User login
Spontaneously Regressing Primary Nodular Melanoma of the Glans Penis
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
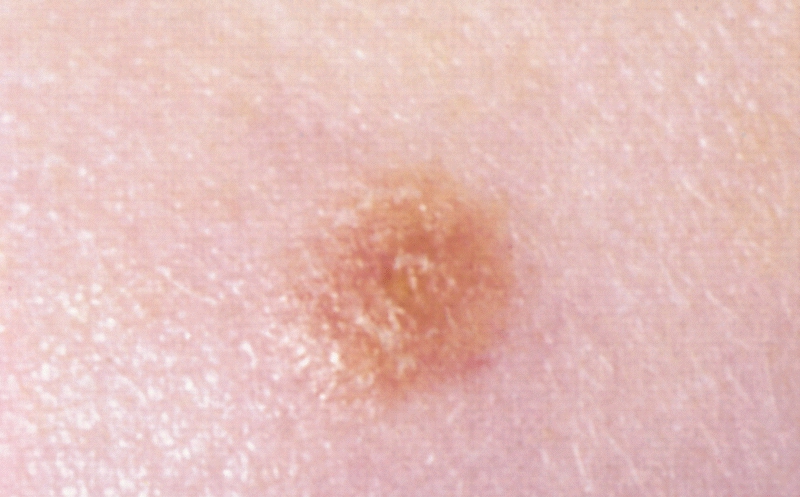
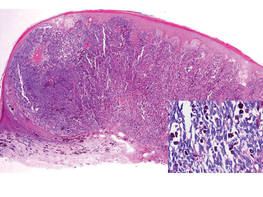
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).
|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |
|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |
|
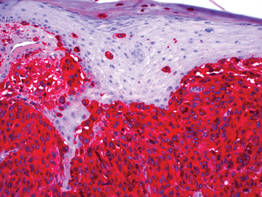
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |
|
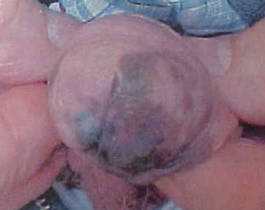
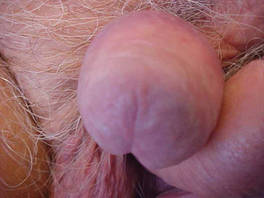
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).

|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |

|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |

|
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |

|
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).

|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |

|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |

|
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |

|
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.
VIDEO: MEK/BRAF combo puts brakes on BRAF-mutated advanced melanoma progression
MADRID – Combining the investigational MEK inhibitor cobimetinib with the BRAF inhibitor vemurafenib nearly halved the risk of progression in previously untreated patients with BRAF-mutated advanced melanoma in the ongoing, phase III coBRIM study.
Median progression-free survival by investigator was 6.2 months with vemurafenib (Zelboraf) and 9.9 months with the combination (Hazard ratio 0.51; P less than .0001); by independent review, median progression-free survival was 6.0 months versus 11.3 months, respectively (HR, 0.60; P = .0003), study author Dr. Grant McArthur reported in a late-breaking abstract at the European Society for Medical Oncology Congress.
Combination therapy also resulted in a significantly higher objective response rate (68% vs. 45%; P less than .0001) and more complete responses (10% vs. 4%) among the 495 patients with unresectable, locally advanced or metastatic BRAF V600 mutation–positive disease.
Overall survival data are not yet mature, but the interim analysis showed a promising 35% reduction in the risk of death at 9 months (HR, 0.65), said Dr. McArthur of the Peter MacCallum Cancer Centre, Melbourne, Australia. The corresponding P value of .046 is descriptive, as it did not cross the prespecified stopping boundary for the interim analysis.
Grade 3 toxicity, albeit fairly common, was slightly higher with combination therapy vs. vemurafenib alone (65% vs. 59%). Adverse event–related treatment discontinuations were similar between arms (13% vs. 12%), he said.
The data, which were simultaneously published in the New England Journal of Medicine (2014 Sept. 29 [doi:10.1056/NEJMoa1408868]) are expected to be submitted to the U.S. Food and Drug Administration later this year for potential approval of cobimetinib. Vemurafenib was approved in the United States for late-stage or unresectable melanoma in August 2011.
In our video interview, Dr. McArthur discusses coBRIM and his suggestions for how clinicians may incorporate this novel strategy into practice.
coBRIM was funded by F. Hoffmann-LaRoche/Genentech. Dr. McArthur reported grants from Roche, Novartis, Pfizer, Millennium, and Celegene, and consulting for Provectus.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Combining the investigational MEK inhibitor cobimetinib with the BRAF inhibitor vemurafenib nearly halved the risk of progression in previously untreated patients with BRAF-mutated advanced melanoma in the ongoing, phase III coBRIM study.
Median progression-free survival by investigator was 6.2 months with vemurafenib (Zelboraf) and 9.9 months with the combination (Hazard ratio 0.51; P less than .0001); by independent review, median progression-free survival was 6.0 months versus 11.3 months, respectively (HR, 0.60; P = .0003), study author Dr. Grant McArthur reported in a late-breaking abstract at the European Society for Medical Oncology Congress.
Combination therapy also resulted in a significantly higher objective response rate (68% vs. 45%; P less than .0001) and more complete responses (10% vs. 4%) among the 495 patients with unresectable, locally advanced or metastatic BRAF V600 mutation–positive disease.
Overall survival data are not yet mature, but the interim analysis showed a promising 35% reduction in the risk of death at 9 months (HR, 0.65), said Dr. McArthur of the Peter MacCallum Cancer Centre, Melbourne, Australia. The corresponding P value of .046 is descriptive, as it did not cross the prespecified stopping boundary for the interim analysis.
Grade 3 toxicity, albeit fairly common, was slightly higher with combination therapy vs. vemurafenib alone (65% vs. 59%). Adverse event–related treatment discontinuations were similar between arms (13% vs. 12%), he said.
The data, which were simultaneously published in the New England Journal of Medicine (2014 Sept. 29 [doi:10.1056/NEJMoa1408868]) are expected to be submitted to the U.S. Food and Drug Administration later this year for potential approval of cobimetinib. Vemurafenib was approved in the United States for late-stage or unresectable melanoma in August 2011.
In our video interview, Dr. McArthur discusses coBRIM and his suggestions for how clinicians may incorporate this novel strategy into practice.
coBRIM was funded by F. Hoffmann-LaRoche/Genentech. Dr. McArthur reported grants from Roche, Novartis, Pfizer, Millennium, and Celegene, and consulting for Provectus.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Combining the investigational MEK inhibitor cobimetinib with the BRAF inhibitor vemurafenib nearly halved the risk of progression in previously untreated patients with BRAF-mutated advanced melanoma in the ongoing, phase III coBRIM study.
Median progression-free survival by investigator was 6.2 months with vemurafenib (Zelboraf) and 9.9 months with the combination (Hazard ratio 0.51; P less than .0001); by independent review, median progression-free survival was 6.0 months versus 11.3 months, respectively (HR, 0.60; P = .0003), study author Dr. Grant McArthur reported in a late-breaking abstract at the European Society for Medical Oncology Congress.
Combination therapy also resulted in a significantly higher objective response rate (68% vs. 45%; P less than .0001) and more complete responses (10% vs. 4%) among the 495 patients with unresectable, locally advanced or metastatic BRAF V600 mutation–positive disease.
Overall survival data are not yet mature, but the interim analysis showed a promising 35% reduction in the risk of death at 9 months (HR, 0.65), said Dr. McArthur of the Peter MacCallum Cancer Centre, Melbourne, Australia. The corresponding P value of .046 is descriptive, as it did not cross the prespecified stopping boundary for the interim analysis.
Grade 3 toxicity, albeit fairly common, was slightly higher with combination therapy vs. vemurafenib alone (65% vs. 59%). Adverse event–related treatment discontinuations were similar between arms (13% vs. 12%), he said.
The data, which were simultaneously published in the New England Journal of Medicine (2014 Sept. 29 [doi:10.1056/NEJMoa1408868]) are expected to be submitted to the U.S. Food and Drug Administration later this year for potential approval of cobimetinib. Vemurafenib was approved in the United States for late-stage or unresectable melanoma in August 2011.
In our video interview, Dr. McArthur discusses coBRIM and his suggestions for how clinicians may incorporate this novel strategy into practice.
coBRIM was funded by F. Hoffmann-LaRoche/Genentech. Dr. McArthur reported grants from Roche, Novartis, Pfizer, Millennium, and Celegene, and consulting for Provectus.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ESMO 2014
More people die from thin melanomas than thick melanomas
EDINBURGH – Contrary to the perception that most melanoma deaths result from thick melanomas, long-term data from Australia show that more people die who initially present with thin melanomas.
Among 4,218 Australians who died from melanoma between 1990 and 2009, thin melanomas (1 mm or less) accounted for 19% of melanoma deaths overall, but increased steadily from 14% of deaths in 1990-1994 to 23% in 2005-2009.
During the most recent time period (2005-2009), more people died from thin melanomas (296 deaths, 23%) than from thick melanomas more than 4 mm in thickness (186 deaths, 14%) or from metastatic presentations (207 deaths, 16%).
The number of deaths in the intermediate thickness categories was also higher for lesions of thickness 1.01-2.0 mm (272 deaths, 21%) than for lesions of thickness 2.01-4.0 mm (267 deaths, 20%).
The patterns of mortality were essentially unchanged when the analyses were restricted to patients with only one primary melanoma, Dr. David Whiteman and Dr. Catherine Olsen of the QIMR Berghofer Medical Research Institute, Brisbane, Australia, reported at the 15th World Congress on Cancers of the Skin.
The melanoma incidence has been rising steadily around the world, mostly because of the greater numbers of thin lesions being diagnosed. The perception that most people who die from melanoma present initially with thick lesions is widespread, but the veracity of this proposition has never been tested because population-based data describing total mortality by the thickness of the primary tumor are scarce, according to the researchers. They used linked histology and death data from the Queensland Cancer Registry, where notification of melanoma has been compulsory since 1982, to calculate age-standardized mortality rates for each year for all melanomas, and by thickness of the first primary lesion. Overall, 67% of patients were male, 68% presented with a single primary lesion, and 68% of all melanomas were thin (1 mm or less).
Deaths from melanoma were most common among those who were in the seventh and eighth decades of life, male, or had a melanoma arising on the trunk. As expected, the intervals from diagnosis to death were significantly shorter for thicker tumors than thinner tumors, Dr. Whiteman and Dr. Olsen noted in the poster presentation.
The average annual rate of change in melanoma mortality increased significantly for men for thin lesions and those of intermediate thickness. Mortality rates from metastatic lesions, however, declined during the observation period.
“From a public health perspective, it can be argued that primary prevention activities aimed at reducing the occurrence of melanoma in the entire population should be accorded the highest priority,” the authors concluded at the meeting, sponsored by the Skin Cancer Foundation.
EDINBURGH – Contrary to the perception that most melanoma deaths result from thick melanomas, long-term data from Australia show that more people die who initially present with thin melanomas.
Among 4,218 Australians who died from melanoma between 1990 and 2009, thin melanomas (1 mm or less) accounted for 19% of melanoma deaths overall, but increased steadily from 14% of deaths in 1990-1994 to 23% in 2005-2009.
During the most recent time period (2005-2009), more people died from thin melanomas (296 deaths, 23%) than from thick melanomas more than 4 mm in thickness (186 deaths, 14%) or from metastatic presentations (207 deaths, 16%).
The number of deaths in the intermediate thickness categories was also higher for lesions of thickness 1.01-2.0 mm (272 deaths, 21%) than for lesions of thickness 2.01-4.0 mm (267 deaths, 20%).
The patterns of mortality were essentially unchanged when the analyses were restricted to patients with only one primary melanoma, Dr. David Whiteman and Dr. Catherine Olsen of the QIMR Berghofer Medical Research Institute, Brisbane, Australia, reported at the 15th World Congress on Cancers of the Skin.
The melanoma incidence has been rising steadily around the world, mostly because of the greater numbers of thin lesions being diagnosed. The perception that most people who die from melanoma present initially with thick lesions is widespread, but the veracity of this proposition has never been tested because population-based data describing total mortality by the thickness of the primary tumor are scarce, according to the researchers. They used linked histology and death data from the Queensland Cancer Registry, where notification of melanoma has been compulsory since 1982, to calculate age-standardized mortality rates for each year for all melanomas, and by thickness of the first primary lesion. Overall, 67% of patients were male, 68% presented with a single primary lesion, and 68% of all melanomas were thin (1 mm or less).
Deaths from melanoma were most common among those who were in the seventh and eighth decades of life, male, or had a melanoma arising on the trunk. As expected, the intervals from diagnosis to death were significantly shorter for thicker tumors than thinner tumors, Dr. Whiteman and Dr. Olsen noted in the poster presentation.
The average annual rate of change in melanoma mortality increased significantly for men for thin lesions and those of intermediate thickness. Mortality rates from metastatic lesions, however, declined during the observation period.
“From a public health perspective, it can be argued that primary prevention activities aimed at reducing the occurrence of melanoma in the entire population should be accorded the highest priority,” the authors concluded at the meeting, sponsored by the Skin Cancer Foundation.
EDINBURGH – Contrary to the perception that most melanoma deaths result from thick melanomas, long-term data from Australia show that more people die who initially present with thin melanomas.
Among 4,218 Australians who died from melanoma between 1990 and 2009, thin melanomas (1 mm or less) accounted for 19% of melanoma deaths overall, but increased steadily from 14% of deaths in 1990-1994 to 23% in 2005-2009.
During the most recent time period (2005-2009), more people died from thin melanomas (296 deaths, 23%) than from thick melanomas more than 4 mm in thickness (186 deaths, 14%) or from metastatic presentations (207 deaths, 16%).
The number of deaths in the intermediate thickness categories was also higher for lesions of thickness 1.01-2.0 mm (272 deaths, 21%) than for lesions of thickness 2.01-4.0 mm (267 deaths, 20%).
The patterns of mortality were essentially unchanged when the analyses were restricted to patients with only one primary melanoma, Dr. David Whiteman and Dr. Catherine Olsen of the QIMR Berghofer Medical Research Institute, Brisbane, Australia, reported at the 15th World Congress on Cancers of the Skin.
The melanoma incidence has been rising steadily around the world, mostly because of the greater numbers of thin lesions being diagnosed. The perception that most people who die from melanoma present initially with thick lesions is widespread, but the veracity of this proposition has never been tested because population-based data describing total mortality by the thickness of the primary tumor are scarce, according to the researchers. They used linked histology and death data from the Queensland Cancer Registry, where notification of melanoma has been compulsory since 1982, to calculate age-standardized mortality rates for each year for all melanomas, and by thickness of the first primary lesion. Overall, 67% of patients were male, 68% presented with a single primary lesion, and 68% of all melanomas were thin (1 mm or less).
Deaths from melanoma were most common among those who were in the seventh and eighth decades of life, male, or had a melanoma arising on the trunk. As expected, the intervals from diagnosis to death were significantly shorter for thicker tumors than thinner tumors, Dr. Whiteman and Dr. Olsen noted in the poster presentation.
The average annual rate of change in melanoma mortality increased significantly for men for thin lesions and those of intermediate thickness. Mortality rates from metastatic lesions, however, declined during the observation period.
“From a public health perspective, it can be argued that primary prevention activities aimed at reducing the occurrence of melanoma in the entire population should be accorded the highest priority,” the authors concluded at the meeting, sponsored by the Skin Cancer Foundation.
AT WCCS 2014
Key clinical point: Thin lesions are associated with substantial mortality, so primary prevention of melanoma should remain the principal strategy.
Major finding: From 2005 to 2009, 23% of residents of Queensland, Australia, died from thin melanomas versus 14% with thick melanomas.
Data source: State cancer registry analysis of 4,218 melanoma deaths in Queensland.
Disclosures: The researchers had no relevant financial conflicts to disclose.
Moles quadruple risk for melanoma
EDINBURGH – Patients with moles had more than four times the risk of developing melanoma, compared with those without moles in a large record-linkage study.
The overall rate ratio for melanoma, based on person-years at risk, was 4.68 among patients with moles recorded in their medical record (95% confidence interval, 4.39-4.98), Dr. Eugene Ong reported at the 15th World Congress on Cancers of the Skin.
Rate ratios were also significantly higher for individuals with moles of both sexes and in all age groups, including those aged younger than 25 years (RR, 3.79), 25-59 years (RR, 5.02), and at least 60 years (RR, 4.68).
Prior research has shown that high numbers of melanocytic or dysplastic nevi are strong risk factors for the development of melanoma. The investigators sought to further characterize the risk of melanoma in persons with melanocytic nevus (MN) using linked hospital and mortality records covering the entire population of England from 1999 to 2011.
The analysis included 271,656 patients with a hospital or day-case record of moles and a control cohort of 10,130,417 persons with no moles recorded. Anyone diagnosed with melanoma within 1 year of study entry was excluded.
Patients with a record of moles had a significantly higher risk of developing melanoma both around the site of the mole and elsewhere on their body, and therefore may benefit from increased surveillance, said Dr. Ong of the University of Oxford, England. For patients with a mole on the trunk, the rate ratio for a melanoma on the trunk was 8.99 (95% CI, 7.69-10.46) and 5.66 for a melanoma elsewhere (95% CI, 4.97-6.42).
The investigators were unable to distinguish between different types of moles or to determine the number of moles in each patient. Further, a mole or moles were the principal reason for hospital contact for 91% of patients, so it’s likely they presented with unusual appearing moles in order for them to warrant recording, Dr. Ong acknowledged.
“So while this study does not suggest that everyone with a single mole is far more likely to develop melanoma, it does illustrate the link between moles and skin cancer. This is why it is vital people check their moles regularly and report any changes to their doctor,” he said in a statement released during the meeting, sponsored in part by the Skin Cancer Foundation.
EDINBURGH – Patients with moles had more than four times the risk of developing melanoma, compared with those without moles in a large record-linkage study.
The overall rate ratio for melanoma, based on person-years at risk, was 4.68 among patients with moles recorded in their medical record (95% confidence interval, 4.39-4.98), Dr. Eugene Ong reported at the 15th World Congress on Cancers of the Skin.
Rate ratios were also significantly higher for individuals with moles of both sexes and in all age groups, including those aged younger than 25 years (RR, 3.79), 25-59 years (RR, 5.02), and at least 60 years (RR, 4.68).
Prior research has shown that high numbers of melanocytic or dysplastic nevi are strong risk factors for the development of melanoma. The investigators sought to further characterize the risk of melanoma in persons with melanocytic nevus (MN) using linked hospital and mortality records covering the entire population of England from 1999 to 2011.
The analysis included 271,656 patients with a hospital or day-case record of moles and a control cohort of 10,130,417 persons with no moles recorded. Anyone diagnosed with melanoma within 1 year of study entry was excluded.
Patients with a record of moles had a significantly higher risk of developing melanoma both around the site of the mole and elsewhere on their body, and therefore may benefit from increased surveillance, said Dr. Ong of the University of Oxford, England. For patients with a mole on the trunk, the rate ratio for a melanoma on the trunk was 8.99 (95% CI, 7.69-10.46) and 5.66 for a melanoma elsewhere (95% CI, 4.97-6.42).
The investigators were unable to distinguish between different types of moles or to determine the number of moles in each patient. Further, a mole or moles were the principal reason for hospital contact for 91% of patients, so it’s likely they presented with unusual appearing moles in order for them to warrant recording, Dr. Ong acknowledged.
“So while this study does not suggest that everyone with a single mole is far more likely to develop melanoma, it does illustrate the link between moles and skin cancer. This is why it is vital people check their moles regularly and report any changes to their doctor,” he said in a statement released during the meeting, sponsored in part by the Skin Cancer Foundation.
EDINBURGH – Patients with moles had more than four times the risk of developing melanoma, compared with those without moles in a large record-linkage study.
The overall rate ratio for melanoma, based on person-years at risk, was 4.68 among patients with moles recorded in their medical record (95% confidence interval, 4.39-4.98), Dr. Eugene Ong reported at the 15th World Congress on Cancers of the Skin.
Rate ratios were also significantly higher for individuals with moles of both sexes and in all age groups, including those aged younger than 25 years (RR, 3.79), 25-59 years (RR, 5.02), and at least 60 years (RR, 4.68).
Prior research has shown that high numbers of melanocytic or dysplastic nevi are strong risk factors for the development of melanoma. The investigators sought to further characterize the risk of melanoma in persons with melanocytic nevus (MN) using linked hospital and mortality records covering the entire population of England from 1999 to 2011.
The analysis included 271,656 patients with a hospital or day-case record of moles and a control cohort of 10,130,417 persons with no moles recorded. Anyone diagnosed with melanoma within 1 year of study entry was excluded.
Patients with a record of moles had a significantly higher risk of developing melanoma both around the site of the mole and elsewhere on their body, and therefore may benefit from increased surveillance, said Dr. Ong of the University of Oxford, England. For patients with a mole on the trunk, the rate ratio for a melanoma on the trunk was 8.99 (95% CI, 7.69-10.46) and 5.66 for a melanoma elsewhere (95% CI, 4.97-6.42).
The investigators were unable to distinguish between different types of moles or to determine the number of moles in each patient. Further, a mole or moles were the principal reason for hospital contact for 91% of patients, so it’s likely they presented with unusual appearing moles in order for them to warrant recording, Dr. Ong acknowledged.
“So while this study does not suggest that everyone with a single mole is far more likely to develop melanoma, it does illustrate the link between moles and skin cancer. This is why it is vital people check their moles regularly and report any changes to their doctor,” he said in a statement released during the meeting, sponsored in part by the Skin Cancer Foundation.
AT THE WCCS 2014
Key clinical point: Individuals with moles are at significantly increased risk for developing melanoma in the same body region as the mole, and in other regions, and could benefit from increased surveillance.
Major finding: The rate ratio for melanoma, based on person-years at risk, was 4.68 times among patients with moles than those without moles (95% CI 4.39-4.98).
Data source: A record-linkage study in 271,656 patients with a diagnosis of moles and 10,130,417 controls without moles.
Disclosures: Dr. Ong had no financial conflicts to disclose.
VIDEO: How red hair and freckles might raise your skin cancer risk
EDINBURGH – Variants in the pigment-associated MC1R gene have been implicated in an increased risk for melanoma and nonmelanoma skin cancers, although the extent of that risk has been inconsistent across studies, according to Dr. Eugene Healy of the University of Southampton (England). In an interview at the 15th World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, Dr. Healy discussed how the MC1R gene variants might impact skin cancer risk and the challenges of pinning down genetic data into practical applications for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EDINBURGH – Variants in the pigment-associated MC1R gene have been implicated in an increased risk for melanoma and nonmelanoma skin cancers, although the extent of that risk has been inconsistent across studies, according to Dr. Eugene Healy of the University of Southampton (England). In an interview at the 15th World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, Dr. Healy discussed how the MC1R gene variants might impact skin cancer risk and the challenges of pinning down genetic data into practical applications for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EDINBURGH – Variants in the pigment-associated MC1R gene have been implicated in an increased risk for melanoma and nonmelanoma skin cancers, although the extent of that risk has been inconsistent across studies, according to Dr. Eugene Healy of the University of Southampton (England). In an interview at the 15th World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, Dr. Healy discussed how the MC1R gene variants might impact skin cancer risk and the challenges of pinning down genetic data into practical applications for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM WCCS 2014
Closing large dermal defects much like a Victorian corset
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EXPERT ANALYSIS FROM WCCS 2014
Senate OKs sunscreen bill
The Senate unanimously approved a bill designed to speed up Food and Drug Administration approval of sunscreen ingredients, adding to the likelihood that the legislation will become law.
The full Senate took quick action on Sept. 17, the same day that the Health, Education, Labor & Pensions (HELP) Committee unanimously voted 12-0 in favor of the Sunscreen Innovation Act (S.2141).
“We have a number of ingredients for sunscreens that have languished at the FDA for years – as long as a decade,” Sen. Johnny Isakson (R-Ga.), a cosponsor of the bill, said at the HELP hearing. “This doesn’t mean Congress makes the decision,” he said, adding that, instead, the law does establish time frames for review.
The legislation “is also about holding the FDA accountable to timelines and reforming the process,” said Sen. Lamar Alexander (R-Tenn.).
The bill would require the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more congressional oversight.
At a recent FDA advisory committee hearing on sunscreen ingredient safety, Dr. Theresa Michele, an FDA official, said that eight ingredients were awaiting approval through what was supposed to be an expedited process. These are the same ingredients that have been under review for 10 years or more. The agency has responded to manufacturers of five of the eight, telling them that so far, there’s not enough data to determine whether they can be marketed, said Dr. Michele.
The Senate joins the House in calling on the FDA to move those approvals along. The full House approved companion legislation (H.R.4250) in late July.
The legislation is supported by the American Academy of Dermatology and by consumer advocates and manufacturers, including the Public Access to SunScreens (PASS) Coalition.
“Congress’s commitment to addressing the skin cancer epidemic in the United States was clearly demonstrated in tonight’s Senate passage of the Sunscreen Innovation Act,” said Michael Werner, PASS Coalition Policy Adviser, in a statement after the vote. “We now call on the House and Senate to swiftly reconcile the differences in their bills and enact final legislation,” Werner said.
Chris Hansen, president of the American Cancer Society Cancer Action Network, said in a statement that if the bill became law, it would add more predictability to FDA reviews.
“The Senate took a critical step yesterday to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer,” he said.
Tim Turnham, executive director of the Melanoma Research Foundation, said in a statement that some of the ingredients under FDA review have been widely available in Europe, Asia, and Central and South America, in some cases for more than 15 years.
“Americans are limited in their choices for compounds that block UV radiation because of the long-standing bureaucratic gridlock at the FDA that prevents new agents from being approved,” said Mr. Turnham.
The House and Senate have to reconcile the two versions of the bill, and then the legislation will have to be approved again by both bodies before being sent to the White House for final approval.
On Twitter @aliciaault
The Senate unanimously approved a bill designed to speed up Food and Drug Administration approval of sunscreen ingredients, adding to the likelihood that the legislation will become law.
The full Senate took quick action on Sept. 17, the same day that the Health, Education, Labor & Pensions (HELP) Committee unanimously voted 12-0 in favor of the Sunscreen Innovation Act (S.2141).
“We have a number of ingredients for sunscreens that have languished at the FDA for years – as long as a decade,” Sen. Johnny Isakson (R-Ga.), a cosponsor of the bill, said at the HELP hearing. “This doesn’t mean Congress makes the decision,” he said, adding that, instead, the law does establish time frames for review.
The legislation “is also about holding the FDA accountable to timelines and reforming the process,” said Sen. Lamar Alexander (R-Tenn.).
The bill would require the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more congressional oversight.
At a recent FDA advisory committee hearing on sunscreen ingredient safety, Dr. Theresa Michele, an FDA official, said that eight ingredients were awaiting approval through what was supposed to be an expedited process. These are the same ingredients that have been under review for 10 years or more. The agency has responded to manufacturers of five of the eight, telling them that so far, there’s not enough data to determine whether they can be marketed, said Dr. Michele.
The Senate joins the House in calling on the FDA to move those approvals along. The full House approved companion legislation (H.R.4250) in late July.
The legislation is supported by the American Academy of Dermatology and by consumer advocates and manufacturers, including the Public Access to SunScreens (PASS) Coalition.
“Congress’s commitment to addressing the skin cancer epidemic in the United States was clearly demonstrated in tonight’s Senate passage of the Sunscreen Innovation Act,” said Michael Werner, PASS Coalition Policy Adviser, in a statement after the vote. “We now call on the House and Senate to swiftly reconcile the differences in their bills and enact final legislation,” Werner said.
Chris Hansen, president of the American Cancer Society Cancer Action Network, said in a statement that if the bill became law, it would add more predictability to FDA reviews.
“The Senate took a critical step yesterday to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer,” he said.
Tim Turnham, executive director of the Melanoma Research Foundation, said in a statement that some of the ingredients under FDA review have been widely available in Europe, Asia, and Central and South America, in some cases for more than 15 years.
“Americans are limited in their choices for compounds that block UV radiation because of the long-standing bureaucratic gridlock at the FDA that prevents new agents from being approved,” said Mr. Turnham.
The House and Senate have to reconcile the two versions of the bill, and then the legislation will have to be approved again by both bodies before being sent to the White House for final approval.
On Twitter @aliciaault
The Senate unanimously approved a bill designed to speed up Food and Drug Administration approval of sunscreen ingredients, adding to the likelihood that the legislation will become law.
The full Senate took quick action on Sept. 17, the same day that the Health, Education, Labor & Pensions (HELP) Committee unanimously voted 12-0 in favor of the Sunscreen Innovation Act (S.2141).
“We have a number of ingredients for sunscreens that have languished at the FDA for years – as long as a decade,” Sen. Johnny Isakson (R-Ga.), a cosponsor of the bill, said at the HELP hearing. “This doesn’t mean Congress makes the decision,” he said, adding that, instead, the law does establish time frames for review.
The legislation “is also about holding the FDA accountable to timelines and reforming the process,” said Sen. Lamar Alexander (R-Tenn.).
The bill would require the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more congressional oversight.
At a recent FDA advisory committee hearing on sunscreen ingredient safety, Dr. Theresa Michele, an FDA official, said that eight ingredients were awaiting approval through what was supposed to be an expedited process. These are the same ingredients that have been under review for 10 years or more. The agency has responded to manufacturers of five of the eight, telling them that so far, there’s not enough data to determine whether they can be marketed, said Dr. Michele.
The Senate joins the House in calling on the FDA to move those approvals along. The full House approved companion legislation (H.R.4250) in late July.
The legislation is supported by the American Academy of Dermatology and by consumer advocates and manufacturers, including the Public Access to SunScreens (PASS) Coalition.
“Congress’s commitment to addressing the skin cancer epidemic in the United States was clearly demonstrated in tonight’s Senate passage of the Sunscreen Innovation Act,” said Michael Werner, PASS Coalition Policy Adviser, in a statement after the vote. “We now call on the House and Senate to swiftly reconcile the differences in their bills and enact final legislation,” Werner said.
Chris Hansen, president of the American Cancer Society Cancer Action Network, said in a statement that if the bill became law, it would add more predictability to FDA reviews.
“The Senate took a critical step yesterday to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer,” he said.
Tim Turnham, executive director of the Melanoma Research Foundation, said in a statement that some of the ingredients under FDA review have been widely available in Europe, Asia, and Central and South America, in some cases for more than 15 years.
“Americans are limited in their choices for compounds that block UV radiation because of the long-standing bureaucratic gridlock at the FDA that prevents new agents from being approved,” said Mr. Turnham.
The House and Senate have to reconcile the two versions of the bill, and then the legislation will have to be approved again by both bodies before being sent to the White House for final approval.
On Twitter @aliciaault
Focus lands on kinase fusions in Spitz tumors
EDINBURGH –Kinase fusions have been well described in hematologic malignancies and are the newest genetic aberration to be identified in Spitz tumors.
Kinase fusions are actually quite common in Spitzoid neoplasms, with 51% of Spitzoid tumors and melanomas harboring a kinase fusion in a recent analysis of 140 tumors (Nat. Commun. 2014;5:3116. doi:10.1038/ncommons4116 ).
The fusions were identified across the entire biological spectrum of Spitzoid neoplasms, including 55% of Spitz nevi, 56% of atypical Spitz tumors, and 39% of Spitzoid melanomas. Therefore, kinase fusions are not useful to distinguish benign from malignant tumors, study author Dr. Thomas Wiesner said at the 15thWorld Congress on Cancers of the Skin.
However, the kinase fusions were mutually exclusive, meaning that only one fusion activates the oncogenic pathway in a tumor. Small molecule kinase inhibitors have become mainstays of modern oncologic therapy in recent years and thus may be useful in Spitz tumors, which typically affect the young.
“These genetic aberrations represent targets for therapeutic interventions and offer investigational treatment options for patients with metastatic disease,” said Dr. Wiesner, a research associate at Memorial Sloan-Kettering Cancer Center in New York City.
ROS1 (c-ros oncogene 1) fusions were the most frequent in the series at 17% and have been shown in mouse models to respond to crizotinib (Xalkori). ALK fusions, present in 10% of tumors, are also sensitive to this drug.
“I think Spitzoid melanoma that is metastatic should be stained for ROS1 or ALK fusions because crizotinib might help these patients,” he said. “We have good evidence that it helps, at least in lung cancer and lymphoma.”
Other drugs target NTRK1 (neurotrophic tyrosine kinase receptor type 1) fusions, which were identified in 16% of Spitzoid tumors, and are in preclinical trials.
The researchers have also published a description of the morphological features that point to the underlying genetic aberrations (Am. J. Surg. Pathol. Jul 2014;38:925-33)). The study, involving 17 patients, aged 2 to 35 years, indicates that BAP1 loss and BRAF mutations are common in epithelioid Spitz tumors, HRAS mutations and gains of 11p are seen in desmoplastic Spitz nevi, while ALK fusions usually point to plexiform Spitz tumors, Dr. Wiesner said.
Clinicians can use the morphological features along with basic immunohistochemistry to identify the distinct subsets of tumors with BAP1 loss or ALK, NTRK1, or ROS1 fusions.
BAP1 loss Spitz tumors are important to identify because they are associated with a hereditary tumor syndrome very similar to Peutz-Jeghers or Muir-Torre syndrome, Dr. Wiesner said.
“When we see multiple epithelioid Spitz tumors in one patient, we have to think about a hereditary tumor syndrome,” he said at the meeting, sponsored by the Skin Cancer Foundation.
EDINBURGH –Kinase fusions have been well described in hematologic malignancies and are the newest genetic aberration to be identified in Spitz tumors.
Kinase fusions are actually quite common in Spitzoid neoplasms, with 51% of Spitzoid tumors and melanomas harboring a kinase fusion in a recent analysis of 140 tumors (Nat. Commun. 2014;5:3116. doi:10.1038/ncommons4116 ).
The fusions were identified across the entire biological spectrum of Spitzoid neoplasms, including 55% of Spitz nevi, 56% of atypical Spitz tumors, and 39% of Spitzoid melanomas. Therefore, kinase fusions are not useful to distinguish benign from malignant tumors, study author Dr. Thomas Wiesner said at the 15thWorld Congress on Cancers of the Skin.
However, the kinase fusions were mutually exclusive, meaning that only one fusion activates the oncogenic pathway in a tumor. Small molecule kinase inhibitors have become mainstays of modern oncologic therapy in recent years and thus may be useful in Spitz tumors, which typically affect the young.
“These genetic aberrations represent targets for therapeutic interventions and offer investigational treatment options for patients with metastatic disease,” said Dr. Wiesner, a research associate at Memorial Sloan-Kettering Cancer Center in New York City.
ROS1 (c-ros oncogene 1) fusions were the most frequent in the series at 17% and have been shown in mouse models to respond to crizotinib (Xalkori). ALK fusions, present in 10% of tumors, are also sensitive to this drug.
“I think Spitzoid melanoma that is metastatic should be stained for ROS1 or ALK fusions because crizotinib might help these patients,” he said. “We have good evidence that it helps, at least in lung cancer and lymphoma.”
Other drugs target NTRK1 (neurotrophic tyrosine kinase receptor type 1) fusions, which were identified in 16% of Spitzoid tumors, and are in preclinical trials.
The researchers have also published a description of the morphological features that point to the underlying genetic aberrations (Am. J. Surg. Pathol. Jul 2014;38:925-33)). The study, involving 17 patients, aged 2 to 35 years, indicates that BAP1 loss and BRAF mutations are common in epithelioid Spitz tumors, HRAS mutations and gains of 11p are seen in desmoplastic Spitz nevi, while ALK fusions usually point to plexiform Spitz tumors, Dr. Wiesner said.
Clinicians can use the morphological features along with basic immunohistochemistry to identify the distinct subsets of tumors with BAP1 loss or ALK, NTRK1, or ROS1 fusions.
BAP1 loss Spitz tumors are important to identify because they are associated with a hereditary tumor syndrome very similar to Peutz-Jeghers or Muir-Torre syndrome, Dr. Wiesner said.
“When we see multiple epithelioid Spitz tumors in one patient, we have to think about a hereditary tumor syndrome,” he said at the meeting, sponsored by the Skin Cancer Foundation.
EDINBURGH –Kinase fusions have been well described in hematologic malignancies and are the newest genetic aberration to be identified in Spitz tumors.
Kinase fusions are actually quite common in Spitzoid neoplasms, with 51% of Spitzoid tumors and melanomas harboring a kinase fusion in a recent analysis of 140 tumors (Nat. Commun. 2014;5:3116. doi:10.1038/ncommons4116 ).
The fusions were identified across the entire biological spectrum of Spitzoid neoplasms, including 55% of Spitz nevi, 56% of atypical Spitz tumors, and 39% of Spitzoid melanomas. Therefore, kinase fusions are not useful to distinguish benign from malignant tumors, study author Dr. Thomas Wiesner said at the 15thWorld Congress on Cancers of the Skin.
However, the kinase fusions were mutually exclusive, meaning that only one fusion activates the oncogenic pathway in a tumor. Small molecule kinase inhibitors have become mainstays of modern oncologic therapy in recent years and thus may be useful in Spitz tumors, which typically affect the young.
“These genetic aberrations represent targets for therapeutic interventions and offer investigational treatment options for patients with metastatic disease,” said Dr. Wiesner, a research associate at Memorial Sloan-Kettering Cancer Center in New York City.
ROS1 (c-ros oncogene 1) fusions were the most frequent in the series at 17% and have been shown in mouse models to respond to crizotinib (Xalkori). ALK fusions, present in 10% of tumors, are also sensitive to this drug.
“I think Spitzoid melanoma that is metastatic should be stained for ROS1 or ALK fusions because crizotinib might help these patients,” he said. “We have good evidence that it helps, at least in lung cancer and lymphoma.”
Other drugs target NTRK1 (neurotrophic tyrosine kinase receptor type 1) fusions, which were identified in 16% of Spitzoid tumors, and are in preclinical trials.
The researchers have also published a description of the morphological features that point to the underlying genetic aberrations (Am. J. Surg. Pathol. Jul 2014;38:925-33)). The study, involving 17 patients, aged 2 to 35 years, indicates that BAP1 loss and BRAF mutations are common in epithelioid Spitz tumors, HRAS mutations and gains of 11p are seen in desmoplastic Spitz nevi, while ALK fusions usually point to plexiform Spitz tumors, Dr. Wiesner said.
Clinicians can use the morphological features along with basic immunohistochemistry to identify the distinct subsets of tumors with BAP1 loss or ALK, NTRK1, or ROS1 fusions.
BAP1 loss Spitz tumors are important to identify because they are associated with a hereditary tumor syndrome very similar to Peutz-Jeghers or Muir-Torre syndrome, Dr. Wiesner said.
“When we see multiple epithelioid Spitz tumors in one patient, we have to think about a hereditary tumor syndrome,” he said at the meeting, sponsored by the Skin Cancer Foundation.
AT THE WCCS 2014
Key clinical point: Kinase fusions are common in Spitz neoplasms and may serve as therapeutic targets.
Major finding: Kinase fusions were identified in 51% of 140 Spitzoid tumors and melanomas.
Data source: Published genetic studies.
Disclosures: Dr. Wiesner reported no conflicts of interest.
Anal high-grade squamous intraepithelial lesions cleared in 42% of gay men
MELBOURNE – High-grade squamous intraepithelial lesions are prevalent among HIV-positive gay men and a significant number of these anal lesions resolve spontaneously, according to data from a longitudinal observational cohort study.
The Study of the Prevention of Anal Cancer (SPANC) is a 3-year prospective study of the natural history of anal HPV infection, which has so far enrolled 350 homosexual men over 35 years old. Dr. Andrew Grulich from the Kirby Institute, University of New South Wales, Sydney, presented trial data at the 20th International AIDS Conference that showed a 42% clearance rate for high-grade squamous intraepithelial lesions (HSIL).
Anal lesions that result from human papillomavirus infection are difficult to treat, and there is no evidence for the effectiveness of treatment, Dr. Grulich said. Further, anal lesions are far less likely than cervical lesions to progress to cancer. However, questions remain about which men are more likely to have persistent high-grade disease and therefore are at a higher risk of progression to cancer.
Interim data from the SPANC study found a 46% prevalence of HSIL among HIV-positive gay men and a 34% prevalence among HIV-negative gay men. The higher rate among HIV-positive individuals was largely driven by a higher prevalence of more advanced anal intraepithelial neoplasia.
Men with persistent infections due to human papillomavirus (HPV) 16 – the subtype most commonly associated with anal cancer – were much less likely to clear the high-grade lesions (hazard ratio = 0.22, 95% confidence interval, 0.11-0.46), as were men with multiple subtypes of HPV.
"What we’ve been able to show is that high-grade disease is very dynamic, with one in six [gay] men getting it and of those who get it about 40% clearing it each year," Dr. Grulich told the conference.
"Not all high-grade disease requires treatment," he said. "These data suggest that treatment can be targeted at those with persistent high-grade disease because much high-grade disease diagnosed on a single occasion will simply go away."
Treatment practices for HSIL vary with some choosing a ‘watch and wait’ approach and others choosing ablative treatment. Dr. Grulich suggested treatment based on "red flags" that suggest a higher risk of progression to cancer.
"If [the patient] is HPV 16–positive, that’s a definite red flag because 90% of anal cancer is caused by that one subtype," Dr. Grulich said. Also, high-grade disease that doesn’t clear is another red flag.
"I think it’s perfectly reasonable, given the state of the science, if you’re doing high-resolution anoscopy and you diagnose (HSIL), to explain to the patient that it’s highly likely to go away but it may not, and therefore get the patient back in about a year," he said.
The study is funded by the National Health and Medical Research Council of Australia, and the Cancer Council NSW. Some authors declared financial ties to pharmaceutical companies including a manufacturer of HPV vaccines.
MELBOURNE – High-grade squamous intraepithelial lesions are prevalent among HIV-positive gay men and a significant number of these anal lesions resolve spontaneously, according to data from a longitudinal observational cohort study.
The Study of the Prevention of Anal Cancer (SPANC) is a 3-year prospective study of the natural history of anal HPV infection, which has so far enrolled 350 homosexual men over 35 years old. Dr. Andrew Grulich from the Kirby Institute, University of New South Wales, Sydney, presented trial data at the 20th International AIDS Conference that showed a 42% clearance rate for high-grade squamous intraepithelial lesions (HSIL).
Anal lesions that result from human papillomavirus infection are difficult to treat, and there is no evidence for the effectiveness of treatment, Dr. Grulich said. Further, anal lesions are far less likely than cervical lesions to progress to cancer. However, questions remain about which men are more likely to have persistent high-grade disease and therefore are at a higher risk of progression to cancer.
Interim data from the SPANC study found a 46% prevalence of HSIL among HIV-positive gay men and a 34% prevalence among HIV-negative gay men. The higher rate among HIV-positive individuals was largely driven by a higher prevalence of more advanced anal intraepithelial neoplasia.
Men with persistent infections due to human papillomavirus (HPV) 16 – the subtype most commonly associated with anal cancer – were much less likely to clear the high-grade lesions (hazard ratio = 0.22, 95% confidence interval, 0.11-0.46), as were men with multiple subtypes of HPV.
"What we’ve been able to show is that high-grade disease is very dynamic, with one in six [gay] men getting it and of those who get it about 40% clearing it each year," Dr. Grulich told the conference.
"Not all high-grade disease requires treatment," he said. "These data suggest that treatment can be targeted at those with persistent high-grade disease because much high-grade disease diagnosed on a single occasion will simply go away."
Treatment practices for HSIL vary with some choosing a ‘watch and wait’ approach and others choosing ablative treatment. Dr. Grulich suggested treatment based on "red flags" that suggest a higher risk of progression to cancer.
"If [the patient] is HPV 16–positive, that’s a definite red flag because 90% of anal cancer is caused by that one subtype," Dr. Grulich said. Also, high-grade disease that doesn’t clear is another red flag.
"I think it’s perfectly reasonable, given the state of the science, if you’re doing high-resolution anoscopy and you diagnose (HSIL), to explain to the patient that it’s highly likely to go away but it may not, and therefore get the patient back in about a year," he said.
The study is funded by the National Health and Medical Research Council of Australia, and the Cancer Council NSW. Some authors declared financial ties to pharmaceutical companies including a manufacturer of HPV vaccines.
MELBOURNE – High-grade squamous intraepithelial lesions are prevalent among HIV-positive gay men and a significant number of these anal lesions resolve spontaneously, according to data from a longitudinal observational cohort study.
The Study of the Prevention of Anal Cancer (SPANC) is a 3-year prospective study of the natural history of anal HPV infection, which has so far enrolled 350 homosexual men over 35 years old. Dr. Andrew Grulich from the Kirby Institute, University of New South Wales, Sydney, presented trial data at the 20th International AIDS Conference that showed a 42% clearance rate for high-grade squamous intraepithelial lesions (HSIL).
Anal lesions that result from human papillomavirus infection are difficult to treat, and there is no evidence for the effectiveness of treatment, Dr. Grulich said. Further, anal lesions are far less likely than cervical lesions to progress to cancer. However, questions remain about which men are more likely to have persistent high-grade disease and therefore are at a higher risk of progression to cancer.
Interim data from the SPANC study found a 46% prevalence of HSIL among HIV-positive gay men and a 34% prevalence among HIV-negative gay men. The higher rate among HIV-positive individuals was largely driven by a higher prevalence of more advanced anal intraepithelial neoplasia.
Men with persistent infections due to human papillomavirus (HPV) 16 – the subtype most commonly associated with anal cancer – were much less likely to clear the high-grade lesions (hazard ratio = 0.22, 95% confidence interval, 0.11-0.46), as were men with multiple subtypes of HPV.
"What we’ve been able to show is that high-grade disease is very dynamic, with one in six [gay] men getting it and of those who get it about 40% clearing it each year," Dr. Grulich told the conference.
"Not all high-grade disease requires treatment," he said. "These data suggest that treatment can be targeted at those with persistent high-grade disease because much high-grade disease diagnosed on a single occasion will simply go away."
Treatment practices for HSIL vary with some choosing a ‘watch and wait’ approach and others choosing ablative treatment. Dr. Grulich suggested treatment based on "red flags" that suggest a higher risk of progression to cancer.
"If [the patient] is HPV 16–positive, that’s a definite red flag because 90% of anal cancer is caused by that one subtype," Dr. Grulich said. Also, high-grade disease that doesn’t clear is another red flag.
"I think it’s perfectly reasonable, given the state of the science, if you’re doing high-resolution anoscopy and you diagnose (HSIL), to explain to the patient that it’s highly likely to go away but it may not, and therefore get the patient back in about a year," he said.
The study is funded by the National Health and Medical Research Council of Australia, and the Cancer Council NSW. Some authors declared financial ties to pharmaceutical companies including a manufacturer of HPV vaccines.
AT AIDS 2014
Key clinical point: Watchful waiting may be an option for gay men with anal high-grade squamous intraepithelial lesions if the lesion is not HPV 16 positive and has not been present for more than a year.
Major finding: Over 40% of anal high-grade squamous intraepithelial lesions clear spontaneously without treatment, with similar clearance rates for HIV-positive and HIV-negative gay men.
Data source: Interim data on 350 gay men over 35 years old enrolled in SPANC, a 3-year prospective study of the natural history of anal HPV infection.
Disclosures: The study is funded by the National Health and Medical Research Council of Australia and the Cancer Council NSW. Some authors declared financial ties to pharmaceutical companies including a manufacturer of HPV vaccines.
Mobile apps get skin in the sun protection game
EDINBURGH – Mobile phone apps that provide real-time, personalized sun risk and protection information are gaining ground in the fight against skin cancer.
The SunSmart app uses geolocation to alert iPhone, Android, and Samsung phones users in Australia when the UV Index reaches 3 or more and protection is required.
"The great advantage of a smartphone app for communicating the UV Index is that it can really communicate very directly to individuals based on their location," Craig Sinclair said at the 15th World Congress on Cancers of the Skin.
The SunSmart app also provides precise information related to the UV index and behavior prompts associated with it. It can, for example, calculate how much sunscreen should be applied based on the user’s height, weight, and choice of clothing; provide reminders to reapply sunscreen every 2 hours; and track whether a user is getting enough vitamin D based on their skin type, time spent outdoors, and clothing choices, said Mr. Sinclair of the Cancer Council Australia, Victoria, developer of the app.
The World UV app, created by the British Association of Dermatologists (BAD) and the United Kingdom’s Met Office (national weather service), takes sun protection one step further by providing a guide to the Fitzpatrick skin classification scale so users can determine the effects of the current UV rating on their specific skin type.
The app uses simple icons to relay the current UV index in real time and whether skin protection is required. Separate tabs allow access to more information on their Fitzpatrick skin type, what type of protection is required, and sun protection tips.
The app uses geolocation to pinpoint users but also allows them to select from more than 10,000 locations worldwide – making it a truly global app, Nina Goad, head of communications at BAD, said at the meeting.
Since its informal launch in 2012, more than 150,000 people in 200 countries have downloaded the free World UV app. The app has been most popular in the United States, United Kingdom, Belgium, Spain, and Australia, she said.
The launch of the SunSmart app in late 2010 has prompted roughly 130,000 Australians to download the free app. An online survey of 78 users in February 2011 showed that 85% felt it was easy to use and 87% said it met or exceeded expectations, Mr. Sinclair said at the meeting, which was sponsored by the Skin Cancer Foundation.
A follow-up survey of 380 persons revealed that 72% "agreed" or "strongly agreed" that using the app improved their sun protection that summer.
Another 86% said that it made them more aware of the times of day when sun protection is required and 81% said they were able to recognize the difference between UV level and temperature, which is "always a difficult issue to communicate," Mr. Sinclair said.
"These are self-reports, so there is undoubtedly some bias associated with them, but nonetheless, showing reasonably promising results," he added.
Both Mr. Sinclair and Ms. Goad said the apps will continue to be refined to increase downloads and return visits but noted that their respective nonprofits do not have the funding for large-scale advertising campaigns.
EDINBURGH – Mobile phone apps that provide real-time, personalized sun risk and protection information are gaining ground in the fight against skin cancer.
The SunSmart app uses geolocation to alert iPhone, Android, and Samsung phones users in Australia when the UV Index reaches 3 or more and protection is required.
"The great advantage of a smartphone app for communicating the UV Index is that it can really communicate very directly to individuals based on their location," Craig Sinclair said at the 15th World Congress on Cancers of the Skin.
The SunSmart app also provides precise information related to the UV index and behavior prompts associated with it. It can, for example, calculate how much sunscreen should be applied based on the user’s height, weight, and choice of clothing; provide reminders to reapply sunscreen every 2 hours; and track whether a user is getting enough vitamin D based on their skin type, time spent outdoors, and clothing choices, said Mr. Sinclair of the Cancer Council Australia, Victoria, developer of the app.
The World UV app, created by the British Association of Dermatologists (BAD) and the United Kingdom’s Met Office (national weather service), takes sun protection one step further by providing a guide to the Fitzpatrick skin classification scale so users can determine the effects of the current UV rating on their specific skin type.
The app uses simple icons to relay the current UV index in real time and whether skin protection is required. Separate tabs allow access to more information on their Fitzpatrick skin type, what type of protection is required, and sun protection tips.
The app uses geolocation to pinpoint users but also allows them to select from more than 10,000 locations worldwide – making it a truly global app, Nina Goad, head of communications at BAD, said at the meeting.
Since its informal launch in 2012, more than 150,000 people in 200 countries have downloaded the free World UV app. The app has been most popular in the United States, United Kingdom, Belgium, Spain, and Australia, she said.
The launch of the SunSmart app in late 2010 has prompted roughly 130,000 Australians to download the free app. An online survey of 78 users in February 2011 showed that 85% felt it was easy to use and 87% said it met or exceeded expectations, Mr. Sinclair said at the meeting, which was sponsored by the Skin Cancer Foundation.
A follow-up survey of 380 persons revealed that 72% "agreed" or "strongly agreed" that using the app improved their sun protection that summer.
Another 86% said that it made them more aware of the times of day when sun protection is required and 81% said they were able to recognize the difference between UV level and temperature, which is "always a difficult issue to communicate," Mr. Sinclair said.
"These are self-reports, so there is undoubtedly some bias associated with them, but nonetheless, showing reasonably promising results," he added.
Both Mr. Sinclair and Ms. Goad said the apps will continue to be refined to increase downloads and return visits but noted that their respective nonprofits do not have the funding for large-scale advertising campaigns.
EDINBURGH – Mobile phone apps that provide real-time, personalized sun risk and protection information are gaining ground in the fight against skin cancer.
The SunSmart app uses geolocation to alert iPhone, Android, and Samsung phones users in Australia when the UV Index reaches 3 or more and protection is required.
"The great advantage of a smartphone app for communicating the UV Index is that it can really communicate very directly to individuals based on their location," Craig Sinclair said at the 15th World Congress on Cancers of the Skin.
The SunSmart app also provides precise information related to the UV index and behavior prompts associated with it. It can, for example, calculate how much sunscreen should be applied based on the user’s height, weight, and choice of clothing; provide reminders to reapply sunscreen every 2 hours; and track whether a user is getting enough vitamin D based on their skin type, time spent outdoors, and clothing choices, said Mr. Sinclair of the Cancer Council Australia, Victoria, developer of the app.
The World UV app, created by the British Association of Dermatologists (BAD) and the United Kingdom’s Met Office (national weather service), takes sun protection one step further by providing a guide to the Fitzpatrick skin classification scale so users can determine the effects of the current UV rating on their specific skin type.
The app uses simple icons to relay the current UV index in real time and whether skin protection is required. Separate tabs allow access to more information on their Fitzpatrick skin type, what type of protection is required, and sun protection tips.
The app uses geolocation to pinpoint users but also allows them to select from more than 10,000 locations worldwide – making it a truly global app, Nina Goad, head of communications at BAD, said at the meeting.
Since its informal launch in 2012, more than 150,000 people in 200 countries have downloaded the free World UV app. The app has been most popular in the United States, United Kingdom, Belgium, Spain, and Australia, she said.
The launch of the SunSmart app in late 2010 has prompted roughly 130,000 Australians to download the free app. An online survey of 78 users in February 2011 showed that 85% felt it was easy to use and 87% said it met or exceeded expectations, Mr. Sinclair said at the meeting, which was sponsored by the Skin Cancer Foundation.
A follow-up survey of 380 persons revealed that 72% "agreed" or "strongly agreed" that using the app improved their sun protection that summer.
Another 86% said that it made them more aware of the times of day when sun protection is required and 81% said they were able to recognize the difference between UV level and temperature, which is "always a difficult issue to communicate," Mr. Sinclair said.
"These are self-reports, so there is undoubtedly some bias associated with them, but nonetheless, showing reasonably promising results," he added.
Both Mr. Sinclair and Ms. Goad said the apps will continue to be refined to increase downloads and return visits but noted that their respective nonprofits do not have the funding for large-scale advertising campaigns.
EXPERT ANALYSIS FROM THE WCCS 2014