User login
Increased hypothyroidism risk seen in young men with HS
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
FROM AAD 20
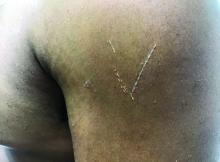
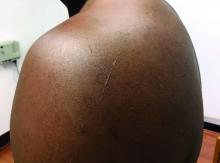
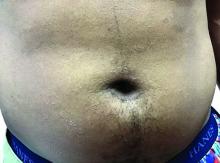
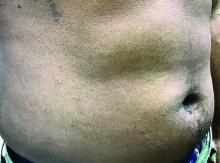
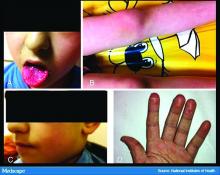
Flat-topped papules on the neck, arms, and trunk
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
Cancer risk elevated in hidradenitis suppurativa patients
.
HS is associated with severe comorbidities, and previous studies have suggested a link between HS and cancer development, wrote Joon Min Jung, MD, of the University of Ulsan College of Medicine, Seoul, Korea, and colleagues.
“The aberrant immune response and chronic inflammation in HS and genetic and environmental factors associated with the disease may all be factors in the development of cancer,” but large, population-based studies of cancer in HS patients are limited, they noted.
In a study published in JAMA Dermatology, the researchers reviewed data from 22,468 adults with HS and 179,734 matched controls, in the Korean National Health Insurance System, seen by physicians between January 2009 and December 2017. The average age of the participants was 34 years, and 64% were male.
Overall, HS patients had a significantly higher risk of cancer compared with controls, with an adjusted hazard ratio (aHR) of 1.28.
As for specific cancers, HS patients had a significantly higher risk for Hodgkin lymphoma (aHR 5.08), oral cavity and pharyngeal cancer (aHR 3.10), central nervous system cancer (aHR 2.40), nonmelanoma skin cancer (aHR 2.06), prostate cancer (aHR 2.05), and colorectal cancer (aHR 1.45).
The risk of any cancer was not significantly different between women with HS and female controls (after adjustment for comorbidities), but was significantly higher among men with HS compared with male controls, also after adjustment for comorbidities (aHR, 1.37). In addition, HS patients in both younger (less than 40 years) and older (aged 40 years and older) age groups had increased cancer risk compared with age-matched controls. Overall cancer risk and the risk of most cancer types were higher among HS patients with moderate to severe disease than in those with mild disease, with the exception of nonmelanoma skin cancer, prostate cancer, lymphoma, and leukemia.
“Overall cancer risk showed a tendency to increase with worsening HS severity, reinforcing the possibility of an association between HS and cancer development,” the researchers noted. “However, we could not identify tendencies in some specific cancers, such as nonmelanoma skin cancer, CNS cancer, and prostate cancer, because the number of occurrences of those cancers was too small in the group with moderate to severe HS.”
The study findings were limited by several factors including the potential underestimate of HS cases in the population and the inability of the study design to adjust for factors including smoking status, alcohol use, and obesity, the researchers noted. However, the results support an increased cancer risk in HS patients and suggest the need to promote lifestyle modifications to reduce risk, and to increase cancer surveillance in these patients, they said. “For early detection of skin cancer, more aggressive histologic examination and a high level of suspicion are required,” they added.
The study was supported by the National Research Foundation of Korea and the Korea Health Technology R&D Project. The researchers had no financial conflicts to disclose.
SOURCE: Jung JM et al. JAMA Dermatol. 2020 May 27. doi: 10.1001/jamadermatol.2020.1422.
.
HS is associated with severe comorbidities, and previous studies have suggested a link between HS and cancer development, wrote Joon Min Jung, MD, of the University of Ulsan College of Medicine, Seoul, Korea, and colleagues.
“The aberrant immune response and chronic inflammation in HS and genetic and environmental factors associated with the disease may all be factors in the development of cancer,” but large, population-based studies of cancer in HS patients are limited, they noted.
In a study published in JAMA Dermatology, the researchers reviewed data from 22,468 adults with HS and 179,734 matched controls, in the Korean National Health Insurance System, seen by physicians between January 2009 and December 2017. The average age of the participants was 34 years, and 64% were male.
Overall, HS patients had a significantly higher risk of cancer compared with controls, with an adjusted hazard ratio (aHR) of 1.28.
As for specific cancers, HS patients had a significantly higher risk for Hodgkin lymphoma (aHR 5.08), oral cavity and pharyngeal cancer (aHR 3.10), central nervous system cancer (aHR 2.40), nonmelanoma skin cancer (aHR 2.06), prostate cancer (aHR 2.05), and colorectal cancer (aHR 1.45).
The risk of any cancer was not significantly different between women with HS and female controls (after adjustment for comorbidities), but was significantly higher among men with HS compared with male controls, also after adjustment for comorbidities (aHR, 1.37). In addition, HS patients in both younger (less than 40 years) and older (aged 40 years and older) age groups had increased cancer risk compared with age-matched controls. Overall cancer risk and the risk of most cancer types were higher among HS patients with moderate to severe disease than in those with mild disease, with the exception of nonmelanoma skin cancer, prostate cancer, lymphoma, and leukemia.
“Overall cancer risk showed a tendency to increase with worsening HS severity, reinforcing the possibility of an association between HS and cancer development,” the researchers noted. “However, we could not identify tendencies in some specific cancers, such as nonmelanoma skin cancer, CNS cancer, and prostate cancer, because the number of occurrences of those cancers was too small in the group with moderate to severe HS.”
The study findings were limited by several factors including the potential underestimate of HS cases in the population and the inability of the study design to adjust for factors including smoking status, alcohol use, and obesity, the researchers noted. However, the results support an increased cancer risk in HS patients and suggest the need to promote lifestyle modifications to reduce risk, and to increase cancer surveillance in these patients, they said. “For early detection of skin cancer, more aggressive histologic examination and a high level of suspicion are required,” they added.
The study was supported by the National Research Foundation of Korea and the Korea Health Technology R&D Project. The researchers had no financial conflicts to disclose.
SOURCE: Jung JM et al. JAMA Dermatol. 2020 May 27. doi: 10.1001/jamadermatol.2020.1422.
.
HS is associated with severe comorbidities, and previous studies have suggested a link between HS and cancer development, wrote Joon Min Jung, MD, of the University of Ulsan College of Medicine, Seoul, Korea, and colleagues.
“The aberrant immune response and chronic inflammation in HS and genetic and environmental factors associated with the disease may all be factors in the development of cancer,” but large, population-based studies of cancer in HS patients are limited, they noted.
In a study published in JAMA Dermatology, the researchers reviewed data from 22,468 adults with HS and 179,734 matched controls, in the Korean National Health Insurance System, seen by physicians between January 2009 and December 2017. The average age of the participants was 34 years, and 64% were male.
Overall, HS patients had a significantly higher risk of cancer compared with controls, with an adjusted hazard ratio (aHR) of 1.28.
As for specific cancers, HS patients had a significantly higher risk for Hodgkin lymphoma (aHR 5.08), oral cavity and pharyngeal cancer (aHR 3.10), central nervous system cancer (aHR 2.40), nonmelanoma skin cancer (aHR 2.06), prostate cancer (aHR 2.05), and colorectal cancer (aHR 1.45).
The risk of any cancer was not significantly different between women with HS and female controls (after adjustment for comorbidities), but was significantly higher among men with HS compared with male controls, also after adjustment for comorbidities (aHR, 1.37). In addition, HS patients in both younger (less than 40 years) and older (aged 40 years and older) age groups had increased cancer risk compared with age-matched controls. Overall cancer risk and the risk of most cancer types were higher among HS patients with moderate to severe disease than in those with mild disease, with the exception of nonmelanoma skin cancer, prostate cancer, lymphoma, and leukemia.
“Overall cancer risk showed a tendency to increase with worsening HS severity, reinforcing the possibility of an association between HS and cancer development,” the researchers noted. “However, we could not identify tendencies in some specific cancers, such as nonmelanoma skin cancer, CNS cancer, and prostate cancer, because the number of occurrences of those cancers was too small in the group with moderate to severe HS.”
The study findings were limited by several factors including the potential underestimate of HS cases in the population and the inability of the study design to adjust for factors including smoking status, alcohol use, and obesity, the researchers noted. However, the results support an increased cancer risk in HS patients and suggest the need to promote lifestyle modifications to reduce risk, and to increase cancer surveillance in these patients, they said. “For early detection of skin cancer, more aggressive histologic examination and a high level of suspicion are required,” they added.
The study was supported by the National Research Foundation of Korea and the Korea Health Technology R&D Project. The researchers had no financial conflicts to disclose.
SOURCE: Jung JM et al. JAMA Dermatol. 2020 May 27. doi: 10.1001/jamadermatol.2020.1422.
FROM JAMA DERMATOLOGY
Erythema and sclerosis predict chronic GVHD clinical response, survival
Erythema associated with chronic graft-versus-host disease (GVHD) after allogenic stem cell transplantation is likely to resolve regardless of when it appears, while sclerosis that develops more than 3 months after a chronic GVHD diagnosis is less likely to resolve, according to research presented during a plenary session at the annual meeting of the Society for Investigative Dermatology, held virtually.
In addition, greater improvement in erythema, as measured by body surface area (BSA) in the study was significantly associated with higher patient survival, Laura X. Baker, BS, a medical student at Vanderbilt University, and coinvestigators at Vanderbilt Dermatology Translational Research Clinic in Nashville, Tenn., said in her presentation.
This new significant association between erythema response and survival could inform future studies,” she said. “Our findings highlight the importance of thorough skin exams in chronic GVHD patients.”
Ms. Baker and colleagues performed a prospective, observational study of 242 patients with chronic GVHD and cutaneous manifestations, enrolled in the Chronic GVHD Consortium across nine centers between 2007 and 2012.
Patients had either erythema or sclerosis at the time of enrollment, which was considered incident if erythema or sclerosis appeared less than 3 months after diagnosis of chronic GVHD, or prevalent if erythema or sclerosis appeared 3 months or later after a chronic GVHD diagnosis. All patients were enrolled in the Chronic GVHD Consortium within 3 years of a transplant and were receiving systemic immunosuppressive therapy.
Transplant clinicians examined patients every 6 months, assessing clinical parameters such as skin involvement. Ms. Baker and colleagues used the 2005 National Institutes of Health (NIH) criteria to assess a complete response, a partial response, or no cutaneous response using measurements made by the transplant clinicians. The NIH criteria recommend calculating the change in BSA at the first follow-up visit to determine these changes (Pavletic S et al. Biol Blood Marrow Transplant. 2006 Mar;12[3]:252-66). Researchers also developed a Cox regression model to evaluate overall survival and non-relapse mortality.
Among those with erythema, 133 patients had incident cases and 52 had prevalent cases of cutaneous chronic GVHD. At first follow-up after a finding of cutaneous involvement, the mean BSA was 4.5%, but the median BSA was zero, “meaning that more than half of the patients had complete disappearance of any erythema by the first follow-up,” Ms. Baker said. By the second follow-up visit, 74% of patients with erythema had complete responses, 9% achieved a partial response, and 18% had no response. A similar complete response rate was seen among patients with prevalent cases.
Among patients with sclerosis, there were 43 incident and 47 prevalent cases. Among patients with incident sclerosis, 68% achieved a clear response, 2% a partial response, and 30% no response. But only 28% of those with prevalent sclerosis had a complete response, 4% had a partial response, and 68% had no response.
Most erythema showed a complete response by the first follow-up, and it was not dependent on time from cGVHD diagnosis, Ms. Baker said. However, while most sclerosis within 3 months of cGVHD diagnosis showed a response, sclerosis present beyond the initial 3 months did not generally respond by the first follow-up.
“These findings could inform clinical care and expectations in addition to guiding the selection of outcome measures and endpoint definitions in clinical trials,” she added.
The researchers also looked at overall survival and nonrelapse mortality among patients with incident and prevalent erythema. After adjustment for age and BSA at enrollment, patients with incident cases of erythema with a complete response had significantly better odds of overall survival compared with patients who had no clinical response (hazard ratio, 0.50; 95% confidence interval; 0.25-1.00; P = .05).
Overall survival was greater in patients with prevalent cases of erythema (HR, 0.29; 95% CI, 0.09-0.87; P = .03). Nonrelapse mortality was also significantly lower among prevalent cases with complete or partial clinical response for erythema (HR, 0.19; 95% CI, 0.06-0.64; P = .01).
In a subgroup analysis, 113 patients with incident cases of erythema that had other organ cGVHD had significantly greater overall survival than did patients without a clinical response (HR, 0.20; 95% CI, 0.08-0.46; P < .005). Median survival distance after the first follow-up between patients with and without a clinical response was 28.9 months among incident cases, and 33.7 months among prevalent erythema cases.
“We knew that erythema is not a direct cause of mortality,” Ms. Baker said. Our results suggest the association between erythema response and survival is important and could inform future study.”
The researchers noted their study was limited by transplant clinicians measuring BSA rather than dermatologists, patients being treated at top transplant centers, and their GVHD diagnosis being within 3 years of a transplant, which could limit generalizability of the findings.
This study was funded by a career development award from the U.S. Department of Veterans Affairs and grants from the National Institutes of Health/National Cancer Institute.
SOURCE: Baker L. SID 2020, Abstract 434.
Erythema associated with chronic graft-versus-host disease (GVHD) after allogenic stem cell transplantation is likely to resolve regardless of when it appears, while sclerosis that develops more than 3 months after a chronic GVHD diagnosis is less likely to resolve, according to research presented during a plenary session at the annual meeting of the Society for Investigative Dermatology, held virtually.
In addition, greater improvement in erythema, as measured by body surface area (BSA) in the study was significantly associated with higher patient survival, Laura X. Baker, BS, a medical student at Vanderbilt University, and coinvestigators at Vanderbilt Dermatology Translational Research Clinic in Nashville, Tenn., said in her presentation.
This new significant association between erythema response and survival could inform future studies,” she said. “Our findings highlight the importance of thorough skin exams in chronic GVHD patients.”
Ms. Baker and colleagues performed a prospective, observational study of 242 patients with chronic GVHD and cutaneous manifestations, enrolled in the Chronic GVHD Consortium across nine centers between 2007 and 2012.
Patients had either erythema or sclerosis at the time of enrollment, which was considered incident if erythema or sclerosis appeared less than 3 months after diagnosis of chronic GVHD, or prevalent if erythema or sclerosis appeared 3 months or later after a chronic GVHD diagnosis. All patients were enrolled in the Chronic GVHD Consortium within 3 years of a transplant and were receiving systemic immunosuppressive therapy.
Transplant clinicians examined patients every 6 months, assessing clinical parameters such as skin involvement. Ms. Baker and colleagues used the 2005 National Institutes of Health (NIH) criteria to assess a complete response, a partial response, or no cutaneous response using measurements made by the transplant clinicians. The NIH criteria recommend calculating the change in BSA at the first follow-up visit to determine these changes (Pavletic S et al. Biol Blood Marrow Transplant. 2006 Mar;12[3]:252-66). Researchers also developed a Cox regression model to evaluate overall survival and non-relapse mortality.
Among those with erythema, 133 patients had incident cases and 52 had prevalent cases of cutaneous chronic GVHD. At first follow-up after a finding of cutaneous involvement, the mean BSA was 4.5%, but the median BSA was zero, “meaning that more than half of the patients had complete disappearance of any erythema by the first follow-up,” Ms. Baker said. By the second follow-up visit, 74% of patients with erythema had complete responses, 9% achieved a partial response, and 18% had no response. A similar complete response rate was seen among patients with prevalent cases.
Among patients with sclerosis, there were 43 incident and 47 prevalent cases. Among patients with incident sclerosis, 68% achieved a clear response, 2% a partial response, and 30% no response. But only 28% of those with prevalent sclerosis had a complete response, 4% had a partial response, and 68% had no response.
Most erythema showed a complete response by the first follow-up, and it was not dependent on time from cGVHD diagnosis, Ms. Baker said. However, while most sclerosis within 3 months of cGVHD diagnosis showed a response, sclerosis present beyond the initial 3 months did not generally respond by the first follow-up.
“These findings could inform clinical care and expectations in addition to guiding the selection of outcome measures and endpoint definitions in clinical trials,” she added.
The researchers also looked at overall survival and nonrelapse mortality among patients with incident and prevalent erythema. After adjustment for age and BSA at enrollment, patients with incident cases of erythema with a complete response had significantly better odds of overall survival compared with patients who had no clinical response (hazard ratio, 0.50; 95% confidence interval; 0.25-1.00; P = .05).
Overall survival was greater in patients with prevalent cases of erythema (HR, 0.29; 95% CI, 0.09-0.87; P = .03). Nonrelapse mortality was also significantly lower among prevalent cases with complete or partial clinical response for erythema (HR, 0.19; 95% CI, 0.06-0.64; P = .01).
In a subgroup analysis, 113 patients with incident cases of erythema that had other organ cGVHD had significantly greater overall survival than did patients without a clinical response (HR, 0.20; 95% CI, 0.08-0.46; P < .005). Median survival distance after the first follow-up between patients with and without a clinical response was 28.9 months among incident cases, and 33.7 months among prevalent erythema cases.
“We knew that erythema is not a direct cause of mortality,” Ms. Baker said. Our results suggest the association between erythema response and survival is important and could inform future study.”
The researchers noted their study was limited by transplant clinicians measuring BSA rather than dermatologists, patients being treated at top transplant centers, and their GVHD diagnosis being within 3 years of a transplant, which could limit generalizability of the findings.
This study was funded by a career development award from the U.S. Department of Veterans Affairs and grants from the National Institutes of Health/National Cancer Institute.
SOURCE: Baker L. SID 2020, Abstract 434.
Erythema associated with chronic graft-versus-host disease (GVHD) after allogenic stem cell transplantation is likely to resolve regardless of when it appears, while sclerosis that develops more than 3 months after a chronic GVHD diagnosis is less likely to resolve, according to research presented during a plenary session at the annual meeting of the Society for Investigative Dermatology, held virtually.
In addition, greater improvement in erythema, as measured by body surface area (BSA) in the study was significantly associated with higher patient survival, Laura X. Baker, BS, a medical student at Vanderbilt University, and coinvestigators at Vanderbilt Dermatology Translational Research Clinic in Nashville, Tenn., said in her presentation.
This new significant association between erythema response and survival could inform future studies,” she said. “Our findings highlight the importance of thorough skin exams in chronic GVHD patients.”
Ms. Baker and colleagues performed a prospective, observational study of 242 patients with chronic GVHD and cutaneous manifestations, enrolled in the Chronic GVHD Consortium across nine centers between 2007 and 2012.
Patients had either erythema or sclerosis at the time of enrollment, which was considered incident if erythema or sclerosis appeared less than 3 months after diagnosis of chronic GVHD, or prevalent if erythema or sclerosis appeared 3 months or later after a chronic GVHD diagnosis. All patients were enrolled in the Chronic GVHD Consortium within 3 years of a transplant and were receiving systemic immunosuppressive therapy.
Transplant clinicians examined patients every 6 months, assessing clinical parameters such as skin involvement. Ms. Baker and colleagues used the 2005 National Institutes of Health (NIH) criteria to assess a complete response, a partial response, or no cutaneous response using measurements made by the transplant clinicians. The NIH criteria recommend calculating the change in BSA at the first follow-up visit to determine these changes (Pavletic S et al. Biol Blood Marrow Transplant. 2006 Mar;12[3]:252-66). Researchers also developed a Cox regression model to evaluate overall survival and non-relapse mortality.
Among those with erythema, 133 patients had incident cases and 52 had prevalent cases of cutaneous chronic GVHD. At first follow-up after a finding of cutaneous involvement, the mean BSA was 4.5%, but the median BSA was zero, “meaning that more than half of the patients had complete disappearance of any erythema by the first follow-up,” Ms. Baker said. By the second follow-up visit, 74% of patients with erythema had complete responses, 9% achieved a partial response, and 18% had no response. A similar complete response rate was seen among patients with prevalent cases.
Among patients with sclerosis, there were 43 incident and 47 prevalent cases. Among patients with incident sclerosis, 68% achieved a clear response, 2% a partial response, and 30% no response. But only 28% of those with prevalent sclerosis had a complete response, 4% had a partial response, and 68% had no response.
Most erythema showed a complete response by the first follow-up, and it was not dependent on time from cGVHD diagnosis, Ms. Baker said. However, while most sclerosis within 3 months of cGVHD diagnosis showed a response, sclerosis present beyond the initial 3 months did not generally respond by the first follow-up.
“These findings could inform clinical care and expectations in addition to guiding the selection of outcome measures and endpoint definitions in clinical trials,” she added.
The researchers also looked at overall survival and nonrelapse mortality among patients with incident and prevalent erythema. After adjustment for age and BSA at enrollment, patients with incident cases of erythema with a complete response had significantly better odds of overall survival compared with patients who had no clinical response (hazard ratio, 0.50; 95% confidence interval; 0.25-1.00; P = .05).
Overall survival was greater in patients with prevalent cases of erythema (HR, 0.29; 95% CI, 0.09-0.87; P = .03). Nonrelapse mortality was also significantly lower among prevalent cases with complete or partial clinical response for erythema (HR, 0.19; 95% CI, 0.06-0.64; P = .01).
In a subgroup analysis, 113 patients with incident cases of erythema that had other organ cGVHD had significantly greater overall survival than did patients without a clinical response (HR, 0.20; 95% CI, 0.08-0.46; P < .005). Median survival distance after the first follow-up between patients with and without a clinical response was 28.9 months among incident cases, and 33.7 months among prevalent erythema cases.
“We knew that erythema is not a direct cause of mortality,” Ms. Baker said. Our results suggest the association between erythema response and survival is important and could inform future study.”
The researchers noted their study was limited by transplant clinicians measuring BSA rather than dermatologists, patients being treated at top transplant centers, and their GVHD diagnosis being within 3 years of a transplant, which could limit generalizability of the findings.
This study was funded by a career development award from the U.S. Department of Veterans Affairs and grants from the National Institutes of Health/National Cancer Institute.
SOURCE: Baker L. SID 2020, Abstract 434.
FROM SID 2020
Most patients with lichen sclerosus receive appropriate treatment
The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
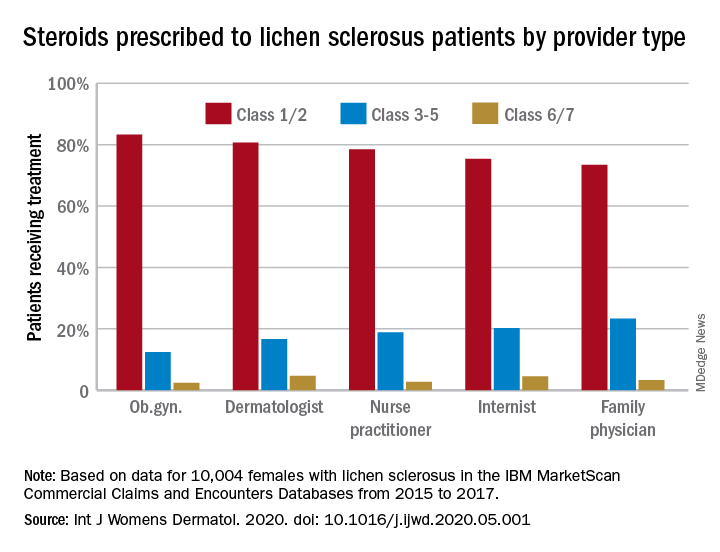
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
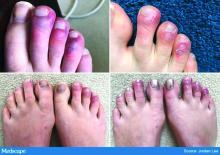
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
A toddler with a fever and desquamating perineal rash
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
An otherwise healthy 18-month-old female presented to the emergency department with 5 days of fever, erythema, fissuring of the lips, conjunctival injection, and a desquamating perineal rash. In addition, she had nasal congestion and cough for which she was started on amoxicillin 2 days prior to presentation given concern for pneumonia.
On exam, she was also noted to have several palpable cervical lymph nodes and edematous hands with overlying erythema. Laboratory evaluation was notable for respiratory syncytial virus positivity by polymerase chain reaction assay, leukocytosis, and elevated inflammatory markers (erythrocyte sedimentation rate and C-reactive protein).
Cell and gene research raise hopes for recessive dystrophic EB treatments
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
REPORTING FROM EB 2020
Case reports illustrate heterogeneity of skin manifestations in COVID patients
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Use of cannabinoids in dermatology here to stay
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.