User login
What hospitalists need to know about COVID-19
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
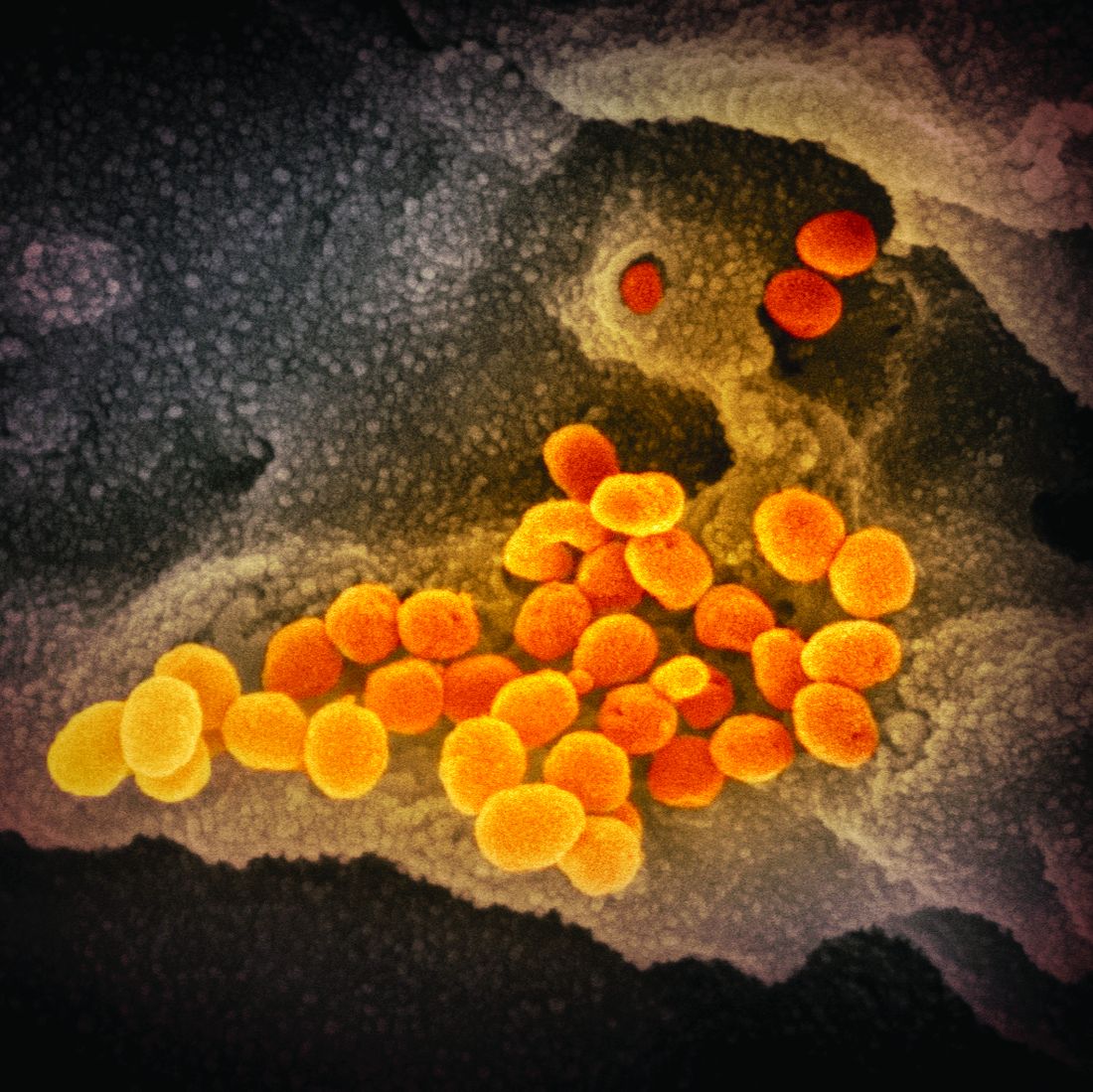
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
Increased risk of infection seen in patients with MS
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
REPORTING FROM ACTRIMS FORUM 2020
Labor & Delivery: An overlooked entry point for the spread of viral infection
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
OB hospitalists have a key role to play
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
ACIP: Flu vaccines for older adults show similar safety profiles
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP advocates pre-exposure Ebola vaccination for high-risk groups
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
COVID-19: Time to ‘take the risk of scaring people’
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
CDC expects eventual community spread of coronavirus in U.S.
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
HBV: Rethink the free pass for immune tolerant patients
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
EXPERT ANALYSIS FROM GUILD 2020
Not So Classic, Classic Kaposi Sarcoma
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.
A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.
Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.

A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.

Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.

A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.

Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
Practice Points
- Classic Kaposi sarcoma is an indolent neoplasm that can be diagnosed in immunocompetent females of Mexican descent.
- Common diagnoses appearing in skin of color may appear morphologically disparate, and a high index of suspicion is needed for correct diagnosis.
Opioid use disorder up in sepsis hospitalizations
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
REPORTING FROM CCC49