User login
Topical anticholinergic for axillary hyperhidrosis shows fewer side effects
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
FROM AAD VMX 2021
Systematic approach to pain helps avoid opioid issues for dermatologists
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
FROM AAD VMX 2021
Boosting the presence of darker skin in rheumatology education
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
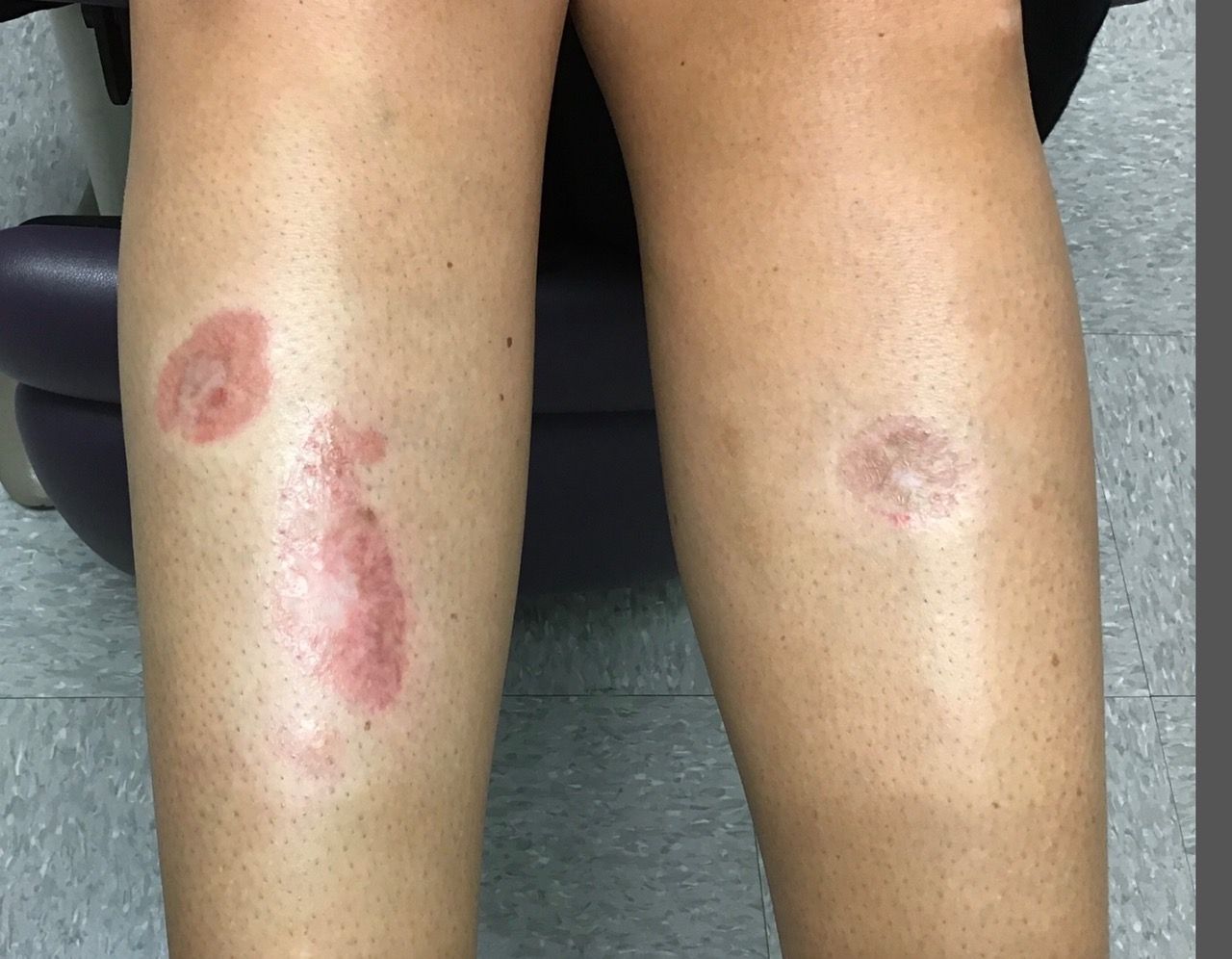
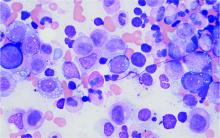
VEXAS syndrome: Implications for dermatologists
When I was a medical student, I always found it gratifying when there was a unifying mechanism that explained the symptoms of a disease. Part of the reason I chose dermatology as a specialty was how frequently we are able to “see” these mechanisms in the skin, both clinically and histologically. What’s even more interesting is that this condition is caused by a postzygotic somatic mutation, an apparently underrecognized cause of disease that we are just now beginning to understand. An example of a postzygotic somatic mutation causing a disease that we all learned about in medical school is paroxysmal nocturnal hemoglobinuria.
Using a “bottom-up” approach, researchers at the National Institutes of Health and in the United Kingdom identified 25 patients with somatic UBA1 mutations and noticed that they had strikingly similar autoinflammatory syndromes. UBA1 encodes ubiquitin E1, which is part of the pathway the breaks down proteins as part of the normal cellular machine. It is localized to the X chromosome, so all 25 affected patients were males, and most were aged between 40 and 70 years. These patients had an autoinflammatory syndrome characterized by fever, chondritis (similar to relapsing polychondritis), vasculitis, and neutrophilic dermatoses. Many patients also had features of myelodysplastic syndrome and plasma cell dyscrasia. The inflammatory pattern in this condition seems to show elevations in tumor necrosis factor, interleukin-6, and interferon-gamma.
So why is this syndrome relevant to dermatology? We are often asked to evaluate patients for neutrophilic dermatosis and vasculitis, and many affected patients had clinical and histologic findings compatible with polyarteritis nodosa and Sweet syndrome. When confronted with a neutrophilic dermatosis, we’ve all been taught to evaluate for myelodysplastic syndrome, which many of these patients appeared to have, at least on the surface. When bone marrow biopsies were done, the myeloid cell precursors that give rise to neutrophils were noted to have prominent cytoplasmic vacuoles, hence the “V” in VEXAS.
In reading the article describing 25 patients with this syndrome, which was published in the New England Journal of Medicine, I was struck by how refractory they were to treatment. Most patients had been treated with systemic steroids, multiple biologics, and several nonbiologic medications that are mainstays of treatment for neutrophilic dermatosis like dapsone and colchicine. I was fortunate enough to speak to Amanda Ombrello, MD, of the National Human Genome Research Institute, one of the lead authors of the paper, who drew my attention to the supplementary appendix, which showed the marked injection-site reactions some patients had to anakinra – yet another reason why a patient might end up in a dermatology clinic. In my mind, all of these features could be a clue to a diagnosis of VEXAS syndrome.
Many patients seemed to fare poorly, with 40% of patients dying before the completion of the study. When it comes to extremely rare diseases, it seems that the more physicians who are aware of the existence of a particular syndrome, the more likely it is a patient will come under our care and be correctly diagnosed.
Dr. Saardi is a dermatologist and internist, and is director of the inpatient dermatology service at the George Washington University Hospital, Washington. He has no disclosures.
When I was a medical student, I always found it gratifying when there was a unifying mechanism that explained the symptoms of a disease. Part of the reason I chose dermatology as a specialty was how frequently we are able to “see” these mechanisms in the skin, both clinically and histologically. What’s even more interesting is that this condition is caused by a postzygotic somatic mutation, an apparently underrecognized cause of disease that we are just now beginning to understand. An example of a postzygotic somatic mutation causing a disease that we all learned about in medical school is paroxysmal nocturnal hemoglobinuria.
Using a “bottom-up” approach, researchers at the National Institutes of Health and in the United Kingdom identified 25 patients with somatic UBA1 mutations and noticed that they had strikingly similar autoinflammatory syndromes. UBA1 encodes ubiquitin E1, which is part of the pathway the breaks down proteins as part of the normal cellular machine. It is localized to the X chromosome, so all 25 affected patients were males, and most were aged between 40 and 70 years. These patients had an autoinflammatory syndrome characterized by fever, chondritis (similar to relapsing polychondritis), vasculitis, and neutrophilic dermatoses. Many patients also had features of myelodysplastic syndrome and plasma cell dyscrasia. The inflammatory pattern in this condition seems to show elevations in tumor necrosis factor, interleukin-6, and interferon-gamma.
So why is this syndrome relevant to dermatology? We are often asked to evaluate patients for neutrophilic dermatosis and vasculitis, and many affected patients had clinical and histologic findings compatible with polyarteritis nodosa and Sweet syndrome. When confronted with a neutrophilic dermatosis, we’ve all been taught to evaluate for myelodysplastic syndrome, which many of these patients appeared to have, at least on the surface. When bone marrow biopsies were done, the myeloid cell precursors that give rise to neutrophils were noted to have prominent cytoplasmic vacuoles, hence the “V” in VEXAS.
In reading the article describing 25 patients with this syndrome, which was published in the New England Journal of Medicine, I was struck by how refractory they were to treatment. Most patients had been treated with systemic steroids, multiple biologics, and several nonbiologic medications that are mainstays of treatment for neutrophilic dermatosis like dapsone and colchicine. I was fortunate enough to speak to Amanda Ombrello, MD, of the National Human Genome Research Institute, one of the lead authors of the paper, who drew my attention to the supplementary appendix, which showed the marked injection-site reactions some patients had to anakinra – yet another reason why a patient might end up in a dermatology clinic. In my mind, all of these features could be a clue to a diagnosis of VEXAS syndrome.
Many patients seemed to fare poorly, with 40% of patients dying before the completion of the study. When it comes to extremely rare diseases, it seems that the more physicians who are aware of the existence of a particular syndrome, the more likely it is a patient will come under our care and be correctly diagnosed.
Dr. Saardi is a dermatologist and internist, and is director of the inpatient dermatology service at the George Washington University Hospital, Washington. He has no disclosures.
When I was a medical student, I always found it gratifying when there was a unifying mechanism that explained the symptoms of a disease. Part of the reason I chose dermatology as a specialty was how frequently we are able to “see” these mechanisms in the skin, both clinically and histologically. What’s even more interesting is that this condition is caused by a postzygotic somatic mutation, an apparently underrecognized cause of disease that we are just now beginning to understand. An example of a postzygotic somatic mutation causing a disease that we all learned about in medical school is paroxysmal nocturnal hemoglobinuria.
Using a “bottom-up” approach, researchers at the National Institutes of Health and in the United Kingdom identified 25 patients with somatic UBA1 mutations and noticed that they had strikingly similar autoinflammatory syndromes. UBA1 encodes ubiquitin E1, which is part of the pathway the breaks down proteins as part of the normal cellular machine. It is localized to the X chromosome, so all 25 affected patients were males, and most were aged between 40 and 70 years. These patients had an autoinflammatory syndrome characterized by fever, chondritis (similar to relapsing polychondritis), vasculitis, and neutrophilic dermatoses. Many patients also had features of myelodysplastic syndrome and plasma cell dyscrasia. The inflammatory pattern in this condition seems to show elevations in tumor necrosis factor, interleukin-6, and interferon-gamma.
So why is this syndrome relevant to dermatology? We are often asked to evaluate patients for neutrophilic dermatosis and vasculitis, and many affected patients had clinical and histologic findings compatible with polyarteritis nodosa and Sweet syndrome. When confronted with a neutrophilic dermatosis, we’ve all been taught to evaluate for myelodysplastic syndrome, which many of these patients appeared to have, at least on the surface. When bone marrow biopsies were done, the myeloid cell precursors that give rise to neutrophils were noted to have prominent cytoplasmic vacuoles, hence the “V” in VEXAS.
In reading the article describing 25 patients with this syndrome, which was published in the New England Journal of Medicine, I was struck by how refractory they were to treatment. Most patients had been treated with systemic steroids, multiple biologics, and several nonbiologic medications that are mainstays of treatment for neutrophilic dermatosis like dapsone and colchicine. I was fortunate enough to speak to Amanda Ombrello, MD, of the National Human Genome Research Institute, one of the lead authors of the paper, who drew my attention to the supplementary appendix, which showed the marked injection-site reactions some patients had to anakinra – yet another reason why a patient might end up in a dermatology clinic. In my mind, all of these features could be a clue to a diagnosis of VEXAS syndrome.
Many patients seemed to fare poorly, with 40% of patients dying before the completion of the study. When it comes to extremely rare diseases, it seems that the more physicians who are aware of the existence of a particular syndrome, the more likely it is a patient will come under our care and be correctly diagnosed.
Dr. Saardi is a dermatologist and internist, and is director of the inpatient dermatology service at the George Washington University Hospital, Washington. He has no disclosures.
Study aims to enhance understanding of ‘tremendously understudied’ prurigo nodularis
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
A woman with a history of diabetes, and plaques on both shins
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
Blacks and Hispanics have higher inpatient use for mycosis fungoides
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
FROM SOC SOCIETY 2021
Despite new ichthyosis treatment recommendations, ‘many questions still exist’
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
FROM THE SPD PRE-AAD MEETING
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
COVID-19’s impact on lupus inpatients examined in study
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
FROM ANNALS OF THE RHEUMATIC DISEASES