User login
LUPUS 2019 Congress: Top takeaways

Join us at #MDedgeChats on Tuesday, April 23, 2019, at 7:00 pm EST, for a Twitter discussion in Rheumatology on some of the top studies reported at the LUPUS 2019 Congress in San Francisco, April 5-8. Our special guests are two rheumatologists with expertise in lupus who attended the congress, Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both with the University of California, San Francisco. They will discuss the ins and outs of the study results presented.
We hope you can join us and invite a colleague, too.
We will discuss the results of the EMBRACE trial, which looks at the efficacy and safety of belimumab (Benlysta) in black patients with systemic lupus erythematosus (SLE); these patients have a higher prevalence of SLE and often higher disease severity but have been poorly represented in past belimumab studies.
We will also chat about the results of a phase 2 trial of baricitinib (Olumiant) in SLE patients.
Finally, we will look at the clinical utility of monitoring hydroxychloroquine levels in SLE patients and how well levels of the drug correlate with disease activity through the results of a meta-analysis of studies that examined these questions.
Topics of conversation
Q1: How does the methodology of the EMBRACE trial differ from past phase 3 belimumab trials?
Q2: How does the EMBRACE trial affect the way you prescribe belimumab?
Q3: Does the hydroxychloroquine level meta-analysis provide persuasive enough evidence to begin measuring it?
Q4: What kinds of interventions show the best evidence for improving hydroxychloroquine adherence?
Q5: Were concerns about the safety of baricitinib in SLE patients reduced by the trial results?
Resources
EMBRACE trial: Efficacy and Safety of Belimumab in Black Race Patients with Systemic Lupus Erythematosus.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Phase 2 trial of baricitinib in SLE.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Meta-analysis examining the clinical significance of monitoring of hydroxychloroquine levels in SLE.
LUPUS 2019 abstract
From LUPUS 2019:
Belimumab a bust for black SLE patients.
Here’s a top strategy for immunosuppressant discontinuation in SLE.
From EULAR 2018:
Baricitinib shows potential as lupus treatment.
About Dr. Yazdany
Dr. Yazdany is an associate professor in the division of rheumatology, department of medicine at UCSF (@UCSFMedicine). She is a rheumatologist and clinical researcher with expertise in systemic lupus erythematosus and health care quality measurement and improvement. Her clinical activities include seeing patients in the UCSF Lupus Clinic, where she serves as codirector, as well as at San Francisco General Hospital. Dr. Yazdany codirects the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She also leads ongoing, longitudinal studies of lupus that are investigating health disparities and outcomes in the condition.
About Dr. Schmajuk
Dr. Schmajuk is an Associate Professor in the Division of Rheumatology, Department of Medicine at UCSF (@UCSFMedicine) and the San Francisco VA Medical Center. She is a rheumatologist and clinical researcher with expertise in systemic #lupus erythematosus, quality of care, and patient safety. Dr. Schmajuk also co-directs the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She leads studies to develop patient-facing disease dashboards with the goal of improving patient-provider communication for patients with RA and SLE.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us at #MDedgeChats on Tuesday, April 23, 2019, at 7:00 pm EST, for a Twitter discussion in Rheumatology on some of the top studies reported at the LUPUS 2019 Congress in San Francisco, April 5-8. Our special guests are two rheumatologists with expertise in lupus who attended the congress, Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both with the University of California, San Francisco. They will discuss the ins and outs of the study results presented.
We hope you can join us and invite a colleague, too.
We will discuss the results of the EMBRACE trial, which looks at the efficacy and safety of belimumab (Benlysta) in black patients with systemic lupus erythematosus (SLE); these patients have a higher prevalence of SLE and often higher disease severity but have been poorly represented in past belimumab studies.
We will also chat about the results of a phase 2 trial of baricitinib (Olumiant) in SLE patients.
Finally, we will look at the clinical utility of monitoring hydroxychloroquine levels in SLE patients and how well levels of the drug correlate with disease activity through the results of a meta-analysis of studies that examined these questions.
Topics of conversation
Q1: How does the methodology of the EMBRACE trial differ from past phase 3 belimumab trials?
Q2: How does the EMBRACE trial affect the way you prescribe belimumab?
Q3: Does the hydroxychloroquine level meta-analysis provide persuasive enough evidence to begin measuring it?
Q4: What kinds of interventions show the best evidence for improving hydroxychloroquine adherence?
Q5: Were concerns about the safety of baricitinib in SLE patients reduced by the trial results?
Resources
EMBRACE trial: Efficacy and Safety of Belimumab in Black Race Patients with Systemic Lupus Erythematosus.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Phase 2 trial of baricitinib in SLE.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Meta-analysis examining the clinical significance of monitoring of hydroxychloroquine levels in SLE.
LUPUS 2019 abstract
From LUPUS 2019:
Belimumab a bust for black SLE patients.
Here’s a top strategy for immunosuppressant discontinuation in SLE.
From EULAR 2018:
Baricitinib shows potential as lupus treatment.
About Dr. Yazdany
Dr. Yazdany is an associate professor in the division of rheumatology, department of medicine at UCSF (@UCSFMedicine). She is a rheumatologist and clinical researcher with expertise in systemic lupus erythematosus and health care quality measurement and improvement. Her clinical activities include seeing patients in the UCSF Lupus Clinic, where she serves as codirector, as well as at San Francisco General Hospital. Dr. Yazdany codirects the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She also leads ongoing, longitudinal studies of lupus that are investigating health disparities and outcomes in the condition.
About Dr. Schmajuk
Dr. Schmajuk is an Associate Professor in the Division of Rheumatology, Department of Medicine at UCSF (@UCSFMedicine) and the San Francisco VA Medical Center. She is a rheumatologist and clinical researcher with expertise in systemic #lupus erythematosus, quality of care, and patient safety. Dr. Schmajuk also co-directs the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She leads studies to develop patient-facing disease dashboards with the goal of improving patient-provider communication for patients with RA and SLE.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us at #MDedgeChats on Tuesday, April 23, 2019, at 7:00 pm EST, for a Twitter discussion in Rheumatology on some of the top studies reported at the LUPUS 2019 Congress in San Francisco, April 5-8. Our special guests are two rheumatologists with expertise in lupus who attended the congress, Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both with the University of California, San Francisco. They will discuss the ins and outs of the study results presented.
We hope you can join us and invite a colleague, too.
We will discuss the results of the EMBRACE trial, which looks at the efficacy and safety of belimumab (Benlysta) in black patients with systemic lupus erythematosus (SLE); these patients have a higher prevalence of SLE and often higher disease severity but have been poorly represented in past belimumab studies.
We will also chat about the results of a phase 2 trial of baricitinib (Olumiant) in SLE patients.
Finally, we will look at the clinical utility of monitoring hydroxychloroquine levels in SLE patients and how well levels of the drug correlate with disease activity through the results of a meta-analysis of studies that examined these questions.
Topics of conversation
Q1: How does the methodology of the EMBRACE trial differ from past phase 3 belimumab trials?
Q2: How does the EMBRACE trial affect the way you prescribe belimumab?
Q3: Does the hydroxychloroquine level meta-analysis provide persuasive enough evidence to begin measuring it?
Q4: What kinds of interventions show the best evidence for improving hydroxychloroquine adherence?
Q5: Were concerns about the safety of baricitinib in SLE patients reduced by the trial results?
Resources
EMBRACE trial: Efficacy and Safety of Belimumab in Black Race Patients with Systemic Lupus Erythematosus.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Phase 2 trial of baricitinib in SLE.
LUPUS 2019 abstract | ClinicalTrials.gov listing
Meta-analysis examining the clinical significance of monitoring of hydroxychloroquine levels in SLE.
LUPUS 2019 abstract
From LUPUS 2019:
Belimumab a bust for black SLE patients.
Here’s a top strategy for immunosuppressant discontinuation in SLE.
From EULAR 2018:
Baricitinib shows potential as lupus treatment.
About Dr. Yazdany
Dr. Yazdany is an associate professor in the division of rheumatology, department of medicine at UCSF (@UCSFMedicine). She is a rheumatologist and clinical researcher with expertise in systemic lupus erythematosus and health care quality measurement and improvement. Her clinical activities include seeing patients in the UCSF Lupus Clinic, where she serves as codirector, as well as at San Francisco General Hospital. Dr. Yazdany codirects the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She also leads ongoing, longitudinal studies of lupus that are investigating health disparities and outcomes in the condition.
About Dr. Schmajuk
Dr. Schmajuk is an Associate Professor in the Division of Rheumatology, Department of Medicine at UCSF (@UCSFMedicine) and the San Francisco VA Medical Center. She is a rheumatologist and clinical researcher with expertise in systemic #lupus erythematosus, quality of care, and patient safety. Dr. Schmajuk also co-directs the Quality and Informatics Lab (quil.ucsf.edu), which uses data to drive improvements in health care delivery and outcomes for people with rheumatic diseases. She leads studies to develop patient-facing disease dashboards with the goal of improving patient-provider communication for patients with RA and SLE.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.
Belimumab a bust for black SLE patients
SAN FRANCISCO – ordered by the Food and Drug Administration.
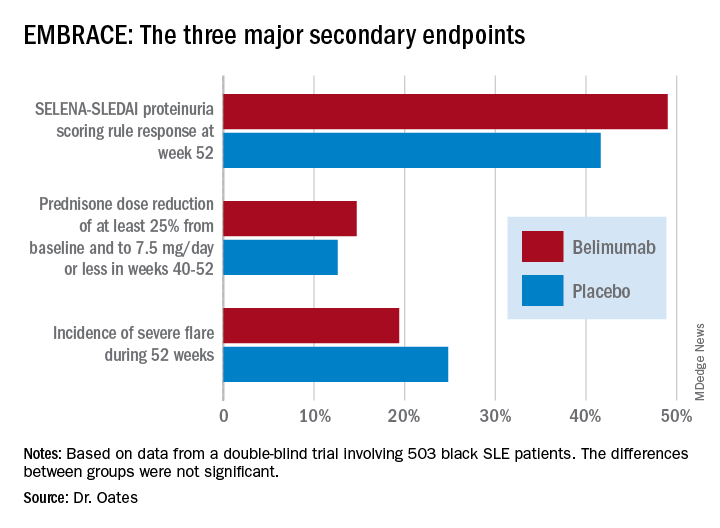
Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
REPORTING FROM LUPUS 2019
Childhood-onset SLE rate doubles in children born in winter
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
REPORTING FROM LUPUS 2019
Think ‘fall prevention’ in SLE patients
SAN FRANCISCO – in a Dutch prospective longitudinal study, Irene E.M. Bultink, MD, PhD, reported at an international congress on systemic lupus erythematosus.
“I think we should focus on fall risk and fall events in lupus. I think that’s a neglected area,” according to Dr. Bultink, a rheumatologist at the University of Amsterdam.
Contrary to the conventional wisdom, in her study, cumulative corticosteroid dose, average daily dosage, and bone loss were not significantly related to fracture risk in these SLE patients. That’s because Dr. Bultink and her fellow rheumatologists took steps to mitigate steroid-induced bone loss.
“We know steroids are very important for bone loss in lupus, but we demonstrated in our long-term follow-up study that it’s very dose dependent. If you use steroids below 7.5 mg daily and you protect your patient with calcium and vitamin D and, if possible, with bisphosphonates, the bone loss is restricted. I think fracture risk in lupus is more related to fall risk and fall incidence than to bone loss and steroid use. That’s what we demonstrated in our study,” she said in an interview.
“Unfortunately, until now, no studies on fall risk and fall events in lupus have been published. It’s not investigated very well. I think that fall risk in lupus patients might be greatly increased, compared to healthy controls,” Dr. Bultink added.
The study included 145 Dutch SLE patients with a mean age of 41 years at baseline. Ninety percent were women; 69% were white. Bone mineral density measurements by dual x-ray bone densitometry and x-rays of the lumbar and thoracic spine were obtained at entry and again after a median of 5 years.
Forty-two incident fractures occurred during a median of 7.2 years of follow-up. This equated to an incidence rate of 2.2 peripheral and 2.0 vertebral fractures per 100 person-years, rates double those in the Dutch general population.
In a logistic regression analysis, white ethnicity was independently associated with a 13.2-fold increased risk of fractures overall. Postmenopausal status conferred a fourfold increased risk. Older age was another important determinant. And patients with a prior stroke were at 15.5-fold greater risk of peripheral fractures.
“The prior stroke subgroup is especially vulnerable. In an earlier population-based study using data from the U.K. we also demonstrated that fractures happened more frequently in SLE patients who had already suffered a stroke (Osteoporos Int. 2014 Apr;25[4]:1275-83). So those are patients who are at very high risk for falling and fractures. But I think there are many reasons why patients with SLE would have an elevated fall risk. They have fatigue. They have muscle weakness, which might be due to vitamin D deficiency or prednisone use or inactivity. And they often have balance problems due to neuropathy or medication,” the rheumatologist observed.
She reported having no financial conflicts regarding her study, carried out free of commercial support.
SAN FRANCISCO – in a Dutch prospective longitudinal study, Irene E.M. Bultink, MD, PhD, reported at an international congress on systemic lupus erythematosus.
“I think we should focus on fall risk and fall events in lupus. I think that’s a neglected area,” according to Dr. Bultink, a rheumatologist at the University of Amsterdam.
Contrary to the conventional wisdom, in her study, cumulative corticosteroid dose, average daily dosage, and bone loss were not significantly related to fracture risk in these SLE patients. That’s because Dr. Bultink and her fellow rheumatologists took steps to mitigate steroid-induced bone loss.
“We know steroids are very important for bone loss in lupus, but we demonstrated in our long-term follow-up study that it’s very dose dependent. If you use steroids below 7.5 mg daily and you protect your patient with calcium and vitamin D and, if possible, with bisphosphonates, the bone loss is restricted. I think fracture risk in lupus is more related to fall risk and fall incidence than to bone loss and steroid use. That’s what we demonstrated in our study,” she said in an interview.
“Unfortunately, until now, no studies on fall risk and fall events in lupus have been published. It’s not investigated very well. I think that fall risk in lupus patients might be greatly increased, compared to healthy controls,” Dr. Bultink added.
The study included 145 Dutch SLE patients with a mean age of 41 years at baseline. Ninety percent were women; 69% were white. Bone mineral density measurements by dual x-ray bone densitometry and x-rays of the lumbar and thoracic spine were obtained at entry and again after a median of 5 years.
Forty-two incident fractures occurred during a median of 7.2 years of follow-up. This equated to an incidence rate of 2.2 peripheral and 2.0 vertebral fractures per 100 person-years, rates double those in the Dutch general population.
In a logistic regression analysis, white ethnicity was independently associated with a 13.2-fold increased risk of fractures overall. Postmenopausal status conferred a fourfold increased risk. Older age was another important determinant. And patients with a prior stroke were at 15.5-fold greater risk of peripheral fractures.
“The prior stroke subgroup is especially vulnerable. In an earlier population-based study using data from the U.K. we also demonstrated that fractures happened more frequently in SLE patients who had already suffered a stroke (Osteoporos Int. 2014 Apr;25[4]:1275-83). So those are patients who are at very high risk for falling and fractures. But I think there are many reasons why patients with SLE would have an elevated fall risk. They have fatigue. They have muscle weakness, which might be due to vitamin D deficiency or prednisone use or inactivity. And they often have balance problems due to neuropathy or medication,” the rheumatologist observed.
She reported having no financial conflicts regarding her study, carried out free of commercial support.
SAN FRANCISCO – in a Dutch prospective longitudinal study, Irene E.M. Bultink, MD, PhD, reported at an international congress on systemic lupus erythematosus.
“I think we should focus on fall risk and fall events in lupus. I think that’s a neglected area,” according to Dr. Bultink, a rheumatologist at the University of Amsterdam.
Contrary to the conventional wisdom, in her study, cumulative corticosteroid dose, average daily dosage, and bone loss were not significantly related to fracture risk in these SLE patients. That’s because Dr. Bultink and her fellow rheumatologists took steps to mitigate steroid-induced bone loss.
“We know steroids are very important for bone loss in lupus, but we demonstrated in our long-term follow-up study that it’s very dose dependent. If you use steroids below 7.5 mg daily and you protect your patient with calcium and vitamin D and, if possible, with bisphosphonates, the bone loss is restricted. I think fracture risk in lupus is more related to fall risk and fall incidence than to bone loss and steroid use. That’s what we demonstrated in our study,” she said in an interview.
“Unfortunately, until now, no studies on fall risk and fall events in lupus have been published. It’s not investigated very well. I think that fall risk in lupus patients might be greatly increased, compared to healthy controls,” Dr. Bultink added.
The study included 145 Dutch SLE patients with a mean age of 41 years at baseline. Ninety percent were women; 69% were white. Bone mineral density measurements by dual x-ray bone densitometry and x-rays of the lumbar and thoracic spine were obtained at entry and again after a median of 5 years.
Forty-two incident fractures occurred during a median of 7.2 years of follow-up. This equated to an incidence rate of 2.2 peripheral and 2.0 vertebral fractures per 100 person-years, rates double those in the Dutch general population.
In a logistic regression analysis, white ethnicity was independently associated with a 13.2-fold increased risk of fractures overall. Postmenopausal status conferred a fourfold increased risk. Older age was another important determinant. And patients with a prior stroke were at 15.5-fold greater risk of peripheral fractures.
“The prior stroke subgroup is especially vulnerable. In an earlier population-based study using data from the U.K. we also demonstrated that fractures happened more frequently in SLE patients who had already suffered a stroke (Osteoporos Int. 2014 Apr;25[4]:1275-83). So those are patients who are at very high risk for falling and fractures. But I think there are many reasons why patients with SLE would have an elevated fall risk. They have fatigue. They have muscle weakness, which might be due to vitamin D deficiency or prednisone use or inactivity. And they often have balance problems due to neuropathy or medication,” the rheumatologist observed.
She reported having no financial conflicts regarding her study, carried out free of commercial support.
REPORTING FROM LUPUS 2019
Young lupus patients need more than medications
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – – and therein lies the importance of introducing interventions beyond simply prescribing appropriate medications, Hermine I. Brunner, MD, asserted at an international congress on systemic lupus erythematosus.
Pilot studies conducted by her research group as well as others suggest that brief cognitive-behavioral interventions, web-based patient and caregiver education, and social media interactions significantly improve the fatigue and depression, poor quality of life, and lack of adherence to medication that are pervasive in young patients with SLE, according to Dr. Brunner, director of the division of rheumatology and professor of pediatrics at the University of Cincinnati and scientific director of the Pediatric Rheumatology Collaborative Study Group.
“Don’t misunderstand: I don’t think we can treat lupus simply with a psychological intervention at the bedside. However, I think doctors would be well advised to offer both psychological interventions and medication when they see young lupus patients, because without the psychological intervention the patients may not feel sufficiently at ease to take their medication. They will not get the benefit of the medications you’ve prescribed,” she said.
Patients with SLE take an average of eight medications daily. Their medication adherence rate is comparable to that of patients with diabetes or many other chronic diseases: that is to say, lousy. When investigators at the University of Texas MD Anderson Cancer Center, Houston, utilized an electronic monitoring system to chart adherence to prescribed oral medications in adults with SLE, they found that over the course of 2 years of follow-up only one-fourth of them had an adherence rate of 80% or better, which is the standard definition of adherence (Lupus. 2012 Oct;21[11]:1158-65).
Treatment adherence is particularly problematic in adolescents and young adults with SLE. They often have great difficulty in mastering the self-management skills required to stay on top of their disease when they have so much else going on during what is a vulnerable and challenging period of development, even for healthy youths.
The texting intervention
Dr. Brunner and her colleagues at Cincinnati Children’s Hospital Medical Center recognized the scope of the nonadherence problem early on. Years ago they started sending text messaging reminders of pending clinic visits to their patients who had a poor track record of showing up for appointments.
“We texted patients 2 weeks before their scheduled visit, 1 week before, and then again the day before the visit,” she explained.
This simple intervention resulted in a 47% reduction in missed appointments, compared with a control group. Also, text recipients were more likely to cancel appointments instead of simply not showing up, an important benefit from a practice management and scheduling standpoint (J Rheumatol. 2012 Jan;39[1]:174-9). Disappointingly, however, the text messaging intervention had no impact on adherence to prescribed use of hydroxychloroquine. This led the investigators to conduct a deeper dive into the roots of the nonadherence problem in childhood-onset lupus.
Disease control, quality of life
Dr. Brunner and her coworkers conducted an in-depth assessment of health-related quality of life in 50 patients with childhood-onset SLE over the course of 6 months. The results were surprising.
“When we looked at the correlation between disease control and quality of life, actually there was none,” according to the pediatric rheumatologist.
Instead, the investigators found that young patients with persistently low quality of life despite objectively measured good disease control scored high for fatigue and depressive symptoms (Lupus. 2018 Jan;27[1]:124-33). This led Dr. Brunner and her coinvestigators to consider developing a practical behavioral intervention to address these potentially modifiable predictors of impaired health-related quality of life in their patient population.
The need for novel approaches was highlighted in focus groups conducted by the investigators, in which patients and their primary caregivers emphasized that current therapeutic strategies don’t adequately address key problems of living with lupus, especially the prominent fatigue, pain, and depressed mood that hamper daily function and personal relationships. Patients said they don’t feel an immediate benefit from taking their medications, so why bother? And parents expressed frustration about how difficult it is to get their teenagers to understand the consequences of nonadherence when they’re at an age when they don’t yet even grasp the concept of their own mortality (Lupus. 2019 Mar. doi: 10.1177/0961203319839478. These observations spurred the Cincinnati investigators to develop a modified cognitive-behavioral therapy (CBT) protocol, known as TEACH, which they believe is the first CBT intervention to specifically target psychological problems in young people with childhood-onset SLE.
The TEACH program
TEACH (Treatment and Education Approach for Childhood-Onset Lupus) is a six-session program that teaches patients and caregivers self-advocacy, relaxation techniques, how to improve sleep hygiene, the importance of engaging in planned pleasant activities, and why taking medications matters. The program content differs depending upon whether the patient is an adolescent or young adult.
Results of a recently published small feasibility study were highly encouraging, showing that 83% of people who enrolled in the program completed it. Posttreatment assessment showed that patients had a marked decrease in depressive symptoms as measured by both the Children’s Depression Inventory and the Beck Depression Inventory. They also showed a significant reduction in fatigue. However, while favorable trends in terms of reduced pain and anxiety symptoms were noted, they didn’t achieve statistical significance (Pediatr Rheumatol Online J. 2019 Feb 18. doi: 10.1186/s12969-019-0307-8). The next step in this project is a planned controlled randomized trial.
A web-based medication adherence program
Researchers at Pennsylvania State University took a different approach. They created a publicly available educational website, www.facinglupustogether.com, aimed at improving self-management skills – and especially medication adherence – in teens and young adults with SLE.
The website contains eight modules: Making the transition and taking charge of my medications, Learning about lupus, Learning about lupus medications, Managing symptoms of lupus, How do I handle lupus and my family, How do I handle lupus and my friends, Lupus and stress, and My personal goals and how I will achieve them. Each takes about 10 minutes to complete.
In a pilot study, 37 patients tackled one module per week and were randomized to respond to questions about the weekly topic either in a journal or by discussing the key points in an online social media forum with other young people with SLE. The idea was to create an intervention that capitalizes on the excellent social media skills possessed by today’s youth. And indeed, incorporation of social media proved to be a winning strategy. Medication adherence for hydroxychloroquine in the group randomized to social media participation jumped from 50% in the 3 months prior to starting the program to 92% in the first 3 months post completion, whereas medication adherence didn’t change significantly in the other study arm. The social media group also experienced significant improvements in self-efficacy, sense of community, acceptance of illness, optimism and control over the future, and other measures of empowerment. The control group did not show significant change in any of these domains (Pediatr Rheumatol Online J. 2018 Mar 14. doi: 10.1186/s12969-018-0232-2).
The TEACH study was sponsored by the National Institutes of Health. The web-based medication adherence program pilot study was supported by the Lupus Foundation of America. What the two approaches share in common is a conviction that, when it comes to addressing pain, fatigue, diminished quality of life, and poor medication adherence in young patients with SLE: “Our medication prescription alone doesn’t do it,” Dr. Brunner said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM LUPUS 2019
Here’s a top strategy for immunosuppressant discontinuation in SLE
SAN FRANCISCO – Long-term maintenance therapy with hydroxychloroquine initiated after patients with systemic lupus erythematosus (SLE) have discontinued immunosuppressant medication during remission greatly reduces their likelihood of disease flare, Margherita Zen, MD, reported at an international congress on systemic lupus erythematosus.
Dr. Zen, a rheumatologist at the University of Padova in Padua, Italy, presented a retrospective study of 319 SLE patients actively followed in the Padova Lupus Cohort. All of them were treated at some point with one or more immunosuppressants, most commonly mycophenolate, azathioprine, or methotrexate. Fifty-six percent of them never discontinued immunosuppressant therapy during long-term follow-up. Of the 139 patients who did, three-quarters of them were in remission at the time of discontinuation, while the remainder discontinued immunosuppressant medication because of intolerance or poor adherence.
The research question of greatest interest to Dr. Zen and her coinvestigators was whether withdrawal of immunosuppressant medication in lupus patients who have responded well and are in remission is a sound strategy or one fraught with an unacceptably high risk of flare.
“The benefits of drug tapering and discontinuation have been proven for glucocorticoids, but there is not enough evidence available for discontinuation of immunosuppressants,” she said.
The good news: 75% of patients who discontinued immunosuppressant therapy while in remission never experienced a flare during a mean 7.6 years of follow-up after discontinuation. And the median time to flare in those who did was 4.75 years. In contrast, more than two-thirds of patients who discontinued immunosuppressants while not in remission experienced an SLE flare, and the mean time to this event was a mere 8 months. In a Cox regression analysis, patients who discontinued their immunosuppressant medication because of intolerance or poor adherence were 6.9-fold more likely to flare during follow-up than were those who quit immunosuppressants while in remission.
Patients who’d been on methotrexate were significantly more likely to flare after discontinuing the drug than were those who’d been on other immunosuppressant medications. However, flare risk post immunosuppressants was unaffected by whether the medication was given for treatment of lupus nephritis, arthritis, neuropsychiatric involvement, vasculitis, hematologic manifestations of the disease, or skin involvement.
In a multivariate logistic regression analysis, by far the strongest independent protective factor against the occurrence of flare after immunosuppressant discontinuation was maintenance therapy with hydroxychloroquine; it was associated with a 76% reduction in flare risk. Put another way, patients not on maintenance antimalarial therapy after immunosuppressant discontinuation were at an adjusted 5.3-fold increased risk of flare. Combining hydroxychloroquine with low-dose prednisone didn’t provide any added benefit beyond the antimalarial alone in terms of protection against flares.
The other protective factor was duration of remission at the time of immunosuppressant discontinuation: the longer, the better. Patients who experienced a lupus flare had been in remission for an average of 28 months when they stopped taking their immunosuppressant medication. Those who remained flare free throughout follow-up had been in remission for an average of 46 months at discontinuation.
Reassuringly, median damage accrual as assessed by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index was similar in patients regardless of whether or not they discontinued immunosuppressant therapy.
Dr. Zen reported having no financial conflicts regarding this study, conducted free of commercial support.
SAN FRANCISCO – Long-term maintenance therapy with hydroxychloroquine initiated after patients with systemic lupus erythematosus (SLE) have discontinued immunosuppressant medication during remission greatly reduces their likelihood of disease flare, Margherita Zen, MD, reported at an international congress on systemic lupus erythematosus.
Dr. Zen, a rheumatologist at the University of Padova in Padua, Italy, presented a retrospective study of 319 SLE patients actively followed in the Padova Lupus Cohort. All of them were treated at some point with one or more immunosuppressants, most commonly mycophenolate, azathioprine, or methotrexate. Fifty-six percent of them never discontinued immunosuppressant therapy during long-term follow-up. Of the 139 patients who did, three-quarters of them were in remission at the time of discontinuation, while the remainder discontinued immunosuppressant medication because of intolerance or poor adherence.
The research question of greatest interest to Dr. Zen and her coinvestigators was whether withdrawal of immunosuppressant medication in lupus patients who have responded well and are in remission is a sound strategy or one fraught with an unacceptably high risk of flare.
“The benefits of drug tapering and discontinuation have been proven for glucocorticoids, but there is not enough evidence available for discontinuation of immunosuppressants,” she said.
The good news: 75% of patients who discontinued immunosuppressant therapy while in remission never experienced a flare during a mean 7.6 years of follow-up after discontinuation. And the median time to flare in those who did was 4.75 years. In contrast, more than two-thirds of patients who discontinued immunosuppressants while not in remission experienced an SLE flare, and the mean time to this event was a mere 8 months. In a Cox regression analysis, patients who discontinued their immunosuppressant medication because of intolerance or poor adherence were 6.9-fold more likely to flare during follow-up than were those who quit immunosuppressants while in remission.
Patients who’d been on methotrexate were significantly more likely to flare after discontinuing the drug than were those who’d been on other immunosuppressant medications. However, flare risk post immunosuppressants was unaffected by whether the medication was given for treatment of lupus nephritis, arthritis, neuropsychiatric involvement, vasculitis, hematologic manifestations of the disease, or skin involvement.
In a multivariate logistic regression analysis, by far the strongest independent protective factor against the occurrence of flare after immunosuppressant discontinuation was maintenance therapy with hydroxychloroquine; it was associated with a 76% reduction in flare risk. Put another way, patients not on maintenance antimalarial therapy after immunosuppressant discontinuation were at an adjusted 5.3-fold increased risk of flare. Combining hydroxychloroquine with low-dose prednisone didn’t provide any added benefit beyond the antimalarial alone in terms of protection against flares.
The other protective factor was duration of remission at the time of immunosuppressant discontinuation: the longer, the better. Patients who experienced a lupus flare had been in remission for an average of 28 months when they stopped taking their immunosuppressant medication. Those who remained flare free throughout follow-up had been in remission for an average of 46 months at discontinuation.
Reassuringly, median damage accrual as assessed by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index was similar in patients regardless of whether or not they discontinued immunosuppressant therapy.
Dr. Zen reported having no financial conflicts regarding this study, conducted free of commercial support.
SAN FRANCISCO – Long-term maintenance therapy with hydroxychloroquine initiated after patients with systemic lupus erythematosus (SLE) have discontinued immunosuppressant medication during remission greatly reduces their likelihood of disease flare, Margherita Zen, MD, reported at an international congress on systemic lupus erythematosus.
Dr. Zen, a rheumatologist at the University of Padova in Padua, Italy, presented a retrospective study of 319 SLE patients actively followed in the Padova Lupus Cohort. All of them were treated at some point with one or more immunosuppressants, most commonly mycophenolate, azathioprine, or methotrexate. Fifty-six percent of them never discontinued immunosuppressant therapy during long-term follow-up. Of the 139 patients who did, three-quarters of them were in remission at the time of discontinuation, while the remainder discontinued immunosuppressant medication because of intolerance or poor adherence.
The research question of greatest interest to Dr. Zen and her coinvestigators was whether withdrawal of immunosuppressant medication in lupus patients who have responded well and are in remission is a sound strategy or one fraught with an unacceptably high risk of flare.
“The benefits of drug tapering and discontinuation have been proven for glucocorticoids, but there is not enough evidence available for discontinuation of immunosuppressants,” she said.
The good news: 75% of patients who discontinued immunosuppressant therapy while in remission never experienced a flare during a mean 7.6 years of follow-up after discontinuation. And the median time to flare in those who did was 4.75 years. In contrast, more than two-thirds of patients who discontinued immunosuppressants while not in remission experienced an SLE flare, and the mean time to this event was a mere 8 months. In a Cox regression analysis, patients who discontinued their immunosuppressant medication because of intolerance or poor adherence were 6.9-fold more likely to flare during follow-up than were those who quit immunosuppressants while in remission.
Patients who’d been on methotrexate were significantly more likely to flare after discontinuing the drug than were those who’d been on other immunosuppressant medications. However, flare risk post immunosuppressants was unaffected by whether the medication was given for treatment of lupus nephritis, arthritis, neuropsychiatric involvement, vasculitis, hematologic manifestations of the disease, or skin involvement.
In a multivariate logistic regression analysis, by far the strongest independent protective factor against the occurrence of flare after immunosuppressant discontinuation was maintenance therapy with hydroxychloroquine; it was associated with a 76% reduction in flare risk. Put another way, patients not on maintenance antimalarial therapy after immunosuppressant discontinuation were at an adjusted 5.3-fold increased risk of flare. Combining hydroxychloroquine with low-dose prednisone didn’t provide any added benefit beyond the antimalarial alone in terms of protection against flares.
The other protective factor was duration of remission at the time of immunosuppressant discontinuation: the longer, the better. Patients who experienced a lupus flare had been in remission for an average of 28 months when they stopped taking their immunosuppressant medication. Those who remained flare free throughout follow-up had been in remission for an average of 46 months at discontinuation.
Reassuringly, median damage accrual as assessed by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index was similar in patients regardless of whether or not they discontinued immunosuppressant therapy.
Dr. Zen reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM LUPUS 2019
ACE inhibitors may improve neuropsychiatric lupus
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
REPORTING FROM LUPUS 2019
Key clinical point: ACE inhibitor therapy may prevent and/or improve neuropsychiatric lupus.
Major finding: The first-ever multicenter randomized trial of ACE inhibitor therapy for neuropsychiatric SLE will soon get underway.
Study details: The planned – and funded – trial will include roughly 70 patients with neuropsychiatric lupus.
Disclosures: The presenter reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
Atmospheric fluctuations tied to lupus flares
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
REPORTING FROM LUPUS 2019
Expert gives tips on timing, managing lupus pregnancies
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
AT LUPUS 2019
EULAR takes SLE guidance to the next level
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
FROM ANNALS OF THE RHEUMATIC DISEASES