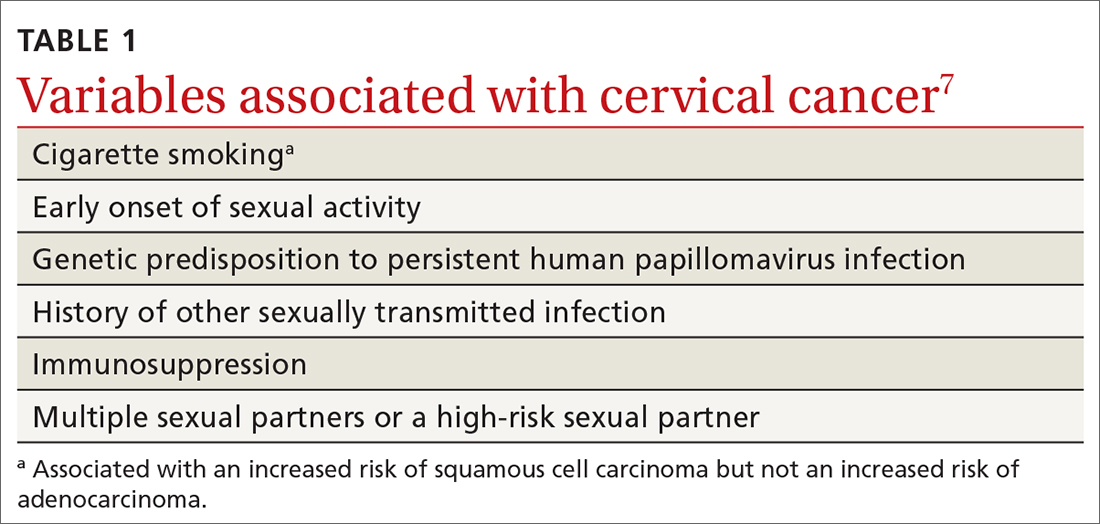
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
Management of abnormal cervical cancer screening results
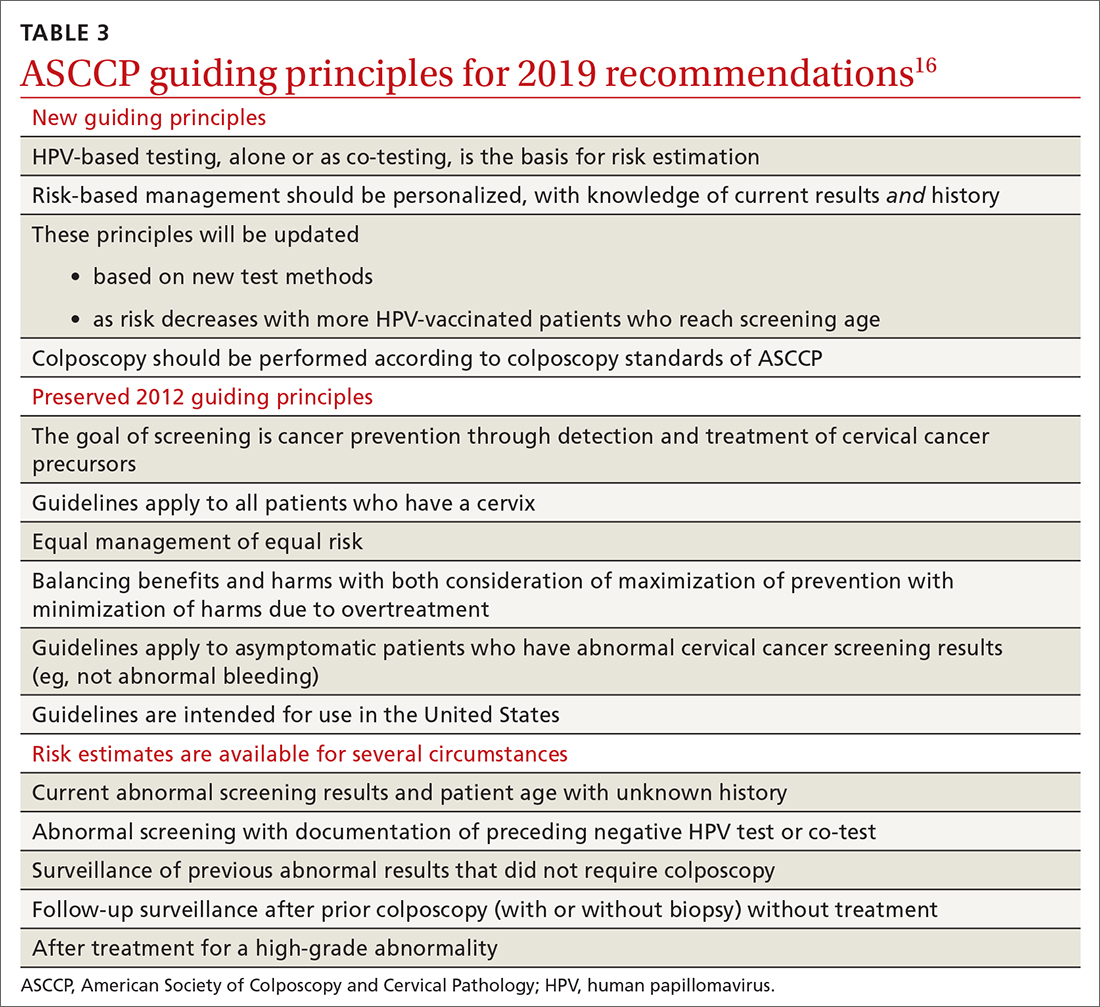
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
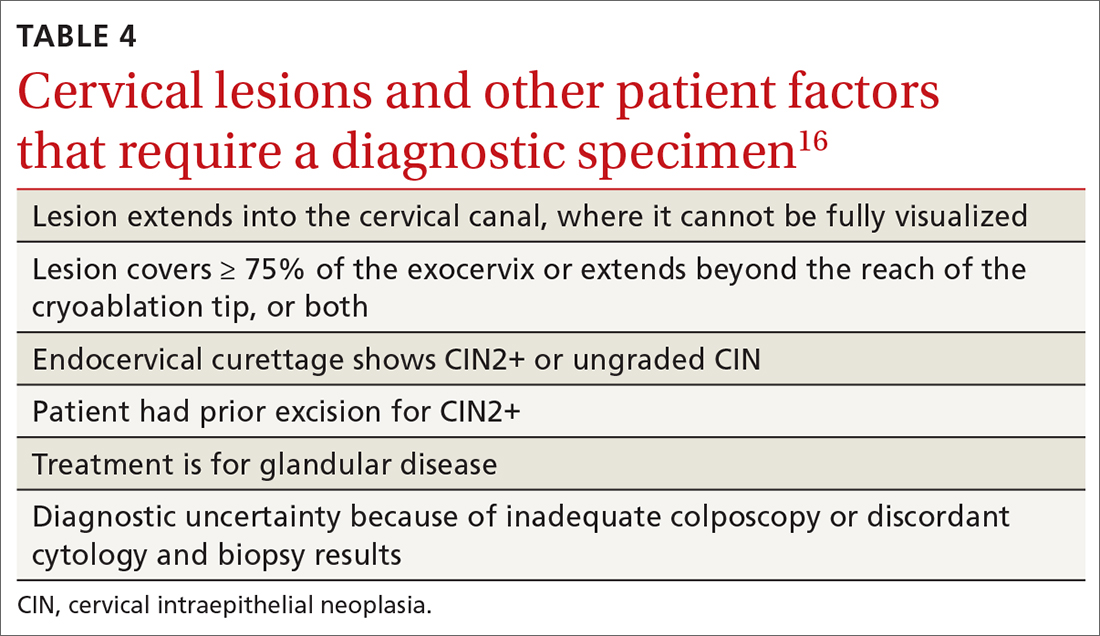
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
PRACTICE RECOMMENDATIONS
› Encourage eligible patients to be vaccinated against human papillomavirus (HPV) because the vaccine is highly effective for preventing cervical dysplasia, especially when given to patients previously unexposed to the virus. A
› Screen for cervical disease with either cytology plus HPV testing or primary HPV testing with secondary triage for cytology; both protocols are more accurate than screening with cervical cytology alone, and allow you to widen the screening interval. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
2021 CDC guidelines on sexually transmitted infections
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
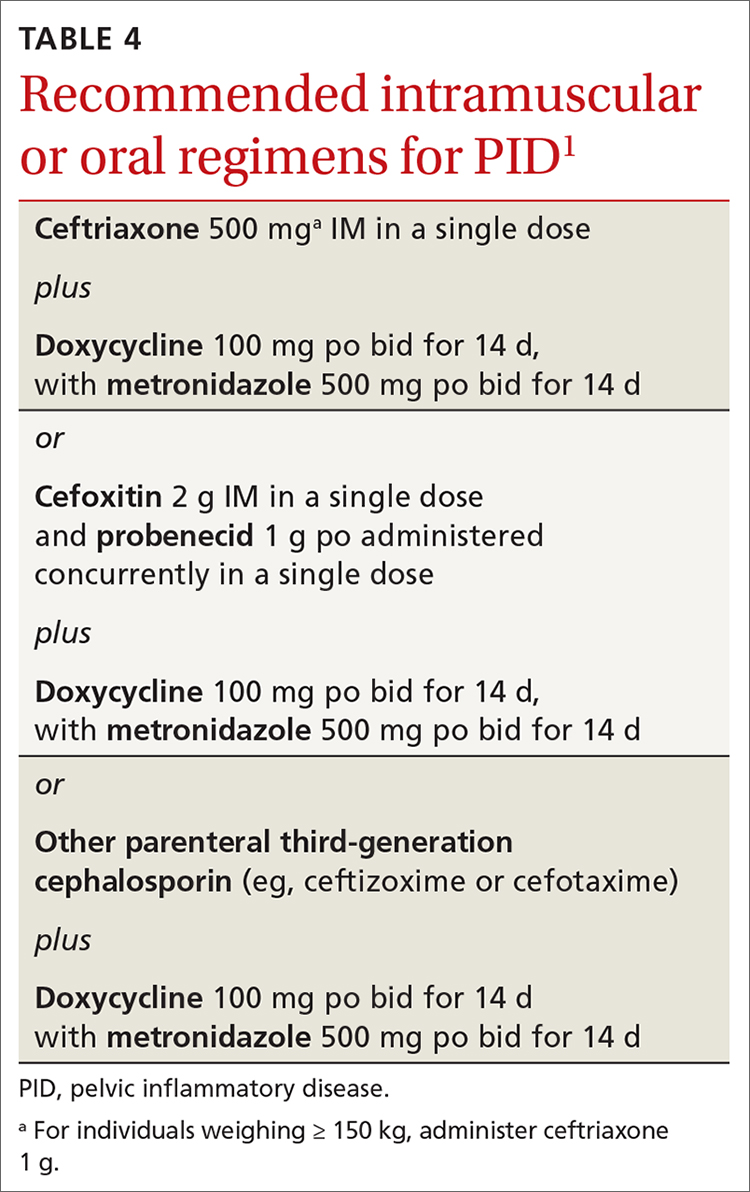
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
New data on rare myocarditis after COVID-19 vaccination
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
Intent to vaccinate kids against COVID higher among vaccinated parents
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
FROM JAMA PEDIATRICS
Children and COVID-19: 7 million cases and still counting
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.
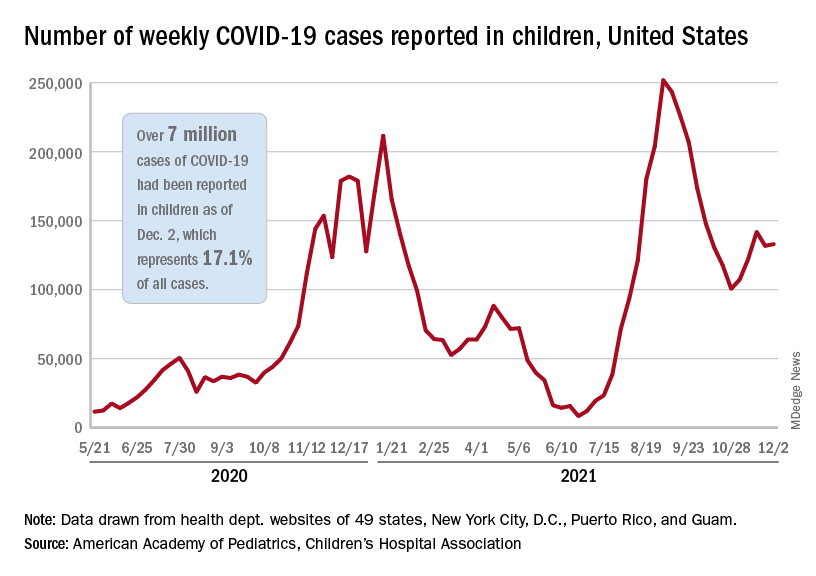
, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Specialists think it’s up to the PCP to recommend flu vaccines. But many patients don’t see a PCP every year
A new survey from the National Foundation for Infectious Diseases shows that, despite the recommendation that patients who have chronic illnesses receive annual flu vaccines, only 45% of these patients do get them. People with chronic diseases are at increased risk for serious flu-related complications, including hospitalization and death.
The survey looked at physicians’ practices toward flu vaccination and communication between health care providers (HCP) and their adult patients with chronic health conditions.
Overall, less than a third of HCPs (31%) said they recommend annual flu vaccination to all of their patients with chronic health conditions. There were some surprising differences between subspecialists. For example, 72% of patients with a heart problem who saw a cardiologist said that physician recommended the flu vaccine. The recommendation rate dropped to 32% of lung patients seeing a pulmonary physician and only 10% of people with diabetes who saw an endocrinologist.
There is quite a large gap between what physicians and patients say about their interactions. Fully 77% of HCPs who recommend annual flu vaccination say they tell patients when they are at higher risk of complications from influenza. Yet only 48% of patients say they have been given such information.
Although it is critically important information for patients to learn, their risk of influenza is often missing from the discussion. For example, patients with heart disease are six times more likely to have a heart attack within 7 days of flu infection. People with diabetes are six times more likely to be hospitalized from flu and three times more likely to die. Similarly, those with asthma or chronic obstructive pulmonary disorder are at a much higher risk of complications.
One problem is that Yet only 65% of patients with one of these chronic illnesses report seeing their primary care physician at least annually.
Much of the disparity between the patient’s perception of what they were told and the physician’s is “how the ‘recommendation’ is actually made,” William Schaffner, MD, NFID’s medical director and professor of medicine at Vanderbilt University, Nashville, Tenn., told this news organization. Dr. Schaffner offered the following example: At the end of the visit, the doctor might say: “It’s that time of the year again – you want to think about getting your flu shot.”
“The doctor thinks they’ve recommended that, but the doctor really has opened the door for you to think about it and leave [yourself] unvaccinated.”
Dr. Schaffner’s alternative? Tell the patient: “‘You’ll get your flu vaccine on the way out. Tom or Sally will give it to you.’ That’s a very different kind of recommendation. And it’s a much greater assurance of providing the vaccine.”
Another major problem, Dr. Schaffner said, is that many specialists “don’t think of vaccination as something that’s included with their routine care” even though they do direct much of the patient’s care. He said that physicians should be more “directive” in their care and that immunizations should be better integrated into routine practice.
Jody Lanard, MD, a retired risk communication consultant who spent many years working with the World Health Organization on disease outbreak communications, said in an interview that this disconnect between physician and patient reports “was really jarring. And it’s actionable!”
She offered several practical suggestions. For one, she said, “the messaging to the specialists has to be very, very empathic. We know you’re already overburdened. And here we’re asking you to do something that you think of as somebody else’s job.” But if your patient gets flu, then your job as the cardiologist or endocrinologist will become more complicated and time-consuming. So getting the patients vaccinated will be a good investment and will make your job easier.
Because of the disparity in patient and physician reports, Dr. Lanard suggested implementing a “feedback mechanism where they [the health care providers] give out the prescription, and then the office calls [the patient] to see if they’ve gotten the shot or not. Because that way it will help correct the mismatch between them thinking that they told the patient and the patient not hearing it.”
Asked about why there might be a big gap between what physicians report they said and what patients heard, Dr. Lanard explained that “physicians often communicate in [a manner] sort of like a checklist. And the patients are focused on one or two things that are high in their minds. And the physician was mentioning some things that are on a separate topic that are not on a patient’s list and it goes right past them.”
Dr. Lanard recommended brief storytelling instead of checklists. For example: “I’ve been treating your diabetes for 10 years. During this last flu season, several of my diabetic patients had a really hard time when they caught the flu. So now I’m trying harder to remember to remind you to get your flu shots.”
She urged HCPs to “make it more personal ... but it can still be scripted in advance as part of something that [you’re] remembering to do during the check.” She added that their professional associations may be able to send them suggested language they can adapt.
Finally, Dr. Lanard cautioned about vaccine myths. “The word myth is so insulting. It’s basically a word that sends the signal that you’re an idiot.”
She advised specialists to avoid the word “myth,” which will make the person defensive. Instead, say something like, “A lot of people, even some of my own family members, think the flu vaccine gives you the flu. ... But it doesn’t. And then you go into the reality.”
Dr. Lanard suggested that specialists implement the follow-up calls and close the feedback loop, saying: “If they did the survey a few years later, I bet that gap would narrow.”
Dr. Schaffner and Dr. Lanard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey from the National Foundation for Infectious Diseases shows that, despite the recommendation that patients who have chronic illnesses receive annual flu vaccines, only 45% of these patients do get them. People with chronic diseases are at increased risk for serious flu-related complications, including hospitalization and death.
The survey looked at physicians’ practices toward flu vaccination and communication between health care providers (HCP) and their adult patients with chronic health conditions.
Overall, less than a third of HCPs (31%) said they recommend annual flu vaccination to all of their patients with chronic health conditions. There were some surprising differences between subspecialists. For example, 72% of patients with a heart problem who saw a cardiologist said that physician recommended the flu vaccine. The recommendation rate dropped to 32% of lung patients seeing a pulmonary physician and only 10% of people with diabetes who saw an endocrinologist.
There is quite a large gap between what physicians and patients say about their interactions. Fully 77% of HCPs who recommend annual flu vaccination say they tell patients when they are at higher risk of complications from influenza. Yet only 48% of patients say they have been given such information.
Although it is critically important information for patients to learn, their risk of influenza is often missing from the discussion. For example, patients with heart disease are six times more likely to have a heart attack within 7 days of flu infection. People with diabetes are six times more likely to be hospitalized from flu and three times more likely to die. Similarly, those with asthma or chronic obstructive pulmonary disorder are at a much higher risk of complications.
One problem is that Yet only 65% of patients with one of these chronic illnesses report seeing their primary care physician at least annually.
Much of the disparity between the patient’s perception of what they were told and the physician’s is “how the ‘recommendation’ is actually made,” William Schaffner, MD, NFID’s medical director and professor of medicine at Vanderbilt University, Nashville, Tenn., told this news organization. Dr. Schaffner offered the following example: At the end of the visit, the doctor might say: “It’s that time of the year again – you want to think about getting your flu shot.”
“The doctor thinks they’ve recommended that, but the doctor really has opened the door for you to think about it and leave [yourself] unvaccinated.”
Dr. Schaffner’s alternative? Tell the patient: “‘You’ll get your flu vaccine on the way out. Tom or Sally will give it to you.’ That’s a very different kind of recommendation. And it’s a much greater assurance of providing the vaccine.”
Another major problem, Dr. Schaffner said, is that many specialists “don’t think of vaccination as something that’s included with their routine care” even though they do direct much of the patient’s care. He said that physicians should be more “directive” in their care and that immunizations should be better integrated into routine practice.
Jody Lanard, MD, a retired risk communication consultant who spent many years working with the World Health Organization on disease outbreak communications, said in an interview that this disconnect between physician and patient reports “was really jarring. And it’s actionable!”
She offered several practical suggestions. For one, she said, “the messaging to the specialists has to be very, very empathic. We know you’re already overburdened. And here we’re asking you to do something that you think of as somebody else’s job.” But if your patient gets flu, then your job as the cardiologist or endocrinologist will become more complicated and time-consuming. So getting the patients vaccinated will be a good investment and will make your job easier.
Because of the disparity in patient and physician reports, Dr. Lanard suggested implementing a “feedback mechanism where they [the health care providers] give out the prescription, and then the office calls [the patient] to see if they’ve gotten the shot or not. Because that way it will help correct the mismatch between them thinking that they told the patient and the patient not hearing it.”
Asked about why there might be a big gap between what physicians report they said and what patients heard, Dr. Lanard explained that “physicians often communicate in [a manner] sort of like a checklist. And the patients are focused on one or two things that are high in their minds. And the physician was mentioning some things that are on a separate topic that are not on a patient’s list and it goes right past them.”
Dr. Lanard recommended brief storytelling instead of checklists. For example: “I’ve been treating your diabetes for 10 years. During this last flu season, several of my diabetic patients had a really hard time when they caught the flu. So now I’m trying harder to remember to remind you to get your flu shots.”
She urged HCPs to “make it more personal ... but it can still be scripted in advance as part of something that [you’re] remembering to do during the check.” She added that their professional associations may be able to send them suggested language they can adapt.
Finally, Dr. Lanard cautioned about vaccine myths. “The word myth is so insulting. It’s basically a word that sends the signal that you’re an idiot.”
She advised specialists to avoid the word “myth,” which will make the person defensive. Instead, say something like, “A lot of people, even some of my own family members, think the flu vaccine gives you the flu. ... But it doesn’t. And then you go into the reality.”
Dr. Lanard suggested that specialists implement the follow-up calls and close the feedback loop, saying: “If they did the survey a few years later, I bet that gap would narrow.”
Dr. Schaffner and Dr. Lanard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey from the National Foundation for Infectious Diseases shows that, despite the recommendation that patients who have chronic illnesses receive annual flu vaccines, only 45% of these patients do get them. People with chronic diseases are at increased risk for serious flu-related complications, including hospitalization and death.
The survey looked at physicians’ practices toward flu vaccination and communication between health care providers (HCP) and their adult patients with chronic health conditions.
Overall, less than a third of HCPs (31%) said they recommend annual flu vaccination to all of their patients with chronic health conditions. There were some surprising differences between subspecialists. For example, 72% of patients with a heart problem who saw a cardiologist said that physician recommended the flu vaccine. The recommendation rate dropped to 32% of lung patients seeing a pulmonary physician and only 10% of people with diabetes who saw an endocrinologist.
There is quite a large gap between what physicians and patients say about their interactions. Fully 77% of HCPs who recommend annual flu vaccination say they tell patients when they are at higher risk of complications from influenza. Yet only 48% of patients say they have been given such information.
Although it is critically important information for patients to learn, their risk of influenza is often missing from the discussion. For example, patients with heart disease are six times more likely to have a heart attack within 7 days of flu infection. People with diabetes are six times more likely to be hospitalized from flu and three times more likely to die. Similarly, those with asthma or chronic obstructive pulmonary disorder are at a much higher risk of complications.
One problem is that Yet only 65% of patients with one of these chronic illnesses report seeing their primary care physician at least annually.
Much of the disparity between the patient’s perception of what they were told and the physician’s is “how the ‘recommendation’ is actually made,” William Schaffner, MD, NFID’s medical director and professor of medicine at Vanderbilt University, Nashville, Tenn., told this news organization. Dr. Schaffner offered the following example: At the end of the visit, the doctor might say: “It’s that time of the year again – you want to think about getting your flu shot.”
“The doctor thinks they’ve recommended that, but the doctor really has opened the door for you to think about it and leave [yourself] unvaccinated.”
Dr. Schaffner’s alternative? Tell the patient: “‘You’ll get your flu vaccine on the way out. Tom or Sally will give it to you.’ That’s a very different kind of recommendation. And it’s a much greater assurance of providing the vaccine.”
Another major problem, Dr. Schaffner said, is that many specialists “don’t think of vaccination as something that’s included with their routine care” even though they do direct much of the patient’s care. He said that physicians should be more “directive” in their care and that immunizations should be better integrated into routine practice.
Jody Lanard, MD, a retired risk communication consultant who spent many years working with the World Health Organization on disease outbreak communications, said in an interview that this disconnect between physician and patient reports “was really jarring. And it’s actionable!”
She offered several practical suggestions. For one, she said, “the messaging to the specialists has to be very, very empathic. We know you’re already overburdened. And here we’re asking you to do something that you think of as somebody else’s job.” But if your patient gets flu, then your job as the cardiologist or endocrinologist will become more complicated and time-consuming. So getting the patients vaccinated will be a good investment and will make your job easier.
Because of the disparity in patient and physician reports, Dr. Lanard suggested implementing a “feedback mechanism where they [the health care providers] give out the prescription, and then the office calls [the patient] to see if they’ve gotten the shot or not. Because that way it will help correct the mismatch between them thinking that they told the patient and the patient not hearing it.”
Asked about why there might be a big gap between what physicians report they said and what patients heard, Dr. Lanard explained that “physicians often communicate in [a manner] sort of like a checklist. And the patients are focused on one or two things that are high in their minds. And the physician was mentioning some things that are on a separate topic that are not on a patient’s list and it goes right past them.”
Dr. Lanard recommended brief storytelling instead of checklists. For example: “I’ve been treating your diabetes for 10 years. During this last flu season, several of my diabetic patients had a really hard time when they caught the flu. So now I’m trying harder to remember to remind you to get your flu shots.”
She urged HCPs to “make it more personal ... but it can still be scripted in advance as part of something that [you’re] remembering to do during the check.” She added that their professional associations may be able to send them suggested language they can adapt.
Finally, Dr. Lanard cautioned about vaccine myths. “The word myth is so insulting. It’s basically a word that sends the signal that you’re an idiot.”
She advised specialists to avoid the word “myth,” which will make the person defensive. Instead, say something like, “A lot of people, even some of my own family members, think the flu vaccine gives you the flu. ... But it doesn’t. And then you go into the reality.”
Dr. Lanard suggested that specialists implement the follow-up calls and close the feedback loop, saying: “If they did the survey a few years later, I bet that gap would narrow.”
Dr. Schaffner and Dr. Lanard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.






