User login
Nivolumab bests dacarbazine for melanoma survival
Nivolumab achieves significant improvements in overall survival and progression-free survival compared with conventional chemotherapy in untreated patients with advanced melanoma, regardless of PD-L1 status, according to data presented at the International Congress for the Society for Melanoma Research.
Data from a randomized, double-blind, placebo-controlled phase III trial showed patients treated with nivolumab – a PD-1 immune checkpoint inhibitor – showed there was a 58% lower incidence of death at 1 year among patients treated with nivolumab compared with those treated with dacarbazine (72.9% vs. 42.1% HR 0.42, 95% CI 0.25-0.73, P < .001).
Median progression-free survival was 5.1 months in the nivolumab arm compared with 2.2 months in the dacarbazine group (HR for death or disease progression: 0.43, 95% CI 0.34-0.56, P < .001). The results were published simultaneously online Nov. 16 in the New England Journal of Medicine.
Researchers randomly assigned 418 previously untreated patients with BRAF mutation-negative advanced melanoma to nivolumab plus placebo, and dacarbazine plus placebo, finding an objective response rate of 40% in patients receiving nivolumab compared with 13.9% in the dacarbazine group.
While PD-L1-positive patients treated with nivolumab showed a greater median response rate compared with PD-L1-negative or indeterminate patients (52.7% vs. 33.1%), both still achieved greater response than either subgroup treated with dacarbazine (N. Engl. J. Med. 2014 Nov. 16 [doi:10.1056/NEJMoa1412082]).
The overall incidence of treatment-related adverse events was similar in the two study arms, although there was a higher incidence of treatment-related grade 3 and 4 adverse events, such as gastrointestinal and hematologic events, in the dacarbazine group.
“It has generally been accepted that immunotherapy is associated with long-term responses in a subset of patients, whereas targeted therapies, such as BRAF inhibitors, are associated with high response rates and a rapid effect, but the responses are often short-lived,” wrote Dr. Caroline Robert of Institut Gustave Roussy, Paris, and her colleagues.
“The present study shows nivolumab is associated with a high response rate, a rapid median time to response (2.1 months, which is similar to the time to response for dacarbazine), and a durable response (the median duration of response was not reached but the duration of follow-up was short),” the investigators said.
The study was supported by Bristol Myers-Squibb. Some authors declared grants, travel expenses, and personal fees from pharmaceutical companies including Bristol Myers-Squibb, and several were employees of the company.
Nivolumab achieves significant improvements in overall survival and progression-free survival compared with conventional chemotherapy in untreated patients with advanced melanoma, regardless of PD-L1 status, according to data presented at the International Congress for the Society for Melanoma Research.
Data from a randomized, double-blind, placebo-controlled phase III trial showed patients treated with nivolumab – a PD-1 immune checkpoint inhibitor – showed there was a 58% lower incidence of death at 1 year among patients treated with nivolumab compared with those treated with dacarbazine (72.9% vs. 42.1% HR 0.42, 95% CI 0.25-0.73, P < .001).
Median progression-free survival was 5.1 months in the nivolumab arm compared with 2.2 months in the dacarbazine group (HR for death or disease progression: 0.43, 95% CI 0.34-0.56, P < .001). The results were published simultaneously online Nov. 16 in the New England Journal of Medicine.
Researchers randomly assigned 418 previously untreated patients with BRAF mutation-negative advanced melanoma to nivolumab plus placebo, and dacarbazine plus placebo, finding an objective response rate of 40% in patients receiving nivolumab compared with 13.9% in the dacarbazine group.
While PD-L1-positive patients treated with nivolumab showed a greater median response rate compared with PD-L1-negative or indeterminate patients (52.7% vs. 33.1%), both still achieved greater response than either subgroup treated with dacarbazine (N. Engl. J. Med. 2014 Nov. 16 [doi:10.1056/NEJMoa1412082]).
The overall incidence of treatment-related adverse events was similar in the two study arms, although there was a higher incidence of treatment-related grade 3 and 4 adverse events, such as gastrointestinal and hematologic events, in the dacarbazine group.
“It has generally been accepted that immunotherapy is associated with long-term responses in a subset of patients, whereas targeted therapies, such as BRAF inhibitors, are associated with high response rates and a rapid effect, but the responses are often short-lived,” wrote Dr. Caroline Robert of Institut Gustave Roussy, Paris, and her colleagues.
“The present study shows nivolumab is associated with a high response rate, a rapid median time to response (2.1 months, which is similar to the time to response for dacarbazine), and a durable response (the median duration of response was not reached but the duration of follow-up was short),” the investigators said.
The study was supported by Bristol Myers-Squibb. Some authors declared grants, travel expenses, and personal fees from pharmaceutical companies including Bristol Myers-Squibb, and several were employees of the company.
Nivolumab achieves significant improvements in overall survival and progression-free survival compared with conventional chemotherapy in untreated patients with advanced melanoma, regardless of PD-L1 status, according to data presented at the International Congress for the Society for Melanoma Research.
Data from a randomized, double-blind, placebo-controlled phase III trial showed patients treated with nivolumab – a PD-1 immune checkpoint inhibitor – showed there was a 58% lower incidence of death at 1 year among patients treated with nivolumab compared with those treated with dacarbazine (72.9% vs. 42.1% HR 0.42, 95% CI 0.25-0.73, P < .001).
Median progression-free survival was 5.1 months in the nivolumab arm compared with 2.2 months in the dacarbazine group (HR for death or disease progression: 0.43, 95% CI 0.34-0.56, P < .001). The results were published simultaneously online Nov. 16 in the New England Journal of Medicine.
Researchers randomly assigned 418 previously untreated patients with BRAF mutation-negative advanced melanoma to nivolumab plus placebo, and dacarbazine plus placebo, finding an objective response rate of 40% in patients receiving nivolumab compared with 13.9% in the dacarbazine group.
While PD-L1-positive patients treated with nivolumab showed a greater median response rate compared with PD-L1-negative or indeterminate patients (52.7% vs. 33.1%), both still achieved greater response than either subgroup treated with dacarbazine (N. Engl. J. Med. 2014 Nov. 16 [doi:10.1056/NEJMoa1412082]).
The overall incidence of treatment-related adverse events was similar in the two study arms, although there was a higher incidence of treatment-related grade 3 and 4 adverse events, such as gastrointestinal and hematologic events, in the dacarbazine group.
“It has generally been accepted that immunotherapy is associated with long-term responses in a subset of patients, whereas targeted therapies, such as BRAF inhibitors, are associated with high response rates and a rapid effect, but the responses are often short-lived,” wrote Dr. Caroline Robert of Institut Gustave Roussy, Paris, and her colleagues.
“The present study shows nivolumab is associated with a high response rate, a rapid median time to response (2.1 months, which is similar to the time to response for dacarbazine), and a durable response (the median duration of response was not reached but the duration of follow-up was short),” the investigators said.
The study was supported by Bristol Myers-Squibb. Some authors declared grants, travel expenses, and personal fees from pharmaceutical companies including Bristol Myers-Squibb, and several were employees of the company.
FROM THE SMR INTERNATIONAL CONGRESS
Key clinical point: Nivolumab achieves significant improvements in overall survival and progression-free survival compared with dacarbazine in untreated patients with advanced melanoma.
Major finding: Nivolumab was associated with a 58% lower incidence of death at 1 year compared with dacarbazine
Data source: Randomized, double-blind, placebo-controlled phase III trial of 418 previously-untreated patients with advanced melanoma.
Disclosures: Several of the researchers disclosed ties with Bristol-Myers Squibb, which funded the study.
Skin cancer treatment costs skyrocket over past decade
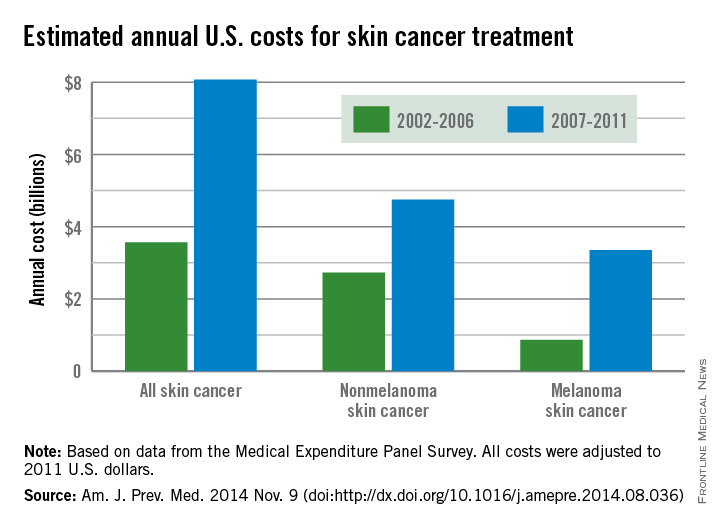
The average annual cost for skin cancer treatment more than doubled from 2002 to 2011, a report from the Centers for Disease Control and Prevention found.
From 2002 to 2006, the average annual skin cancer treatment cost was $3.6 billion, while for 2007-2011, the average annual cost was $8.1 billion, an increase of about 126%. The cost of nonmelanoma skin cancers increased 74%, from $2.7 billion to $4.8 billion, but the average annual cost for melanoma cancers increased about 280%, from $864 million to $3.3 billion, according to the CDC (Am. J. Prev. Med. 2014 Nov. 9 [doi:10.1016/j.amepre.2014.08.036]).
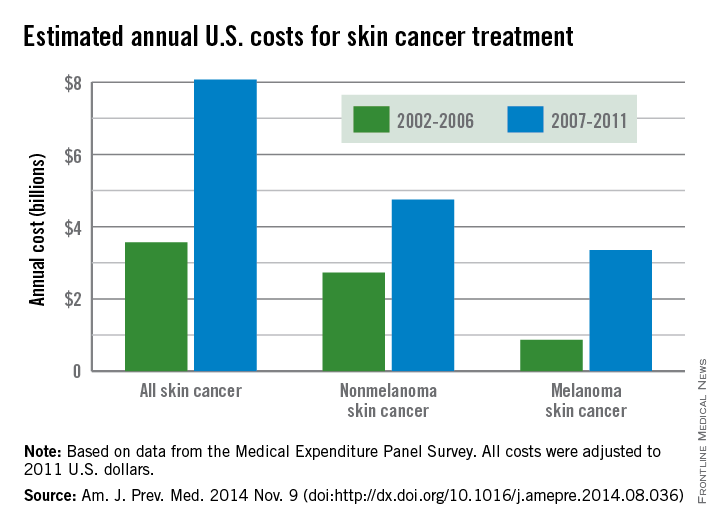
From 2002 to 2006, the average annual number of adults treated for skin cancer was 3.4 million, which increased to an average annual number of 4.9 million for 2007-2011. The average annual cost per person for all skin cancers increased by 57%, from $1,044 for 2002-2006 to $1,643 for 2007-2011, while the average cost for melanomas more than doubled from $2,320 to $4,780. The increase in annual cost for nonmelanoma skin cancers was more modest; only a 25% increase, from $882 to $1,105, was noted between the two time periods, the CDC reported.
The average annual cost for all cancer treatment rose from $67.3 billion for 2002-2006 to $87.8 billion for 2007-2011, an increase of $20.5 billion. While skin cancer treatment costs represented only 5% of all treatment costs in 2002-2006, the increase in skin cancer costs was 22% of the total increase, so from 2007 to 2011, skin cancer represented 9% of all treatment costs, according to data from the Medical Expenditure Panel Survey.
The average annual cost for skin cancer treatment more than doubled from 2002 to 2011, a report from the Centers for Disease Control and Prevention found.
From 2002 to 2006, the average annual skin cancer treatment cost was $3.6 billion, while for 2007-2011, the average annual cost was $8.1 billion, an increase of about 126%. The cost of nonmelanoma skin cancers increased 74%, from $2.7 billion to $4.8 billion, but the average annual cost for melanoma cancers increased about 280%, from $864 million to $3.3 billion, according to the CDC (Am. J. Prev. Med. 2014 Nov. 9 [doi:10.1016/j.amepre.2014.08.036]).

From 2002 to 2006, the average annual number of adults treated for skin cancer was 3.4 million, which increased to an average annual number of 4.9 million for 2007-2011. The average annual cost per person for all skin cancers increased by 57%, from $1,044 for 2002-2006 to $1,643 for 2007-2011, while the average cost for melanomas more than doubled from $2,320 to $4,780. The increase in annual cost for nonmelanoma skin cancers was more modest; only a 25% increase, from $882 to $1,105, was noted between the two time periods, the CDC reported.
The average annual cost for all cancer treatment rose from $67.3 billion for 2002-2006 to $87.8 billion for 2007-2011, an increase of $20.5 billion. While skin cancer treatment costs represented only 5% of all treatment costs in 2002-2006, the increase in skin cancer costs was 22% of the total increase, so from 2007 to 2011, skin cancer represented 9% of all treatment costs, according to data from the Medical Expenditure Panel Survey.
The average annual cost for skin cancer treatment more than doubled from 2002 to 2011, a report from the Centers for Disease Control and Prevention found.
From 2002 to 2006, the average annual skin cancer treatment cost was $3.6 billion, while for 2007-2011, the average annual cost was $8.1 billion, an increase of about 126%. The cost of nonmelanoma skin cancers increased 74%, from $2.7 billion to $4.8 billion, but the average annual cost for melanoma cancers increased about 280%, from $864 million to $3.3 billion, according to the CDC (Am. J. Prev. Med. 2014 Nov. 9 [doi:10.1016/j.amepre.2014.08.036]).

From 2002 to 2006, the average annual number of adults treated for skin cancer was 3.4 million, which increased to an average annual number of 4.9 million for 2007-2011. The average annual cost per person for all skin cancers increased by 57%, from $1,044 for 2002-2006 to $1,643 for 2007-2011, while the average cost for melanomas more than doubled from $2,320 to $4,780. The increase in annual cost for nonmelanoma skin cancers was more modest; only a 25% increase, from $882 to $1,105, was noted between the two time periods, the CDC reported.
The average annual cost for all cancer treatment rose from $67.3 billion for 2002-2006 to $87.8 billion for 2007-2011, an increase of $20.5 billion. While skin cancer treatment costs represented only 5% of all treatment costs in 2002-2006, the increase in skin cancer costs was 22% of the total increase, so from 2007 to 2011, skin cancer represented 9% of all treatment costs, according to data from the Medical Expenditure Panel Survey.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Health-Related Quality of Life in Skin Cancer Patients
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
Risk Factors for Malignant Melanoma and Preventive Methods
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
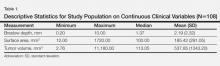
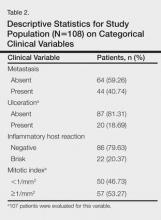
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Practice Points
- Our study revealed the following common risk factors associated with higher melanoma incidence: light eye color (ie, blue, green, gray), Fitzpatrick skin types I and II, frequent sunburns during childhood and adolescence, and higher level of education.
- Prevention campaigns should be implemented to improve awareness of melanoma to reduce exposure to UV radiation among high-risk patient populations.
Tumor Volume: An Adjunct Prognostic Factor in Cutaneous Melanoma
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
Practice Points
- Measurement of melanoma tumor volume using clinical area (length • width of the lesion before diagnostic biopsy) multiplied by Breslow depth may provide additional prognostic information.
- Further study is needed to validate the use of tumor volume as an adjunct to established histopathologic prognostic factors in cutaneous melanoma.
Sargramostim addition improves survival in metastatic melanoma
The addition of sargramostim to ipilimumab therapy for unresectable stage III or IV melanoma may improve overall survival and toxicity, but it does not appear to affect progression-free survival, results from a phase II randomized trial suggest.
Patients randomized to receive ipilimumab plus sargramostim showed significantly lower mortality at 1 year, compared with those treated with ipilimumab alone (hazard ratio, 0.64), and they experienced significantly fewer grade 3 or above treatment-related toxicities, particularly gastrointestinal toxicities such as colonic perforation.
“The improved toxicity profile must be considered as contributing to the improved survival even in light of the survival advantage remaining when patients who discontinued therapy due to toxicity are excluded,” wrote Dr. F. Stephen Hodi of the Dana-Farber Cancer Institute, Boston, and colleagues.
Researchers stressed that the study, which enrolled a total of 245 patients, is ongoing, and that the findings need to be confirmed in larger studies with longer follow-up, according to a paper published online Nov. 4 (JAMA 2014 [doi:10.1001/jama.2014.13943].
The study was supported by Public Health Service grants, the Eastern Cooperative Oncology Group, the U.S. National Cancer Institute, the U.S. National Institutes of Health, the U.S. Department of Health & Human Services, Bristol-Myers Squibb, and Genzyme. Some authors reported grants, personal fees, and support from pharmaceutical companies, including Bristol-Myers Squibb and Genzyme.
The addition of sargramostim to ipilimumab therapy for unresectable stage III or IV melanoma may improve overall survival and toxicity, but it does not appear to affect progression-free survival, results from a phase II randomized trial suggest.
Patients randomized to receive ipilimumab plus sargramostim showed significantly lower mortality at 1 year, compared with those treated with ipilimumab alone (hazard ratio, 0.64), and they experienced significantly fewer grade 3 or above treatment-related toxicities, particularly gastrointestinal toxicities such as colonic perforation.
“The improved toxicity profile must be considered as contributing to the improved survival even in light of the survival advantage remaining when patients who discontinued therapy due to toxicity are excluded,” wrote Dr. F. Stephen Hodi of the Dana-Farber Cancer Institute, Boston, and colleagues.
Researchers stressed that the study, which enrolled a total of 245 patients, is ongoing, and that the findings need to be confirmed in larger studies with longer follow-up, according to a paper published online Nov. 4 (JAMA 2014 [doi:10.1001/jama.2014.13943].
The study was supported by Public Health Service grants, the Eastern Cooperative Oncology Group, the U.S. National Cancer Institute, the U.S. National Institutes of Health, the U.S. Department of Health & Human Services, Bristol-Myers Squibb, and Genzyme. Some authors reported grants, personal fees, and support from pharmaceutical companies, including Bristol-Myers Squibb and Genzyme.
The addition of sargramostim to ipilimumab therapy for unresectable stage III or IV melanoma may improve overall survival and toxicity, but it does not appear to affect progression-free survival, results from a phase II randomized trial suggest.
Patients randomized to receive ipilimumab plus sargramostim showed significantly lower mortality at 1 year, compared with those treated with ipilimumab alone (hazard ratio, 0.64), and they experienced significantly fewer grade 3 or above treatment-related toxicities, particularly gastrointestinal toxicities such as colonic perforation.
“The improved toxicity profile must be considered as contributing to the improved survival even in light of the survival advantage remaining when patients who discontinued therapy due to toxicity are excluded,” wrote Dr. F. Stephen Hodi of the Dana-Farber Cancer Institute, Boston, and colleagues.
Researchers stressed that the study, which enrolled a total of 245 patients, is ongoing, and that the findings need to be confirmed in larger studies with longer follow-up, according to a paper published online Nov. 4 (JAMA 2014 [doi:10.1001/jama.2014.13943].
The study was supported by Public Health Service grants, the Eastern Cooperative Oncology Group, the U.S. National Cancer Institute, the U.S. National Institutes of Health, the U.S. Department of Health & Human Services, Bristol-Myers Squibb, and Genzyme. Some authors reported grants, personal fees, and support from pharmaceutical companies, including Bristol-Myers Squibb and Genzyme.
FROM JAMA
Key clinical point: Sargramostim plus ipilimumab improves overall survival and toxicity profile, compared with ipilimumab alone.
Major finding: Patients treated with sargramostim plus ipilimumab showed a 36% reduction in mortality at one year, compared with those on ipilimumab alone.
Data source: Randomized controlled trial in 245 patients with unresectable stage III or IV melanoma.
Disclosures: The study was supported by Public Health Service grants, the Eastern Cooperative Oncology Group, the U.S. National Cancer Institute, the U.S. National Institutes of Health, the U.S. Department of Health and Human Services, Bristol-Myers Squibb, and Genzyme. Some authors reported grants, personal fees, and support from pharmaceutical companies, including Bristol-Myers Squibb and Genzyme.
Nine weeks of biochemotherapy effective for high-risk melanoma
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
Key clinical point: A 9-week course of biochemotherapy markedly improved relapse-free survival in high-risk melanoma, compared with standard-of-care 1-year treatment.
Major finding: The median relapse-free survival was 4.0 years for multiagent biochemotherapy, compared with only 1.9 years for high-dose interferon alone.
Data source: A phase III randomized clinical trial involving 402 patients aged 10-74 years who had high-risk melanoma and were followed for a median of 7.2 years.
Disclosures: This study was supported in part by numerous Public Health Service grants from the National Cancer Institute and by Novartis. Dr. Flaherty reported ties to Novartis and Merck, and his associates reported ties to numerous industry sources.
COMBI-v affirms combination MEK, BRAF blockade in melanoma
MADRID – More complete blockade of the MAP kinase pathway with both a BRAF and MEK inhibitor decreased the risk of death by one-third among patients with advanced BRAF mutation–positive melanoma in the phase III COMBI-v study.
After a median follow-up of 10-11 months, the primary endpoint of overall survival was 17.2 months with the BRAF inhibitor vemurafenib (Zelboraf) alone, but had not been reached with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) (hazard ratio, 0.69; P = .005).
“This combination has met all the endpoints that were looked for, and I’d like to remind you that we compared this combination to an already very active drug,” study author Dr. Caroline Robert, head of dermatology at the Institut Gustave Roussy in Paris, said during a presidential session at the European Society for Medical Oncology Congress.
Patients in the combination arm had significantly better median progression-free survival than those given vemurafenib monotherapy (11.4 vs. 7.3 months; HR, 0.56; P < .001), as well as higher response rates (64% vs. 51%; P < .001), more complete responses (13% vs. 8%), and a longer duration of response (13.8 vs. 7.5 months).
COMBI-v was stopped early for efficacy at the interim analysis, which de facto became the final overall survival analysis presented by Dr. Robert.
The study evenly randomized 704 patients with stage IIIC or IV BRAF V600E or V600K mutation–positive melanoma to first-line therapy with twice-daily dabrafenib 150 mg plus daily trametinib 2 mg or twice-daily vemurafenib 960 mg.
The study sponsor, GlaxoSmithKline, gained accelerated approval in the United States in January 2014 for use of combination dabrafenib and trametinib in unresectable BRAF V600E or V600K mutation–positive melanoma based on positive response data.
The strategy of up-front BRAF and MEK inhibition in BRAF-mutant unresectable disease was supported by data presented in the same session from the coBRIM study, where treatment with vemurafenib and the investigational MEK inhibitor cobimetinib improved progression-free survival, response rates, and overall survival, although the overall survival data are immature. Listen to our interview with coBRIM study author Dr. Grant McArthur.
Dr. Christian Blank, from the Netherlands Cancer Institute in Amsterdam, who was invited to discuss both studies, commented that the combinations used in COMBI-v and coBRIM had similar toxicity to that of single-agent treatment, and that if mature data confirm the presented observations, combined BRAF and MEK inhibition is the new standard therapy for patients with BRAF V600 melanoma.
In COMBI-v, patients receiving dabrafenib plus trametinib, as compared with vemurafenib alone, had more pyrexia (53% vs. 21%), chills (31% vs. 8%), and decreases in ejection fraction (8% vs. 0%), although the latter was easily reversible without sequelae, Dr. Robert stressed. The addition of a MEK inhibitor, however, reduced BRAF inhibitor–related adverse skin events such as cutaneous squamous cell carcinoma and keratoacanthoma (1% vs. 18%), as well as photosensitivity (4% vs. 36%).
GlaxoSmithKline funded the study. Dr. Robert reported serving as a consultant to GlaxoSmithKline, Roche, Bristol-Myers Squibb, Amgen, Novartis, and Merck. Dr. Blank disclosed an advisory role with GSK, Roche, BMS, MSD, and Novartis, honoraria from GSK, Roche, BMS, and MSD, and a research grant from Novartis.
MADRID – More complete blockade of the MAP kinase pathway with both a BRAF and MEK inhibitor decreased the risk of death by one-third among patients with advanced BRAF mutation–positive melanoma in the phase III COMBI-v study.
After a median follow-up of 10-11 months, the primary endpoint of overall survival was 17.2 months with the BRAF inhibitor vemurafenib (Zelboraf) alone, but had not been reached with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) (hazard ratio, 0.69; P = .005).
“This combination has met all the endpoints that were looked for, and I’d like to remind you that we compared this combination to an already very active drug,” study author Dr. Caroline Robert, head of dermatology at the Institut Gustave Roussy in Paris, said during a presidential session at the European Society for Medical Oncology Congress.
Patients in the combination arm had significantly better median progression-free survival than those given vemurafenib monotherapy (11.4 vs. 7.3 months; HR, 0.56; P < .001), as well as higher response rates (64% vs. 51%; P < .001), more complete responses (13% vs. 8%), and a longer duration of response (13.8 vs. 7.5 months).
COMBI-v was stopped early for efficacy at the interim analysis, which de facto became the final overall survival analysis presented by Dr. Robert.
The study evenly randomized 704 patients with stage IIIC or IV BRAF V600E or V600K mutation–positive melanoma to first-line therapy with twice-daily dabrafenib 150 mg plus daily trametinib 2 mg or twice-daily vemurafenib 960 mg.
The study sponsor, GlaxoSmithKline, gained accelerated approval in the United States in January 2014 for use of combination dabrafenib and trametinib in unresectable BRAF V600E or V600K mutation–positive melanoma based on positive response data.
The strategy of up-front BRAF and MEK inhibition in BRAF-mutant unresectable disease was supported by data presented in the same session from the coBRIM study, where treatment with vemurafenib and the investigational MEK inhibitor cobimetinib improved progression-free survival, response rates, and overall survival, although the overall survival data are immature. Listen to our interview with coBRIM study author Dr. Grant McArthur.
Dr. Christian Blank, from the Netherlands Cancer Institute in Amsterdam, who was invited to discuss both studies, commented that the combinations used in COMBI-v and coBRIM had similar toxicity to that of single-agent treatment, and that if mature data confirm the presented observations, combined BRAF and MEK inhibition is the new standard therapy for patients with BRAF V600 melanoma.
In COMBI-v, patients receiving dabrafenib plus trametinib, as compared with vemurafenib alone, had more pyrexia (53% vs. 21%), chills (31% vs. 8%), and decreases in ejection fraction (8% vs. 0%), although the latter was easily reversible without sequelae, Dr. Robert stressed. The addition of a MEK inhibitor, however, reduced BRAF inhibitor–related adverse skin events such as cutaneous squamous cell carcinoma and keratoacanthoma (1% vs. 18%), as well as photosensitivity (4% vs. 36%).
GlaxoSmithKline funded the study. Dr. Robert reported serving as a consultant to GlaxoSmithKline, Roche, Bristol-Myers Squibb, Amgen, Novartis, and Merck. Dr. Blank disclosed an advisory role with GSK, Roche, BMS, MSD, and Novartis, honoraria from GSK, Roche, BMS, and MSD, and a research grant from Novartis.
MADRID – More complete blockade of the MAP kinase pathway with both a BRAF and MEK inhibitor decreased the risk of death by one-third among patients with advanced BRAF mutation–positive melanoma in the phase III COMBI-v study.
After a median follow-up of 10-11 months, the primary endpoint of overall survival was 17.2 months with the BRAF inhibitor vemurafenib (Zelboraf) alone, but had not been reached with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) (hazard ratio, 0.69; P = .005).
“This combination has met all the endpoints that were looked for, and I’d like to remind you that we compared this combination to an already very active drug,” study author Dr. Caroline Robert, head of dermatology at the Institut Gustave Roussy in Paris, said during a presidential session at the European Society for Medical Oncology Congress.
Patients in the combination arm had significantly better median progression-free survival than those given vemurafenib monotherapy (11.4 vs. 7.3 months; HR, 0.56; P < .001), as well as higher response rates (64% vs. 51%; P < .001), more complete responses (13% vs. 8%), and a longer duration of response (13.8 vs. 7.5 months).
COMBI-v was stopped early for efficacy at the interim analysis, which de facto became the final overall survival analysis presented by Dr. Robert.
The study evenly randomized 704 patients with stage IIIC or IV BRAF V600E or V600K mutation–positive melanoma to first-line therapy with twice-daily dabrafenib 150 mg plus daily trametinib 2 mg or twice-daily vemurafenib 960 mg.
The study sponsor, GlaxoSmithKline, gained accelerated approval in the United States in January 2014 for use of combination dabrafenib and trametinib in unresectable BRAF V600E or V600K mutation–positive melanoma based on positive response data.
The strategy of up-front BRAF and MEK inhibition in BRAF-mutant unresectable disease was supported by data presented in the same session from the coBRIM study, where treatment with vemurafenib and the investigational MEK inhibitor cobimetinib improved progression-free survival, response rates, and overall survival, although the overall survival data are immature. Listen to our interview with coBRIM study author Dr. Grant McArthur.
Dr. Christian Blank, from the Netherlands Cancer Institute in Amsterdam, who was invited to discuss both studies, commented that the combinations used in COMBI-v and coBRIM had similar toxicity to that of single-agent treatment, and that if mature data confirm the presented observations, combined BRAF and MEK inhibition is the new standard therapy for patients with BRAF V600 melanoma.
In COMBI-v, patients receiving dabrafenib plus trametinib, as compared with vemurafenib alone, had more pyrexia (53% vs. 21%), chills (31% vs. 8%), and decreases in ejection fraction (8% vs. 0%), although the latter was easily reversible without sequelae, Dr. Robert stressed. The addition of a MEK inhibitor, however, reduced BRAF inhibitor–related adverse skin events such as cutaneous squamous cell carcinoma and keratoacanthoma (1% vs. 18%), as well as photosensitivity (4% vs. 36%).
GlaxoSmithKline funded the study. Dr. Robert reported serving as a consultant to GlaxoSmithKline, Roche, Bristol-Myers Squibb, Amgen, Novartis, and Merck. Dr. Blank disclosed an advisory role with GSK, Roche, BMS, MSD, and Novartis, honoraria from GSK, Roche, BMS, and MSD, and a research grant from Novartis.
AT ESMO 2014
Key clinical point: A BRAF and MEK inhibitor combination has greater efficacy than a BRAF inhibitor alone in BRAF mutation–positive melanoma.
Major finding: Overall survival was 17.2 months with vemurafenib alone, but had not been reached with combination dabrafenib and trametinib (hazard ratio, 0.69; P = .005).
Data source: Open-label, phase III study in 704 patients with advanced melanoma.
Disclosures: GlaxoSmithKline funded the study. Dr. Robert reported serving as a consultant to GlaxoSmithKline, Roche, Bristol-Myers Squibb, Amgen, Novartis, and Merck. Dr. Blank disclosed an advisory role with GSK, Roche, BMS, MSD and Novartis, honoraria from GSK, Roche, BMS, and MSD, and a research grant from Novartis.
Investigational nivolumab beats chemo in pretreated advanced melanoma
MADRID – The investigational anti-PD-1 drug nivolumab bested investigator’s choice of chemotherapy in pretreated advanced melanoma in the phase III CheckMate-037 trial.
The coprimary end point of objective response rate by central review was 32% with nivolumab vs. 11% with investigator’s choice chemotherapy among patients with unresectable metastatic melanoma that progressed despite prior ipilimumab or a BRAF inhibitor, if BRAF mutation-positive.
The median duration of response for chemotherapy was 3.6 months, but has not been reached with nivolumab. Five patients on chemotherapy continue to respond, whereas 95% of nivolumab responders (36/38) continue in remission with a minimum follow-up of 24 weeks, Dr. Jeffrey Weber reported during a presidential symposium at the European Society for Medical Oncology Congress.
Grade 3-4 treatment-related adverse events also were significantly lower with nivolumab than with chemotherapy (9% vs. 31%), as were toxicity-related treatment discontinuations (2% vs. 8%).
“Overall nivolumab was superior to chemotherapy in terms of toxicity and response rate in patients that fail prior ipilimumab and in my view should replace chemotherapy in practice for second-line or even third-line melanoma,” said Dr. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center of Excellence, Moffitt Cancer Center, Tampa, Fla.
Survival data are pending, but the impressive data on duration of response suggest there will be significant prolongation of progression-free and overall survival with the programmed death (PD)-1 blocking antibody when the analysis of those data is mature, Dr. Weber said in a statement.
Nivolumab is under priority review with the Food and Drug Administration and accelerated assessment with the European Medicines Agency based on these data. The drug is already approved for unresectable melanoma in Japan, where it is sold under the trade name Opdivo.
Dr. Weber told reporters in a press briefing that he rarely uses chemotherapy in his practice for patients with melanoma. This sentiment was echoed by invited discussant Ignacio Melero of the University of Navarra, Pamplona, Spain. “The trends that one can foresee are that we will be moving PD-1 blockade up front in treatment and will probably get rid of chemotherapy or save it as a last-ditch effort,” said Dr. Melero.
He added that the “best is yet to come,” and that this is particularly true about immunotherapy combinations. Dr. Melero highlighted recent phase I data for pembrolizumab (Keytruda), which just gained approval in September as the first PD-1 checkpoint inhibitor in the United States in advanced melanoma. The overall response rate with pembrolizumab was in the same range as nivolumab at 26% and overall survival was “impressive,” with the median not yet reached after 14 months in patients who also had ipilimumab-refractory advanced melanoma (Lancet 2014;384:1109-17).
CheckMate-037 randomized 405 patients in a 2:1 fashion to intravenous nivolumab 3 mg/kg or investigator’s choice of chemotherapy regimens: dacarbazine 1,000 mg/m2 or carboplatin AUC 6 plus paclitaxel 175 mg/m2 . Response data were based on 120 patients in the nivolumab and 47 in the chemotherapy arm, and safety data were based on the entire population. The best overall response in the nivolumab arm was complete response in 3%, partial response in 28%, and stable disease in 23%, compared with 0%, 11%, and 34%, respectively, in the chemotherapy arm.
Subgroup analysis revealed consistently higher clinical activity with nivolumab regardless of pretreatment PD-ligand 1 expression status, BRAF mutation status, or prior ipilimumab benefit, Dr. Weber said.
Ten patients (8%) given nivolumab had an immune-related response pattern involving 30% or more reduction in target lesion tumor burden.
Dr. Weber reported serving on the advisory board for Genentech, Merck, and the study sponsor, Bristol-Myers Squibb. His institution also receives research funding from BMS and Genentech.
MADRID – The investigational anti-PD-1 drug nivolumab bested investigator’s choice of chemotherapy in pretreated advanced melanoma in the phase III CheckMate-037 trial.
The coprimary end point of objective response rate by central review was 32% with nivolumab vs. 11% with investigator’s choice chemotherapy among patients with unresectable metastatic melanoma that progressed despite prior ipilimumab or a BRAF inhibitor, if BRAF mutation-positive.
The median duration of response for chemotherapy was 3.6 months, but has not been reached with nivolumab. Five patients on chemotherapy continue to respond, whereas 95% of nivolumab responders (36/38) continue in remission with a minimum follow-up of 24 weeks, Dr. Jeffrey Weber reported during a presidential symposium at the European Society for Medical Oncology Congress.
Grade 3-4 treatment-related adverse events also were significantly lower with nivolumab than with chemotherapy (9% vs. 31%), as were toxicity-related treatment discontinuations (2% vs. 8%).
“Overall nivolumab was superior to chemotherapy in terms of toxicity and response rate in patients that fail prior ipilimumab and in my view should replace chemotherapy in practice for second-line or even third-line melanoma,” said Dr. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center of Excellence, Moffitt Cancer Center, Tampa, Fla.
Survival data are pending, but the impressive data on duration of response suggest there will be significant prolongation of progression-free and overall survival with the programmed death (PD)-1 blocking antibody when the analysis of those data is mature, Dr. Weber said in a statement.
Nivolumab is under priority review with the Food and Drug Administration and accelerated assessment with the European Medicines Agency based on these data. The drug is already approved for unresectable melanoma in Japan, where it is sold under the trade name Opdivo.
Dr. Weber told reporters in a press briefing that he rarely uses chemotherapy in his practice for patients with melanoma. This sentiment was echoed by invited discussant Ignacio Melero of the University of Navarra, Pamplona, Spain. “The trends that one can foresee are that we will be moving PD-1 blockade up front in treatment and will probably get rid of chemotherapy or save it as a last-ditch effort,” said Dr. Melero.
He added that the “best is yet to come,” and that this is particularly true about immunotherapy combinations. Dr. Melero highlighted recent phase I data for pembrolizumab (Keytruda), which just gained approval in September as the first PD-1 checkpoint inhibitor in the United States in advanced melanoma. The overall response rate with pembrolizumab was in the same range as nivolumab at 26% and overall survival was “impressive,” with the median not yet reached after 14 months in patients who also had ipilimumab-refractory advanced melanoma (Lancet 2014;384:1109-17).
CheckMate-037 randomized 405 patients in a 2:1 fashion to intravenous nivolumab 3 mg/kg or investigator’s choice of chemotherapy regimens: dacarbazine 1,000 mg/m2 or carboplatin AUC 6 plus paclitaxel 175 mg/m2 . Response data were based on 120 patients in the nivolumab and 47 in the chemotherapy arm, and safety data were based on the entire population. The best overall response in the nivolumab arm was complete response in 3%, partial response in 28%, and stable disease in 23%, compared with 0%, 11%, and 34%, respectively, in the chemotherapy arm.
Subgroup analysis revealed consistently higher clinical activity with nivolumab regardless of pretreatment PD-ligand 1 expression status, BRAF mutation status, or prior ipilimumab benefit, Dr. Weber said.
Ten patients (8%) given nivolumab had an immune-related response pattern involving 30% or more reduction in target lesion tumor burden.
Dr. Weber reported serving on the advisory board for Genentech, Merck, and the study sponsor, Bristol-Myers Squibb. His institution also receives research funding from BMS and Genentech.
MADRID – The investigational anti-PD-1 drug nivolumab bested investigator’s choice of chemotherapy in pretreated advanced melanoma in the phase III CheckMate-037 trial.
The coprimary end point of objective response rate by central review was 32% with nivolumab vs. 11% with investigator’s choice chemotherapy among patients with unresectable metastatic melanoma that progressed despite prior ipilimumab or a BRAF inhibitor, if BRAF mutation-positive.
The median duration of response for chemotherapy was 3.6 months, but has not been reached with nivolumab. Five patients on chemotherapy continue to respond, whereas 95% of nivolumab responders (36/38) continue in remission with a minimum follow-up of 24 weeks, Dr. Jeffrey Weber reported during a presidential symposium at the European Society for Medical Oncology Congress.
Grade 3-4 treatment-related adverse events also were significantly lower with nivolumab than with chemotherapy (9% vs. 31%), as were toxicity-related treatment discontinuations (2% vs. 8%).
“Overall nivolumab was superior to chemotherapy in terms of toxicity and response rate in patients that fail prior ipilimumab and in my view should replace chemotherapy in practice for second-line or even third-line melanoma,” said Dr. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center of Excellence, Moffitt Cancer Center, Tampa, Fla.
Survival data are pending, but the impressive data on duration of response suggest there will be significant prolongation of progression-free and overall survival with the programmed death (PD)-1 blocking antibody when the analysis of those data is mature, Dr. Weber said in a statement.
Nivolumab is under priority review with the Food and Drug Administration and accelerated assessment with the European Medicines Agency based on these data. The drug is already approved for unresectable melanoma in Japan, where it is sold under the trade name Opdivo.
Dr. Weber told reporters in a press briefing that he rarely uses chemotherapy in his practice for patients with melanoma. This sentiment was echoed by invited discussant Ignacio Melero of the University of Navarra, Pamplona, Spain. “The trends that one can foresee are that we will be moving PD-1 blockade up front in treatment and will probably get rid of chemotherapy or save it as a last-ditch effort,” said Dr. Melero.
He added that the “best is yet to come,” and that this is particularly true about immunotherapy combinations. Dr. Melero highlighted recent phase I data for pembrolizumab (Keytruda), which just gained approval in September as the first PD-1 checkpoint inhibitor in the United States in advanced melanoma. The overall response rate with pembrolizumab was in the same range as nivolumab at 26% and overall survival was “impressive,” with the median not yet reached after 14 months in patients who also had ipilimumab-refractory advanced melanoma (Lancet 2014;384:1109-17).
CheckMate-037 randomized 405 patients in a 2:1 fashion to intravenous nivolumab 3 mg/kg or investigator’s choice of chemotherapy regimens: dacarbazine 1,000 mg/m2 or carboplatin AUC 6 plus paclitaxel 175 mg/m2 . Response data were based on 120 patients in the nivolumab and 47 in the chemotherapy arm, and safety data were based on the entire population. The best overall response in the nivolumab arm was complete response in 3%, partial response in 28%, and stable disease in 23%, compared with 0%, 11%, and 34%, respectively, in the chemotherapy arm.
Subgroup analysis revealed consistently higher clinical activity with nivolumab regardless of pretreatment PD-ligand 1 expression status, BRAF mutation status, or prior ipilimumab benefit, Dr. Weber said.
Ten patients (8%) given nivolumab had an immune-related response pattern involving 30% or more reduction in target lesion tumor burden.
Dr. Weber reported serving on the advisory board for Genentech, Merck, and the study sponsor, Bristol-Myers Squibb. His institution also receives research funding from BMS and Genentech.
AT ESMO 2014
Key clinical point: The trend in care for treating advanced melanoma will be moving PD-1 blockade up front in treatment with less reliance on chemotherapy.
Major finding: The median duration of response for chemotherapy was 3.6 months, but has not been reached with nivolumab. Five patients on chemotherapy continue to respond, whereas 95% of nivolumab responders (36/38) continue in remission with a minimum follow-up of 24 weeks.
Data source: Phase III study in 405 patients with previously treated advanced melanoma.
Disclosures: Dr. Weber reported serving on the advisory board for Genentech, Merck, and the study sponsor, Bristol-Myers Squibb. His institution also receives research funding from BMS and Genentech.
VIDEO: A new system for staging squamous cell carcinoma
ORLANDO – Squamous cell carcinoma has a direct cost of nearly $300 million a year. Although squamous cell carcinoma (SCC) is not as deadly as other forms of skin cancer, it is a serious public health issue, according to Dr. Chrysalyne D. Schmults.
Dr. Schmults of Harvard Medical School and the Brigham and Women’s Hospital, Boston, published a study earlier this year introducing a new staging system for SCC, called the Brigham and Women’s Hospital (BWH) tumor staging system.
The BWH system, Dr. Schmults and her colleagues wrote, “offers improved distinctiveness, homogeneity, and monotonicity” over both the American Joint Committee on Cancer and International Union Against Cancer staging systems, although larger studies are needed.
In a video interview at the annual meeting of the Florida Society of Dermatologic Surgeons, Dr. Schmults explained the BWH staging system and shared her clinical advice and recommendations for managing SCC.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
ORLANDO – Squamous cell carcinoma has a direct cost of nearly $300 million a year. Although squamous cell carcinoma (SCC) is not as deadly as other forms of skin cancer, it is a serious public health issue, according to Dr. Chrysalyne D. Schmults.
Dr. Schmults of Harvard Medical School and the Brigham and Women’s Hospital, Boston, published a study earlier this year introducing a new staging system for SCC, called the Brigham and Women’s Hospital (BWH) tumor staging system.
The BWH system, Dr. Schmults and her colleagues wrote, “offers improved distinctiveness, homogeneity, and monotonicity” over both the American Joint Committee on Cancer and International Union Against Cancer staging systems, although larger studies are needed.
In a video interview at the annual meeting of the Florida Society of Dermatologic Surgeons, Dr. Schmults explained the BWH staging system and shared her clinical advice and recommendations for managing SCC.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
ORLANDO – Squamous cell carcinoma has a direct cost of nearly $300 million a year. Although squamous cell carcinoma (SCC) is not as deadly as other forms of skin cancer, it is a serious public health issue, according to Dr. Chrysalyne D. Schmults.
Dr. Schmults of Harvard Medical School and the Brigham and Women’s Hospital, Boston, published a study earlier this year introducing a new staging system for SCC, called the Brigham and Women’s Hospital (BWH) tumor staging system.
The BWH system, Dr. Schmults and her colleagues wrote, “offers improved distinctiveness, homogeneity, and monotonicity” over both the American Joint Committee on Cancer and International Union Against Cancer staging systems, although larger studies are needed.
In a video interview at the annual meeting of the Florida Society of Dermatologic Surgeons, Dr. Schmults explained the BWH staging system and shared her clinical advice and recommendations for managing SCC.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
AT FSDS 2014