User login
Comorbid respiratory disease key predictor of NTM-PD
(NTM-PD), data from a systematic review of 99 studies indicate.
NTM-PD is frequently underdiagnosed, and data on specific risk factors are lacking, especially for high-risk individuals with preexisting respiratory diseases, wrote Michael R. Loebinger, PhD, of Imperial College London, and colleagues.
“NTM-PD can be a substantial burden for patients, contributing to lung function decline and reduced health-related quality of life, and is associated with significant morbidity and mortality,” they said.
In a study published in the journal Chest, the researchers identified 99 studies published between 2011 and 2021. Of these, 24 reported an association between risk factors and NTM-PD among patients with respiratory disease compared with patients without NTM-PD and with healthy control persons without NTM-PD; these studies were included in the meta-analysis.
Overall, comorbid respiratory disease was significantly associated with an increased risk of NTM-PD, with odds ratios ranging from 4.15 for asthma to 21.43 for bronchiectasis. Other conditions significantly associated with NTM-PD risk included history of tuberculosis (odds ratio, 12.69), interstitial lung disease (OR, 6.39), and chronic obstructive pulmonary disease (COPD) (OR, 6.63).
Other factors associated with increased NTM-PD risk included inhaled corticosteroids (OR, 4.46), oral corticosteroids (OR, 3.37), and other immunosuppressants (OR, 2.60). Additional risk factors were use of anti–tumor necrosis factor-alpha for rheumatoid arthritis (OR, 2.13), solid tumors (OR, 4.66), current pneumonia (OR, 5.54), cardiovascular disease (OR, 1.73), and low body mass index (OR, 3.04).
Additional marginal or nonsignificant associations with NTM-PD risk were found for lung function, diabetes, renal disease, cancer, healthy weight, and infection with either Pseudomonas aeruginosa or Staphylococcus aureus.
Possible protective factors, though not significant, included increasing or high BMI and long-term macrolide use.
Bronchiectasis, which is associated with the highest risk of NTM-PD, was assessed in four studies. It was evaluated less frequently because it was often considered a reason for study exclusion, the researchers wrote in their discussion.
“However, many studies report high numbers of patients with nodular bronchiectatic NTM-PD and is suggested to be almost universal in patients with noncavitary NTM-PD,” they said.
The most common risk factors for NTM-PD in the included studies were the use of immunosuppressants, female sex, COPD comorbidity, and history of suspected tuberculosis.
The findings were limited by several factors, including the high level of heterogeneity among the included studies, the lack of data on attributable risk, and inconsistent definitions of NTM-PD, the researchers noted. However, the results may be useful for highlighting risk factors that could be used to identify high-risk patients and to promote early diagnosis and treatment, they said. In addition, long-term studies are needed regarding the impact of multiple potential risk factors on individual risk for NTM-PD among patients with respiratory disease, they concluded.
The study was supported by Insmed BV. Dr. Loebinger has relationships with Insmed, AstraZeneca, Chiesi, Savara, Parion, Zambon, 30T, Electromed, Recode, AN2 Therapeutics, and Armata.
A version of this article first appeared on Medscape.com.
(NTM-PD), data from a systematic review of 99 studies indicate.
NTM-PD is frequently underdiagnosed, and data on specific risk factors are lacking, especially for high-risk individuals with preexisting respiratory diseases, wrote Michael R. Loebinger, PhD, of Imperial College London, and colleagues.
“NTM-PD can be a substantial burden for patients, contributing to lung function decline and reduced health-related quality of life, and is associated with significant morbidity and mortality,” they said.
In a study published in the journal Chest, the researchers identified 99 studies published between 2011 and 2021. Of these, 24 reported an association between risk factors and NTM-PD among patients with respiratory disease compared with patients without NTM-PD and with healthy control persons without NTM-PD; these studies were included in the meta-analysis.
Overall, comorbid respiratory disease was significantly associated with an increased risk of NTM-PD, with odds ratios ranging from 4.15 for asthma to 21.43 for bronchiectasis. Other conditions significantly associated with NTM-PD risk included history of tuberculosis (odds ratio, 12.69), interstitial lung disease (OR, 6.39), and chronic obstructive pulmonary disease (COPD) (OR, 6.63).
Other factors associated with increased NTM-PD risk included inhaled corticosteroids (OR, 4.46), oral corticosteroids (OR, 3.37), and other immunosuppressants (OR, 2.60). Additional risk factors were use of anti–tumor necrosis factor-alpha for rheumatoid arthritis (OR, 2.13), solid tumors (OR, 4.66), current pneumonia (OR, 5.54), cardiovascular disease (OR, 1.73), and low body mass index (OR, 3.04).
Additional marginal or nonsignificant associations with NTM-PD risk were found for lung function, diabetes, renal disease, cancer, healthy weight, and infection with either Pseudomonas aeruginosa or Staphylococcus aureus.
Possible protective factors, though not significant, included increasing or high BMI and long-term macrolide use.
Bronchiectasis, which is associated with the highest risk of NTM-PD, was assessed in four studies. It was evaluated less frequently because it was often considered a reason for study exclusion, the researchers wrote in their discussion.
“However, many studies report high numbers of patients with nodular bronchiectatic NTM-PD and is suggested to be almost universal in patients with noncavitary NTM-PD,” they said.
The most common risk factors for NTM-PD in the included studies were the use of immunosuppressants, female sex, COPD comorbidity, and history of suspected tuberculosis.
The findings were limited by several factors, including the high level of heterogeneity among the included studies, the lack of data on attributable risk, and inconsistent definitions of NTM-PD, the researchers noted. However, the results may be useful for highlighting risk factors that could be used to identify high-risk patients and to promote early diagnosis and treatment, they said. In addition, long-term studies are needed regarding the impact of multiple potential risk factors on individual risk for NTM-PD among patients with respiratory disease, they concluded.
The study was supported by Insmed BV. Dr. Loebinger has relationships with Insmed, AstraZeneca, Chiesi, Savara, Parion, Zambon, 30T, Electromed, Recode, AN2 Therapeutics, and Armata.
A version of this article first appeared on Medscape.com.
(NTM-PD), data from a systematic review of 99 studies indicate.
NTM-PD is frequently underdiagnosed, and data on specific risk factors are lacking, especially for high-risk individuals with preexisting respiratory diseases, wrote Michael R. Loebinger, PhD, of Imperial College London, and colleagues.
“NTM-PD can be a substantial burden for patients, contributing to lung function decline and reduced health-related quality of life, and is associated with significant morbidity and mortality,” they said.
In a study published in the journal Chest, the researchers identified 99 studies published between 2011 and 2021. Of these, 24 reported an association between risk factors and NTM-PD among patients with respiratory disease compared with patients without NTM-PD and with healthy control persons without NTM-PD; these studies were included in the meta-analysis.
Overall, comorbid respiratory disease was significantly associated with an increased risk of NTM-PD, with odds ratios ranging from 4.15 for asthma to 21.43 for bronchiectasis. Other conditions significantly associated with NTM-PD risk included history of tuberculosis (odds ratio, 12.69), interstitial lung disease (OR, 6.39), and chronic obstructive pulmonary disease (COPD) (OR, 6.63).
Other factors associated with increased NTM-PD risk included inhaled corticosteroids (OR, 4.46), oral corticosteroids (OR, 3.37), and other immunosuppressants (OR, 2.60). Additional risk factors were use of anti–tumor necrosis factor-alpha for rheumatoid arthritis (OR, 2.13), solid tumors (OR, 4.66), current pneumonia (OR, 5.54), cardiovascular disease (OR, 1.73), and low body mass index (OR, 3.04).
Additional marginal or nonsignificant associations with NTM-PD risk were found for lung function, diabetes, renal disease, cancer, healthy weight, and infection with either Pseudomonas aeruginosa or Staphylococcus aureus.
Possible protective factors, though not significant, included increasing or high BMI and long-term macrolide use.
Bronchiectasis, which is associated with the highest risk of NTM-PD, was assessed in four studies. It was evaluated less frequently because it was often considered a reason for study exclusion, the researchers wrote in their discussion.
“However, many studies report high numbers of patients with nodular bronchiectatic NTM-PD and is suggested to be almost universal in patients with noncavitary NTM-PD,” they said.
The most common risk factors for NTM-PD in the included studies were the use of immunosuppressants, female sex, COPD comorbidity, and history of suspected tuberculosis.
The findings were limited by several factors, including the high level of heterogeneity among the included studies, the lack of data on attributable risk, and inconsistent definitions of NTM-PD, the researchers noted. However, the results may be useful for highlighting risk factors that could be used to identify high-risk patients and to promote early diagnosis and treatment, they said. In addition, long-term studies are needed regarding the impact of multiple potential risk factors on individual risk for NTM-PD among patients with respiratory disease, they concluded.
The study was supported by Insmed BV. Dr. Loebinger has relationships with Insmed, AstraZeneca, Chiesi, Savara, Parion, Zambon, 30T, Electromed, Recode, AN2 Therapeutics, and Armata.
A version of this article first appeared on Medscape.com.
CDC signs off on RSV vaccine for older adults
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
Severe strep infections rebound after pandemic lull
Severe infections caused by group A streptococcus bacteria are on the rise in countries around the world, including the United States, according to new data from the Centers for Disease Control and Prevention.
Group A strep bacteria usually cause mild illnesses like strep throat and scarlet fever. But they can also cause more severe diseases, like the flesh-eating disease necrotizing fasciitis and streptococcal toxic shock syndrome, known as invasive group A strep infections.
These infections fell by 25% during the COVID-19 pandemic and were especially low in children. The number of milder infections also dropped. But in 2022, severe infections came roaring back, particularly in children.
such as Colorado and Minnesota.
Now in 2023, invasive infections are high in children in some parts of the country, even after respiratory viruses like the flu and respiratory syncytial virus (RSV) decreased in those areas. Some parts of the country also saw high rates of invasive infections in older adults.
Less severe strep A infections in children have returned to levels similar to or higher than those seen in prepandemic years.
A similar postpandemic resurgence in invasive infections has also been seen in other countries, including Canada, the United Kingdom, France, and Denmark.
Strep A is a very common bacteria that causes only mild or no symptoms in most people, and severe infections are usually quite rare. They tend to affect the most vulnerable people: those who have another virus, multiple chronic conditions, or an open wound.
People should watch for fever, headaches, or confusion during a strep infection, which all might signal a more severe illness.
A version of this article first appeared on Medscape.com.
Severe infections caused by group A streptococcus bacteria are on the rise in countries around the world, including the United States, according to new data from the Centers for Disease Control and Prevention.
Group A strep bacteria usually cause mild illnesses like strep throat and scarlet fever. But they can also cause more severe diseases, like the flesh-eating disease necrotizing fasciitis and streptococcal toxic shock syndrome, known as invasive group A strep infections.
These infections fell by 25% during the COVID-19 pandemic and were especially low in children. The number of milder infections also dropped. But in 2022, severe infections came roaring back, particularly in children.
such as Colorado and Minnesota.
Now in 2023, invasive infections are high in children in some parts of the country, even after respiratory viruses like the flu and respiratory syncytial virus (RSV) decreased in those areas. Some parts of the country also saw high rates of invasive infections in older adults.
Less severe strep A infections in children have returned to levels similar to or higher than those seen in prepandemic years.
A similar postpandemic resurgence in invasive infections has also been seen in other countries, including Canada, the United Kingdom, France, and Denmark.
Strep A is a very common bacteria that causes only mild or no symptoms in most people, and severe infections are usually quite rare. They tend to affect the most vulnerable people: those who have another virus, multiple chronic conditions, or an open wound.
People should watch for fever, headaches, or confusion during a strep infection, which all might signal a more severe illness.
A version of this article first appeared on Medscape.com.
Severe infections caused by group A streptococcus bacteria are on the rise in countries around the world, including the United States, according to new data from the Centers for Disease Control and Prevention.
Group A strep bacteria usually cause mild illnesses like strep throat and scarlet fever. But they can also cause more severe diseases, like the flesh-eating disease necrotizing fasciitis and streptococcal toxic shock syndrome, known as invasive group A strep infections.
These infections fell by 25% during the COVID-19 pandemic and were especially low in children. The number of milder infections also dropped. But in 2022, severe infections came roaring back, particularly in children.
such as Colorado and Minnesota.
Now in 2023, invasive infections are high in children in some parts of the country, even after respiratory viruses like the flu and respiratory syncytial virus (RSV) decreased in those areas. Some parts of the country also saw high rates of invasive infections in older adults.
Less severe strep A infections in children have returned to levels similar to or higher than those seen in prepandemic years.
A similar postpandemic resurgence in invasive infections has also been seen in other countries, including Canada, the United Kingdom, France, and Denmark.
Strep A is a very common bacteria that causes only mild or no symptoms in most people, and severe infections are usually quite rare. They tend to affect the most vulnerable people: those who have another virus, multiple chronic conditions, or an open wound.
People should watch for fever, headaches, or confusion during a strep infection, which all might signal a more severe illness.
A version of this article first appeared on Medscape.com.
FDA passes on olorofim despite critical need for antifungals
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
Low-calorie tastes sweeter with a little salt
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
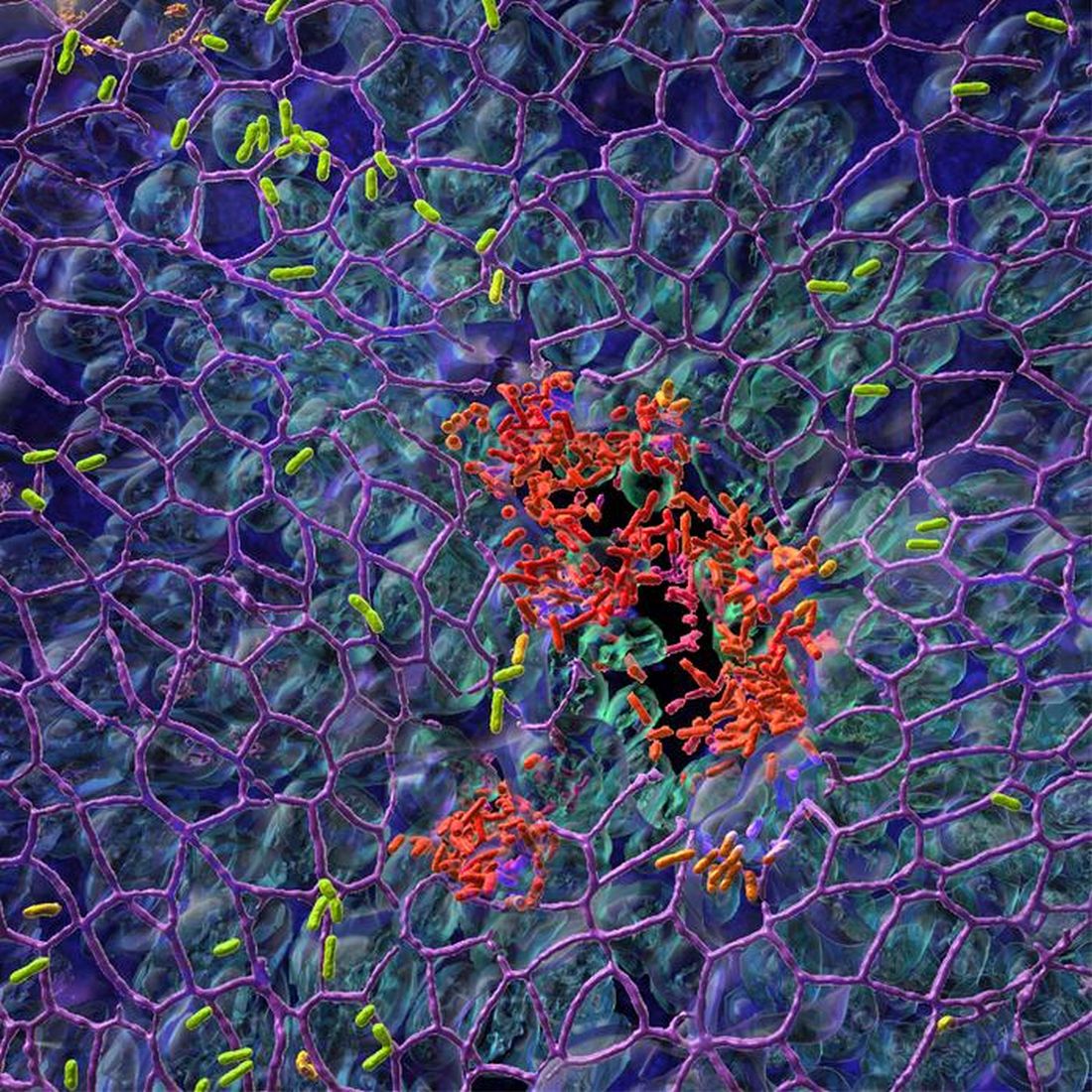
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Proteomics reveals potential targets for drug-resistant TB
TOPLINE:
Downregulation of plasma exosome-derived apolipoproteins APOA1, APOB, and APOC1 indicates DR-TB status and lipid metabolism regulation in pathogenesis.
METHODOLOGY:
Group case-controlled study assessed 17 drug resistant tuberculosis (DR-TB) and 33 non–drug resistant TB (NDR-TB) patients at The Fourth People’s Hospital of Taiyuan, China, from November 2018 to March 2019.
Plasma exosome purity and quality was determined by transmission electron microscopy, nanoparticle tracking analysis, and Western blot markers.
Proteins purified from plasma exosomes were characterized by SDS-Page with Western blotting and liquid chromatography coupled with tandem mass spectrometry techniques.
Functional proteomic differential analysis was achieved using the UniProt-GOA, Kyoto Encyclopedia of Genes and Genomes (KEGG), and STRING databases.
TAKEAWAYS:
DR-TB patients tended to be older than NDR-TB patients.
Isolated plasma exosomes were morphologically characterized as being “close to pure.”
Differential gene expression analysis revealed 16 upregulated and 10 downregulated proteins from DR-TB compared with NDR-TB patient-derived plasma exosomes.
through their functions in lipid metabolism and protein transport.
IN PRACTICE:
Key apolipoproteins “may be involved in the pathogenesis of DR-TB via accelerating the formation of foamy macrophages and reducing the cellular uptake of anti-TB drugs.”
STUDY DETAILS:
The study led by Mingrui Wu of Shanxi (China) Medical University and colleagues was published in the July 2023 issue of Tuberculosis.
LIMITATIONS:
This study is limited by an enrollment bias of at least twice as many men to women patients for both DR-TB and NDR-TB categories, reporting of some incomplete data collection characterizing the study population, and small sample size, which did not permit stratified analysis of the five types of DR-TB.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
TOPLINE:
Downregulation of plasma exosome-derived apolipoproteins APOA1, APOB, and APOC1 indicates DR-TB status and lipid metabolism regulation in pathogenesis.
METHODOLOGY:
Group case-controlled study assessed 17 drug resistant tuberculosis (DR-TB) and 33 non–drug resistant TB (NDR-TB) patients at The Fourth People’s Hospital of Taiyuan, China, from November 2018 to March 2019.
Plasma exosome purity and quality was determined by transmission electron microscopy, nanoparticle tracking analysis, and Western blot markers.
Proteins purified from plasma exosomes were characterized by SDS-Page with Western blotting and liquid chromatography coupled with tandem mass spectrometry techniques.
Functional proteomic differential analysis was achieved using the UniProt-GOA, Kyoto Encyclopedia of Genes and Genomes (KEGG), and STRING databases.
TAKEAWAYS:
DR-TB patients tended to be older than NDR-TB patients.
Isolated plasma exosomes were morphologically characterized as being “close to pure.”
Differential gene expression analysis revealed 16 upregulated and 10 downregulated proteins from DR-TB compared with NDR-TB patient-derived plasma exosomes.
through their functions in lipid metabolism and protein transport.
IN PRACTICE:
Key apolipoproteins “may be involved in the pathogenesis of DR-TB via accelerating the formation of foamy macrophages and reducing the cellular uptake of anti-TB drugs.”
STUDY DETAILS:
The study led by Mingrui Wu of Shanxi (China) Medical University and colleagues was published in the July 2023 issue of Tuberculosis.
LIMITATIONS:
This study is limited by an enrollment bias of at least twice as many men to women patients for both DR-TB and NDR-TB categories, reporting of some incomplete data collection characterizing the study population, and small sample size, which did not permit stratified analysis of the five types of DR-TB.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
TOPLINE:
Downregulation of plasma exosome-derived apolipoproteins APOA1, APOB, and APOC1 indicates DR-TB status and lipid metabolism regulation in pathogenesis.
METHODOLOGY:
Group case-controlled study assessed 17 drug resistant tuberculosis (DR-TB) and 33 non–drug resistant TB (NDR-TB) patients at The Fourth People’s Hospital of Taiyuan, China, from November 2018 to March 2019.
Plasma exosome purity and quality was determined by transmission electron microscopy, nanoparticle tracking analysis, and Western blot markers.
Proteins purified from plasma exosomes were characterized by SDS-Page with Western blotting and liquid chromatography coupled with tandem mass spectrometry techniques.
Functional proteomic differential analysis was achieved using the UniProt-GOA, Kyoto Encyclopedia of Genes and Genomes (KEGG), and STRING databases.
TAKEAWAYS:
DR-TB patients tended to be older than NDR-TB patients.
Isolated plasma exosomes were morphologically characterized as being “close to pure.”
Differential gene expression analysis revealed 16 upregulated and 10 downregulated proteins from DR-TB compared with NDR-TB patient-derived plasma exosomes.
through their functions in lipid metabolism and protein transport.
IN PRACTICE:
Key apolipoproteins “may be involved in the pathogenesis of DR-TB via accelerating the formation of foamy macrophages and reducing the cellular uptake of anti-TB drugs.”
STUDY DETAILS:
The study led by Mingrui Wu of Shanxi (China) Medical University and colleagues was published in the July 2023 issue of Tuberculosis.
LIMITATIONS:
This study is limited by an enrollment bias of at least twice as many men to women patients for both DR-TB and NDR-TB categories, reporting of some incomplete data collection characterizing the study population, and small sample size, which did not permit stratified analysis of the five types of DR-TB.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New tool uses nanotechnology to speed up diagnostic testing of infectious disease
A new tool promises to expedite detection of infectious disease, according to researchers from McGill University, Montreal.
The diagnostic platform, called The device was tested for several respiratory viruses and bacteria, including the H1N1 influenza virus and SARS-CoV-2. It achieved 95% accuracy at identifying COVID-19 and its variants in 48 human saliva samples.
“COVID was something that opened our eyes, and now we have to think more seriously about point-of-care diagnostics,” Sara Mahshid, PhD, assistant professor of biomedical engineering and Canada Research Chair in Nano-Biosensing Devices at McGill University, said in an interview. The technology could become important for a range of medical applications, especially in low-resource areas.
The development was detailed in an article in Nature Nanotechnology.
Nonclinical setting
The COVID-19 pandemic has demonstrated the need for fast and accurate testing that can be used outside of a clinical setting. The gold-standard diagnostic method is PCR testing, but its accuracy comes with a trade-off. PCR testing involves a lengthy protocol and requires a centralized testing facility.
With QolorEX, the investigators aimed to develop a new test that achieves the accuracy of PCR in an automated tool that can be used outside of a testing facility or hospital setting. Dr. Mahshid noted a particular need for a tool that could be used in congregate settings, such as airports, schools, or restaurants.
The device is compact enough to sit on a tabletop or bench and can be used easily in group settings, according to Dr. Mahshid. In the future, she hopes to further miniaturize the device to make it more scalable for widespread use.
Requiring only a saliva sample, the tool is easy to use. Unlike current COVID-19 rapid tests, which involve several steps, the system is automated and does not require manually mixing reagents. After collecting a sample, a user taps a button in a smartphone or computer application. The device handles the rest.
“We’re not chemists who understand how to mix these solutions,” Dr. Mahshid said. Avoiding those extra steps may reduce the false positives and false negatives caused by user error.
Fast results
QolorEX can return results in 13 minutes, like a rapid antigen test does. Like a PCR test, the device uses nucleic acid amplification. But PCR tests typically take much longer. The sample analysis alone takes 1.5-2 hours.
The new test accelerates the reaction by injecting light-excited “hot” electrons from the surface of a nanoplasmonic sensor. The device then uses imaging and a machine learning algorithm to quantify a color transformation that occurs when a pathogen is present.
The fast, reliable results make the system potentially appropriate for use in places such as airports. Previously, passengers had to wait 24 hours for a negative COVID test before boarding a plane. A device such as QolorEX would allow screening on site.
The ability of the tool to distinguish between bacterial and viral infections so quickly is “an application that is both important and extremely difficult to achieve,” according to Nikhil Bhalla, PhD, in a research briefing. Dr. Bhalla is a lecturer in electronic engineering at Ulster University, Belfast, Ireland.
The researchers hope that by delivering results quickly, the device will help reduce the spread of respiratory diseases and possibly save lives.
‘Sensitive and specific’
The primary benefit of the tool is its ability to return results quickly while having low false positive and false negative rates, according to Leyla Soleymani, PhD, of McMaster University, Hamilton, Ont. “It is hard to come by rapid tests that are both sensitive and specific, compared to PCR,” Dr. Soleymani told this news organization.
Although QolorEX was developed to detect COVID-19 and other infectious diseases, the uses of the device are not limited to the pathogens tested. The tool can be applied to a range of tests that currently use PCR technology. Dr. Mahshid and her team are considering several other applications of the technology, such as analyzing therapeutics for antimicrobial-resistant pathogens prioritized by the World Health Organization. The technology may also have potential for detecting cancer and bacterial infections, Dr. Mahshid said in an interview.
But to Dr. Soleymani, the most exciting application remains its use in diagnosing infectious diseases. She noted, however, that it’s unclear whether the price of the device will be too high for widespread home use. It may be more practical for family physician clinics and other facilities.
Before the device becomes commercially available, more testing is needed to validate the results, which are based on a limited number of samples that were available in a research setting.
The study was supported by the MI4 Emergency COVID-19 Research Funding, Natural Sciences and Engineering Research Council of Canada, Canadian Institutes of Health Research, Canada Foundation for Innovation, and McGill University. Dr. Mahshid and Dr. Soleymani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new tool promises to expedite detection of infectious disease, according to researchers from McGill University, Montreal.
The diagnostic platform, called The device was tested for several respiratory viruses and bacteria, including the H1N1 influenza virus and SARS-CoV-2. It achieved 95% accuracy at identifying COVID-19 and its variants in 48 human saliva samples.
“COVID was something that opened our eyes, and now we have to think more seriously about point-of-care diagnostics,” Sara Mahshid, PhD, assistant professor of biomedical engineering and Canada Research Chair in Nano-Biosensing Devices at McGill University, said in an interview. The technology could become important for a range of medical applications, especially in low-resource areas.
The development was detailed in an article in Nature Nanotechnology.
Nonclinical setting
The COVID-19 pandemic has demonstrated the need for fast and accurate testing that can be used outside of a clinical setting. The gold-standard diagnostic method is PCR testing, but its accuracy comes with a trade-off. PCR testing involves a lengthy protocol and requires a centralized testing facility.
With QolorEX, the investigators aimed to develop a new test that achieves the accuracy of PCR in an automated tool that can be used outside of a testing facility or hospital setting. Dr. Mahshid noted a particular need for a tool that could be used in congregate settings, such as airports, schools, or restaurants.
The device is compact enough to sit on a tabletop or bench and can be used easily in group settings, according to Dr. Mahshid. In the future, she hopes to further miniaturize the device to make it more scalable for widespread use.
Requiring only a saliva sample, the tool is easy to use. Unlike current COVID-19 rapid tests, which involve several steps, the system is automated and does not require manually mixing reagents. After collecting a sample, a user taps a button in a smartphone or computer application. The device handles the rest.
“We’re not chemists who understand how to mix these solutions,” Dr. Mahshid said. Avoiding those extra steps may reduce the false positives and false negatives caused by user error.
Fast results
QolorEX can return results in 13 minutes, like a rapid antigen test does. Like a PCR test, the device uses nucleic acid amplification. But PCR tests typically take much longer. The sample analysis alone takes 1.5-2 hours.
The new test accelerates the reaction by injecting light-excited “hot” electrons from the surface of a nanoplasmonic sensor. The device then uses imaging and a machine learning algorithm to quantify a color transformation that occurs when a pathogen is present.
The fast, reliable results make the system potentially appropriate for use in places such as airports. Previously, passengers had to wait 24 hours for a negative COVID test before boarding a plane. A device such as QolorEX would allow screening on site.
The ability of the tool to distinguish between bacterial and viral infections so quickly is “an application that is both important and extremely difficult to achieve,” according to Nikhil Bhalla, PhD, in a research briefing. Dr. Bhalla is a lecturer in electronic engineering at Ulster University, Belfast, Ireland.
The researchers hope that by delivering results quickly, the device will help reduce the spread of respiratory diseases and possibly save lives.
‘Sensitive and specific’
The primary benefit of the tool is its ability to return results quickly while having low false positive and false negative rates, according to Leyla Soleymani, PhD, of McMaster University, Hamilton, Ont. “It is hard to come by rapid tests that are both sensitive and specific, compared to PCR,” Dr. Soleymani told this news organization.
Although QolorEX was developed to detect COVID-19 and other infectious diseases, the uses of the device are not limited to the pathogens tested. The tool can be applied to a range of tests that currently use PCR technology. Dr. Mahshid and her team are considering several other applications of the technology, such as analyzing therapeutics for antimicrobial-resistant pathogens prioritized by the World Health Organization. The technology may also have potential for detecting cancer and bacterial infections, Dr. Mahshid said in an interview.
But to Dr. Soleymani, the most exciting application remains its use in diagnosing infectious diseases. She noted, however, that it’s unclear whether the price of the device will be too high for widespread home use. It may be more practical for family physician clinics and other facilities.
Before the device becomes commercially available, more testing is needed to validate the results, which are based on a limited number of samples that were available in a research setting.
The study was supported by the MI4 Emergency COVID-19 Research Funding, Natural Sciences and Engineering Research Council of Canada, Canadian Institutes of Health Research, Canada Foundation for Innovation, and McGill University. Dr. Mahshid and Dr. Soleymani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new tool promises to expedite detection of infectious disease, according to researchers from McGill University, Montreal.
The diagnostic platform, called The device was tested for several respiratory viruses and bacteria, including the H1N1 influenza virus and SARS-CoV-2. It achieved 95% accuracy at identifying COVID-19 and its variants in 48 human saliva samples.
“COVID was something that opened our eyes, and now we have to think more seriously about point-of-care diagnostics,” Sara Mahshid, PhD, assistant professor of biomedical engineering and Canada Research Chair in Nano-Biosensing Devices at McGill University, said in an interview. The technology could become important for a range of medical applications, especially in low-resource areas.
The development was detailed in an article in Nature Nanotechnology.
Nonclinical setting
The COVID-19 pandemic has demonstrated the need for fast and accurate testing that can be used outside of a clinical setting. The gold-standard diagnostic method is PCR testing, but its accuracy comes with a trade-off. PCR testing involves a lengthy protocol and requires a centralized testing facility.
With QolorEX, the investigators aimed to develop a new test that achieves the accuracy of PCR in an automated tool that can be used outside of a testing facility or hospital setting. Dr. Mahshid noted a particular need for a tool that could be used in congregate settings, such as airports, schools, or restaurants.
The device is compact enough to sit on a tabletop or bench and can be used easily in group settings, according to Dr. Mahshid. In the future, she hopes to further miniaturize the device to make it more scalable for widespread use.
Requiring only a saliva sample, the tool is easy to use. Unlike current COVID-19 rapid tests, which involve several steps, the system is automated and does not require manually mixing reagents. After collecting a sample, a user taps a button in a smartphone or computer application. The device handles the rest.
“We’re not chemists who understand how to mix these solutions,” Dr. Mahshid said. Avoiding those extra steps may reduce the false positives and false negatives caused by user error.
Fast results
QolorEX can return results in 13 minutes, like a rapid antigen test does. Like a PCR test, the device uses nucleic acid amplification. But PCR tests typically take much longer. The sample analysis alone takes 1.5-2 hours.
The new test accelerates the reaction by injecting light-excited “hot” electrons from the surface of a nanoplasmonic sensor. The device then uses imaging and a machine learning algorithm to quantify a color transformation that occurs when a pathogen is present.
The fast, reliable results make the system potentially appropriate for use in places such as airports. Previously, passengers had to wait 24 hours for a negative COVID test before boarding a plane. A device such as QolorEX would allow screening on site.
The ability of the tool to distinguish between bacterial and viral infections so quickly is “an application that is both important and extremely difficult to achieve,” according to Nikhil Bhalla, PhD, in a research briefing. Dr. Bhalla is a lecturer in electronic engineering at Ulster University, Belfast, Ireland.
The researchers hope that by delivering results quickly, the device will help reduce the spread of respiratory diseases and possibly save lives.
‘Sensitive and specific’
The primary benefit of the tool is its ability to return results quickly while having low false positive and false negative rates, according to Leyla Soleymani, PhD, of McMaster University, Hamilton, Ont. “It is hard to come by rapid tests that are both sensitive and specific, compared to PCR,” Dr. Soleymani told this news organization.
Although QolorEX was developed to detect COVID-19 and other infectious diseases, the uses of the device are not limited to the pathogens tested. The tool can be applied to a range of tests that currently use PCR technology. Dr. Mahshid and her team are considering several other applications of the technology, such as analyzing therapeutics for antimicrobial-resistant pathogens prioritized by the World Health Organization. The technology may also have potential for detecting cancer and bacterial infections, Dr. Mahshid said in an interview.
But to Dr. Soleymani, the most exciting application remains its use in diagnosing infectious diseases. She noted, however, that it’s unclear whether the price of the device will be too high for widespread home use. It may be more practical for family physician clinics and other facilities.
Before the device becomes commercially available, more testing is needed to validate the results, which are based on a limited number of samples that were available in a research setting.
The study was supported by the MI4 Emergency COVID-19 Research Funding, Natural Sciences and Engineering Research Council of Canada, Canadian Institutes of Health Research, Canada Foundation for Innovation, and McGill University. Dr. Mahshid and Dr. Soleymani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NATURE NANOTECHNOLOGY
Erythematous Dermal Facial Plaques in a Neutropenic Patient
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.
Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.
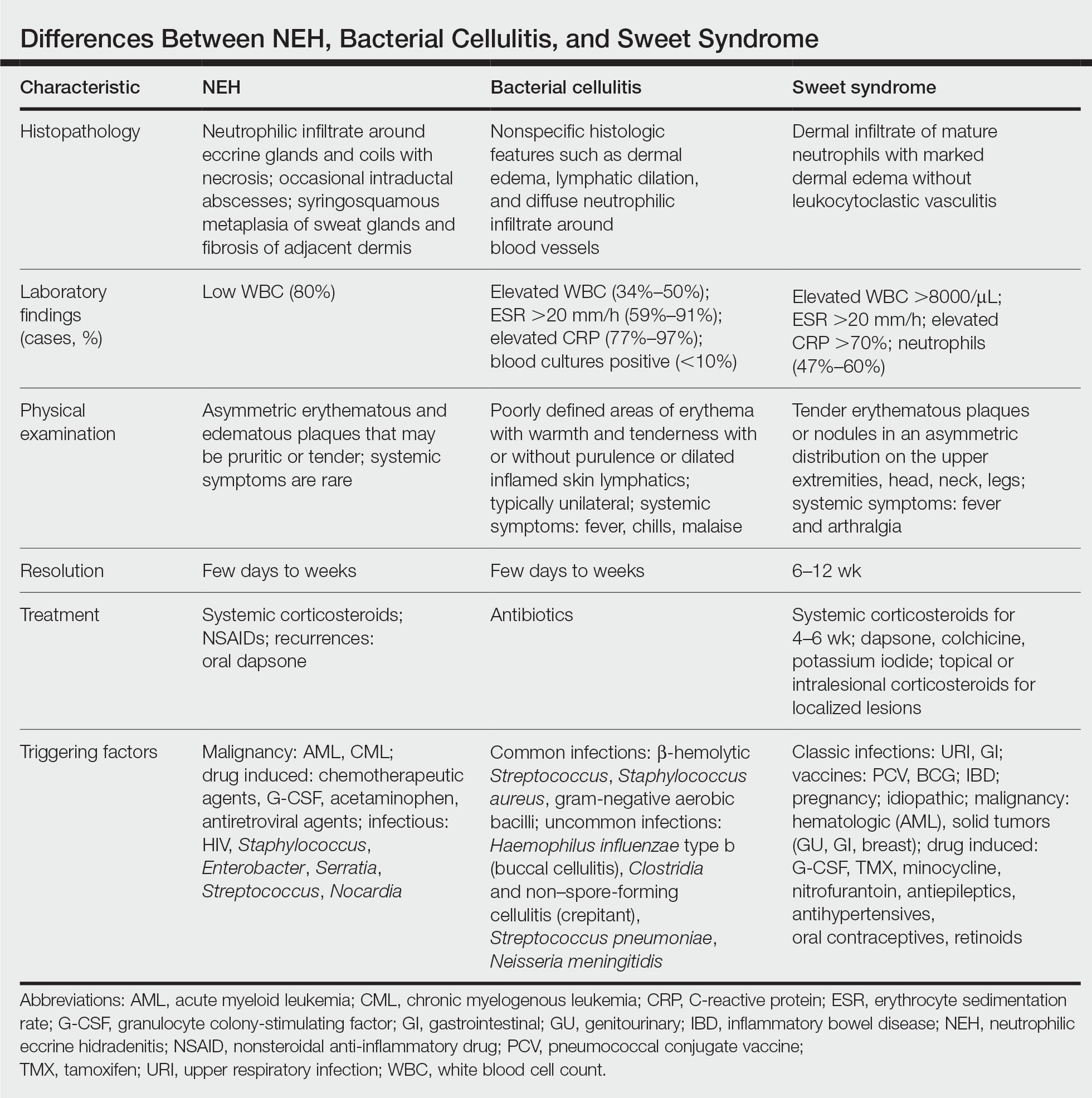
Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.

Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.

Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.

Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.

Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
A 50-year-old woman undergoing cytarabine induction therapy for acute myeloid leukemia developed tender, erythematous, dermal plaques on the nasal dorsum, left medial eyebrow, left preauricular cheek, and right cheek. The rash erupted 7 days after receiving the cytarabine induction regimen. She had a fever (temperature, 39.9 °C [103.8 °F]) and also was neutropenic.
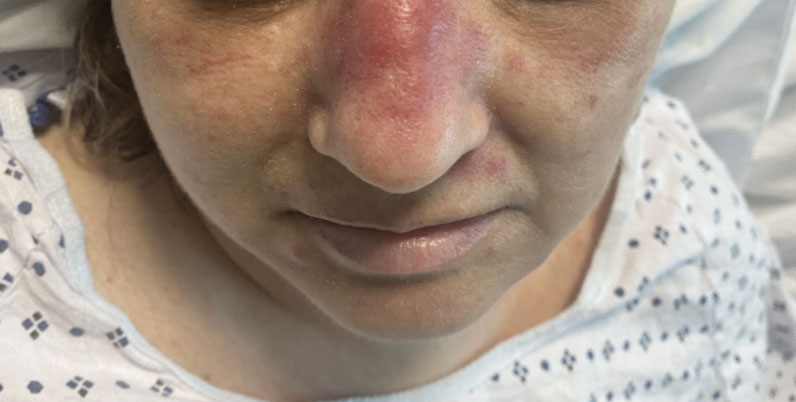
Bordetella parapertussis reemerges as a cause of respiratory illness in children
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
FDA warns of tattoo ink tied to dangerous infections
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.