User login
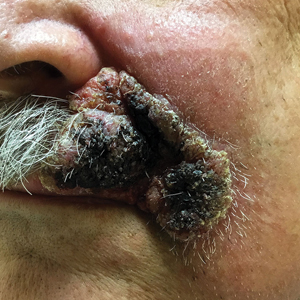
Expanding Verrucous Plaque on the Face
The Diagnosis: Blastomycosis
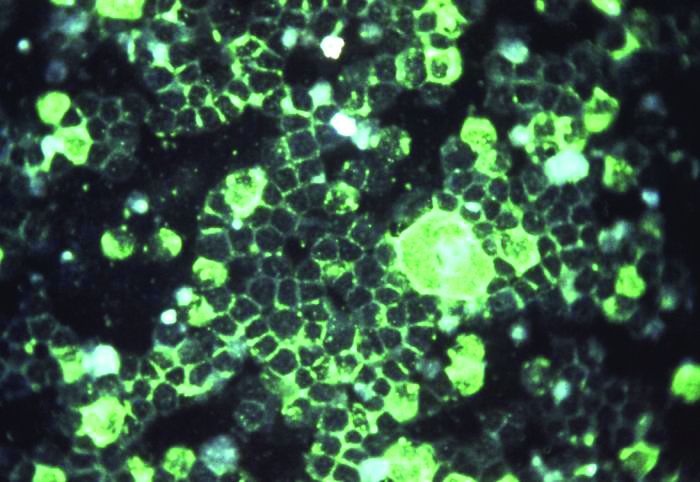
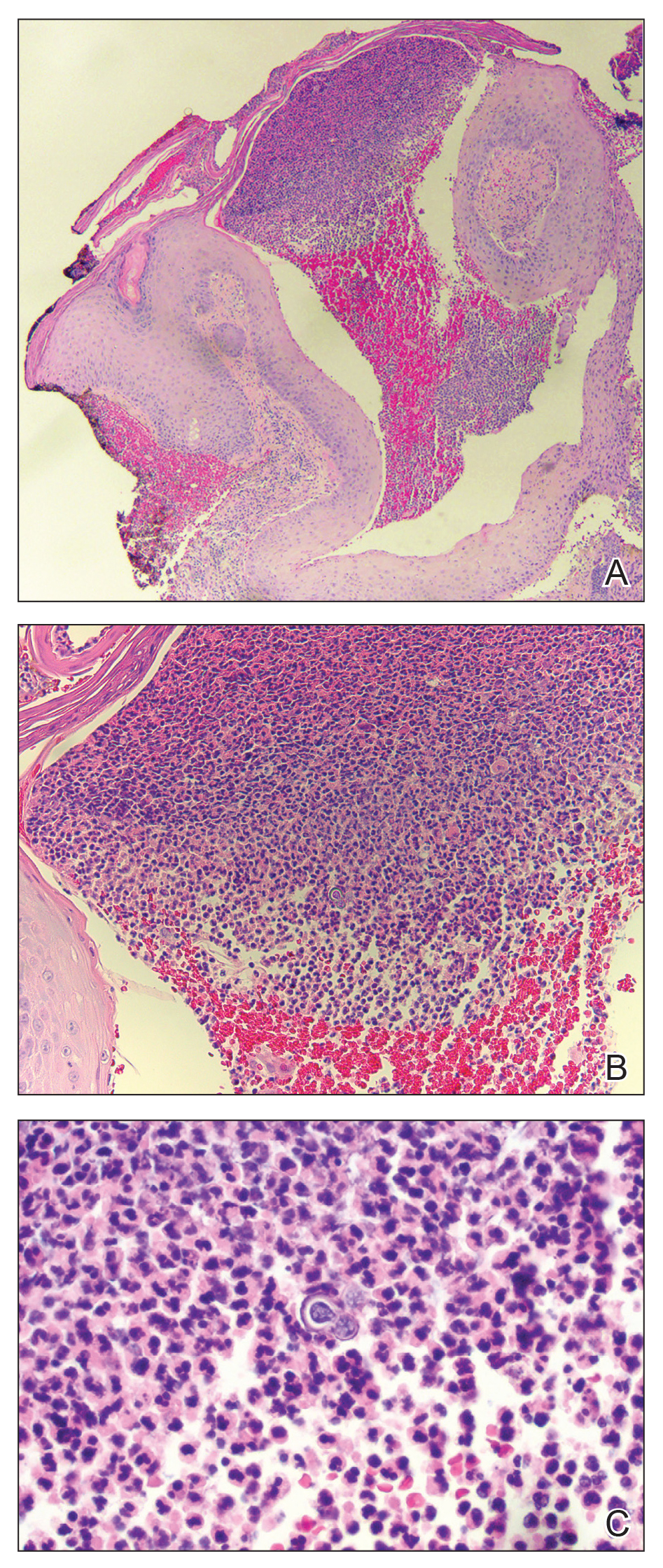
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
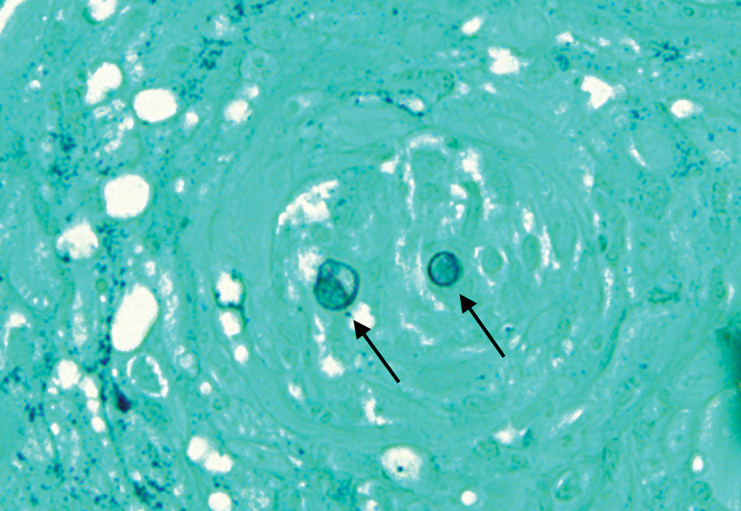
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
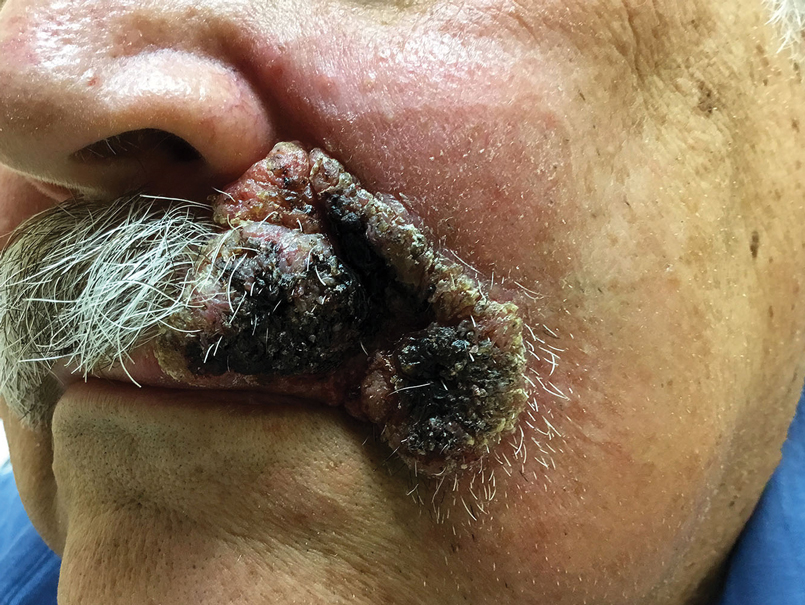
A 69-year-old man presented with a slowly expanding, verrucous plaque on the left side of the upper cutaneous lip of 4 months’ duration. The lesion reportedly began as an abscess and had undergone incision and drainage followed by multiple courses of oral antibiotics that were unsuccessful prior to presentation to our clinic. The patient’s hobbies included gardening near his summer home in the mountains of western North Carolina, where he resided when the lesion appeared. Physical examination revealed an approximately 6×4-cm verrucous plaque with central ulceration on the left side of the upper cutaneous and vermilion lip extending to the nasolabial fold. A review of systems was negative for any systemic symptoms. Routine laboratory tests and computed tomography of the head and neck were normal.
No increase seen in children’s cumulative COVID-19 burden
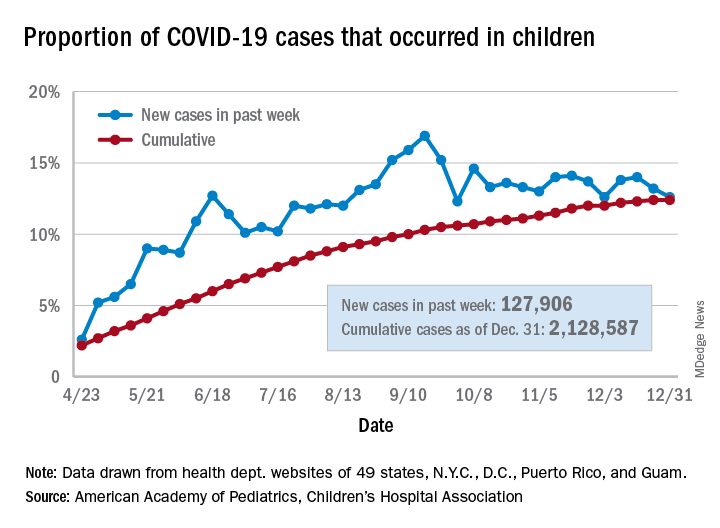
Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.
COVID-19 vaccines: The rollout, the risks, and the reason to still wear a mask
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
Skin Cancer Management During the COVID-19 Pandemic
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.

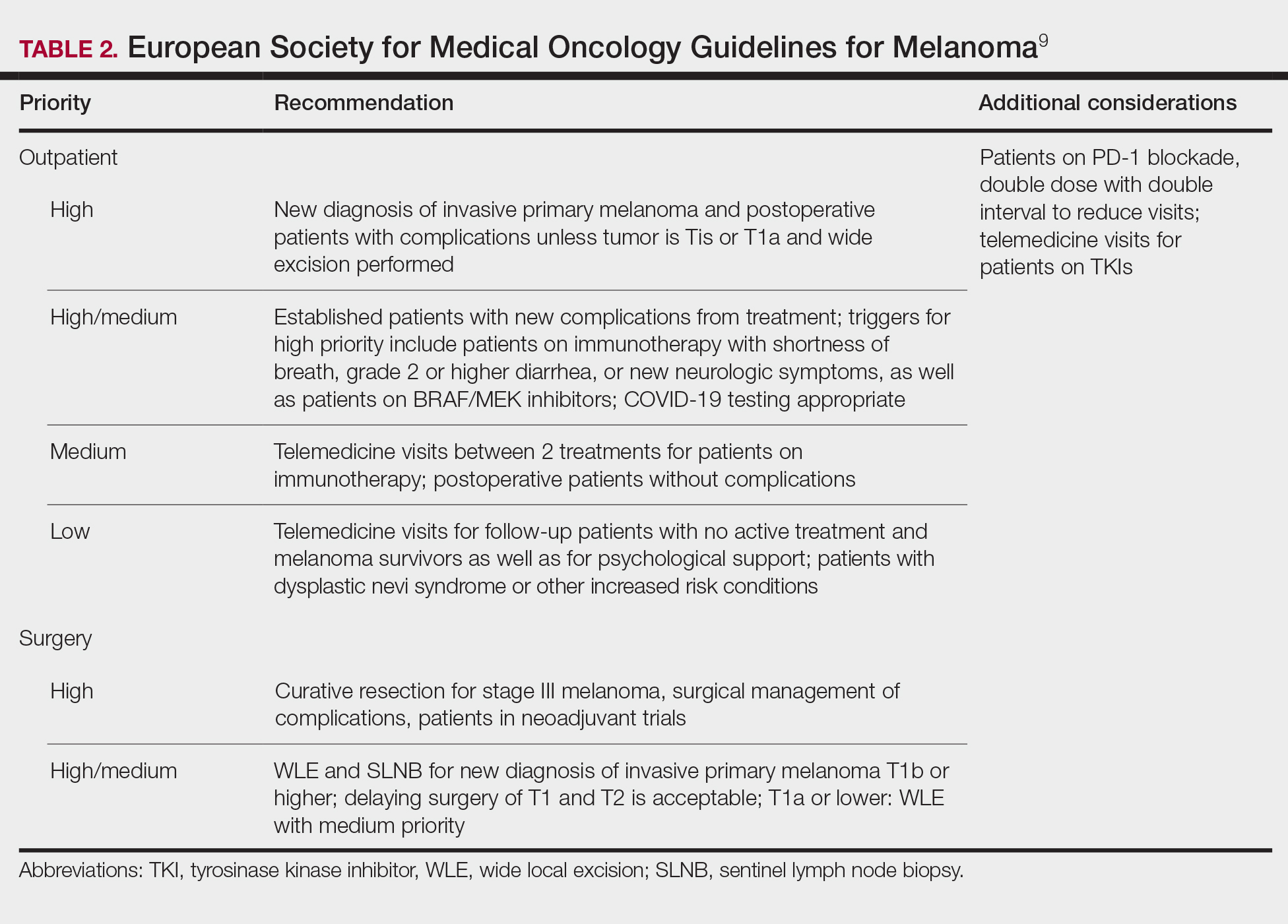

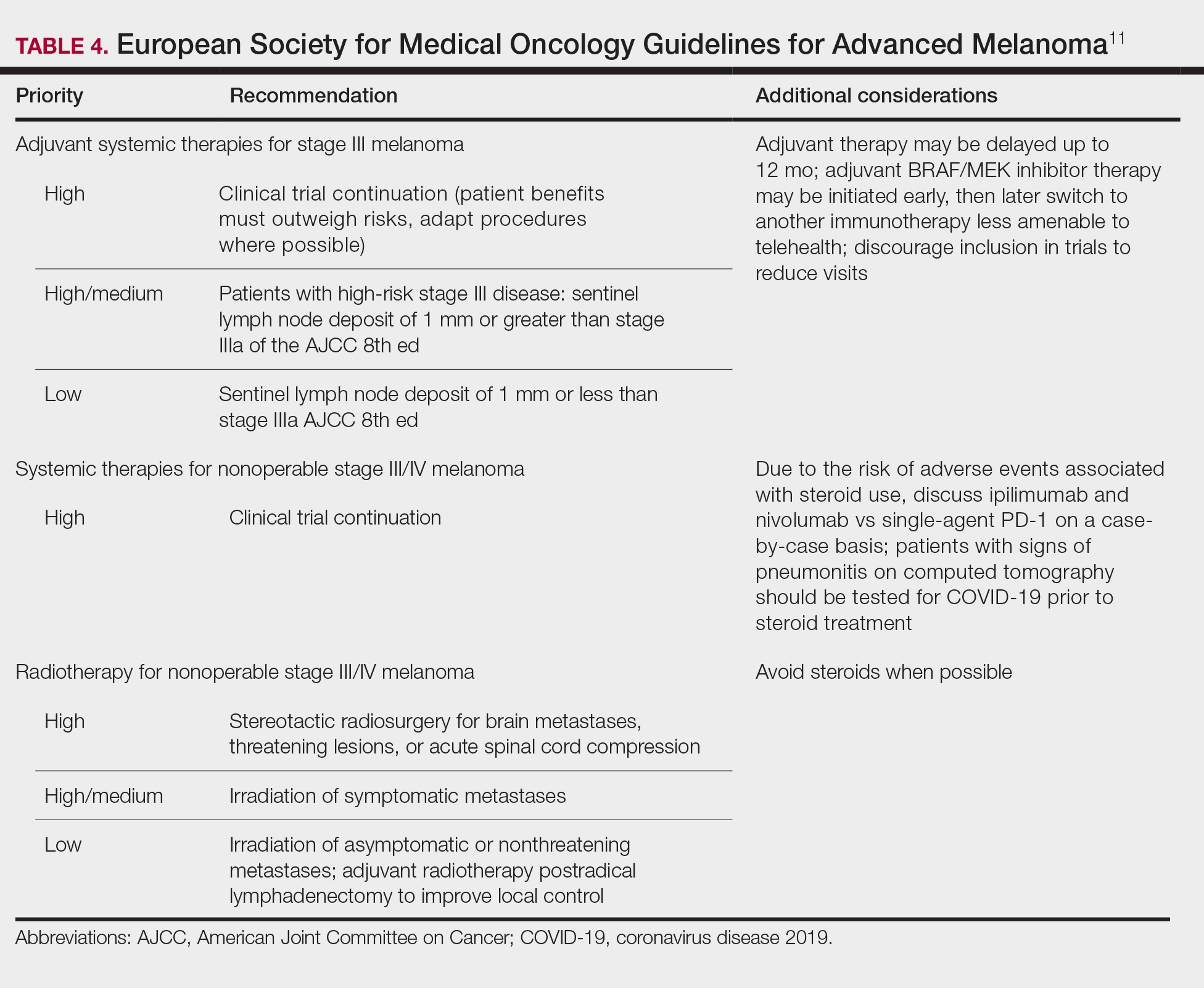
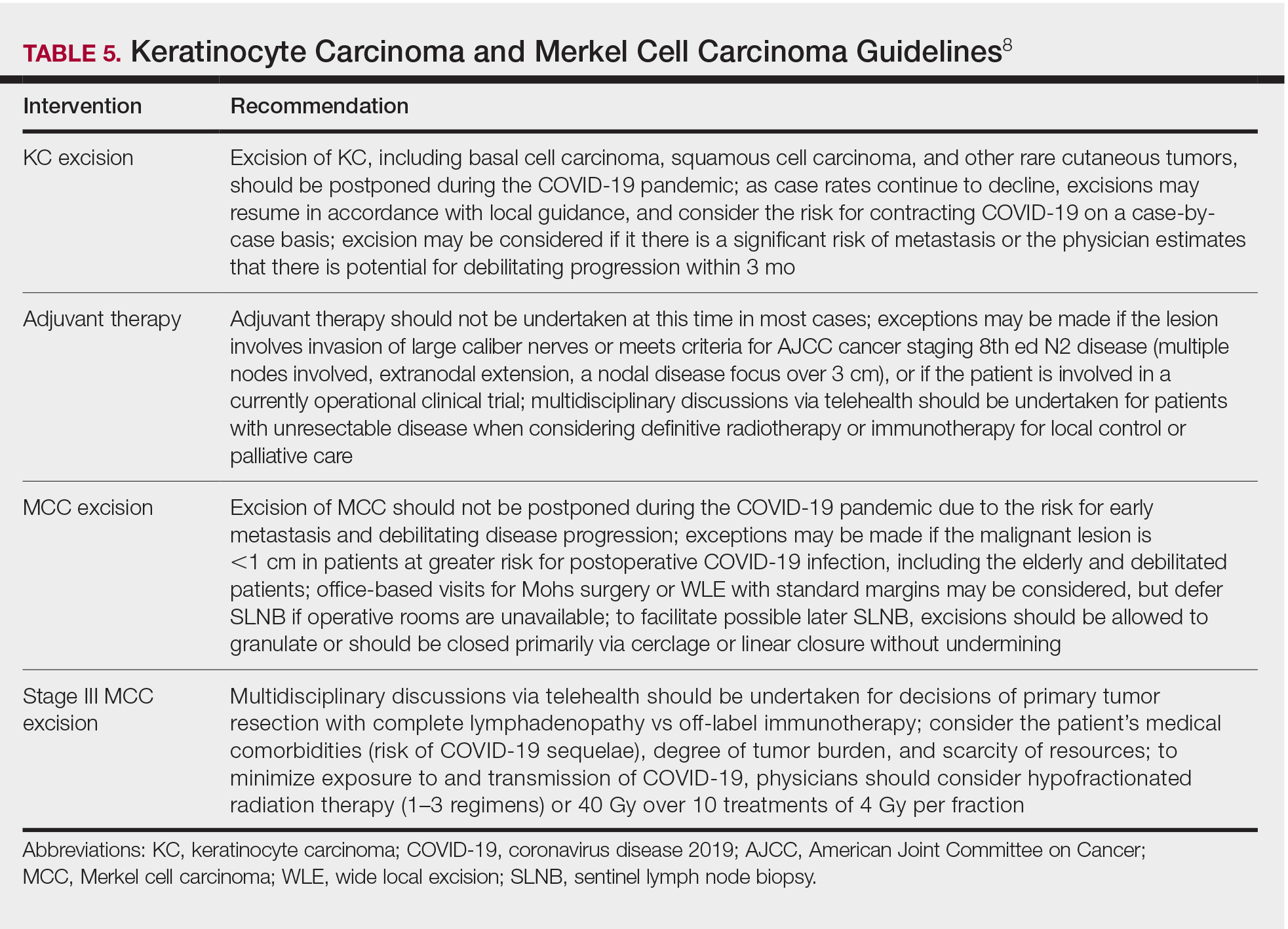
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.





Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.





Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
Practice Points
- Consider the rate of cases and transmission in your area during a pandemic surge when triaging surgical and nonsurgical cases.
- If performing head and neck surgical procedures or cosmetic procedures in which the patient cannot wear a mask, consider testing them 24 to 48 hours before the procedure.
- Follow Centers for Disease Control and Prevention (CDC) guidelines concerning screening asymptomatic patients. Also, follow CDC guidelines on testing patients who have had prior infections.
- Ensure proper personal protective equipment for yourself and staff, including the use of properly fitting N95 respirators and face shields.
Many health plans now must cover full cost of expensive HIV prevention drugs
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Skin Cancer Screening and Prevention During the COVID-19 Pandemic
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
Practice Points
- It is important for dermatologists to maintain skin cancer screening and prevention efforts during the coronavirus disease 2019 pandemic.
- Patient populations at increased risk for skin cancer should be prioritized for in-person evaluations, but teledermatology should be considered for initial examination in new patients and patients at average risk for skin cancer.
- Teledermatology presents a learning curve for dermatologists and patients, but the confidence level will increase, and evidence-based data will pave the way to enhance this experience.
Bariatric surgery might reduce severity of COVID-19 infection
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New pediatric cases down as U.S. tops 2 million children with COVID-19
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
HPV vaccine appears effective for treating warts, particularly in children
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Getting closer to a lifesaving RSV vaccine
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
FROM ESPID 2020