User login
Friable Nodule on the Back
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
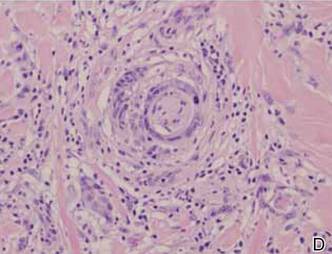
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
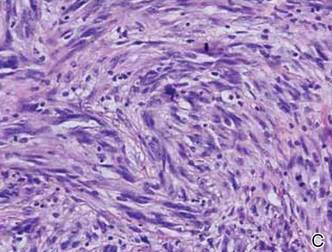
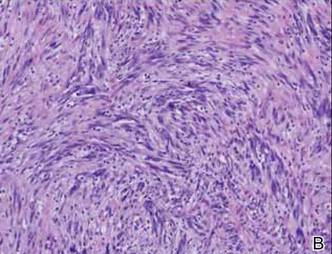
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
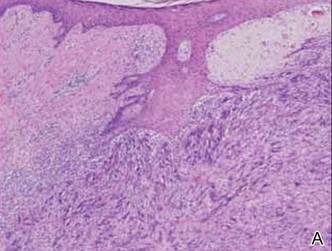
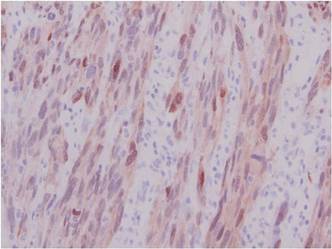
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
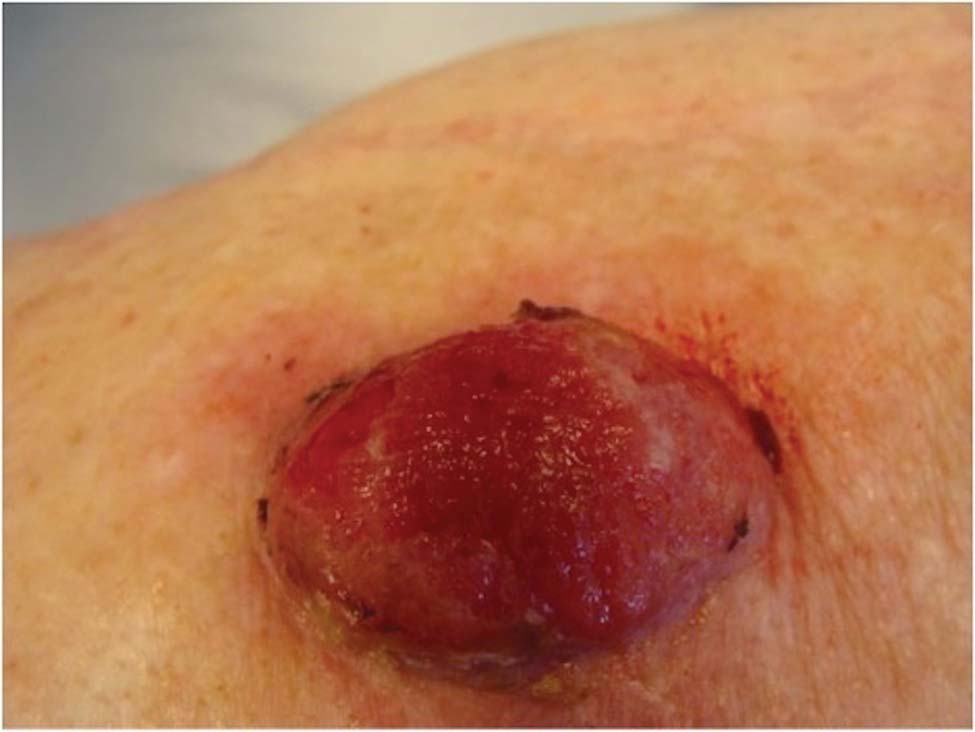
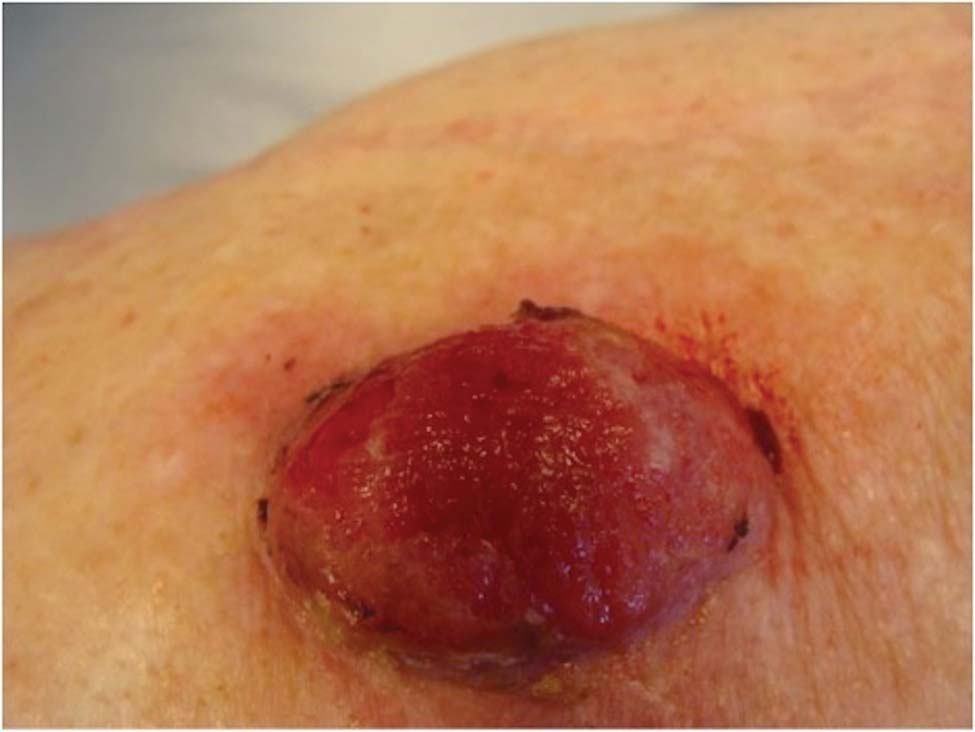
A 78-year-old woman presented with a large friable, sharply demarcated nodule of 3 months’ duration on the left side of the back. The lesion occasionally bled but was otherwise asymptomatic. There was no perilesional paresthesia. The patient’s medical history included hypertension, depression, chronic obstructive pulmonary disease, breast cancer, osteoporosis, and aortic valve disease.
C-reactive protein signals melanoma progression
Among patients with early or advanced melanoma, elevated blood levels of C-reactive protein (CRP) predicted disease recurrence and poorer survival. In a subset of patients who had sequential blood draws, CRP levels indicated melanoma disease progression, according to a study published online March 16 in the Journal of Clinical Oncology.
CRP measurements for 1,144 patients demonstrated that elevated CRP was associated with increased overall risk of death (hazard ratio, 1.44/U increase of logarithmic CRP; 95% confidence interval, 1.30-1.59; P < .001), reported Dr. Shenying Fang of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
“These data provide strong evidence that CRP is an independent prognostic biomarker in patients with melanoma, including those with early-stage disease as well as those with advanced-stage disease. A markedly elevated CRP level in particular seems to identify a subgroup of patients at high risk for disease recurrence and death,” they said (J. Clin. Onc. 2015 March 16 [doi: 10.1200/JCO.2014.58.0209]).
Investigators demonstrated a dose effect by dividing CRP levels into quintiles and showing patients in the highest quintile had significantly poorer overall survival than did those in the lowest quintile (HR, 4.14; 2.58-6.64), and significant trends across quintiles. Recursive partitioning indicated the best CRP cutoff value was 10.94 mg/L, close to the commonly used clinical cutoff of 10 mg/L. CRP levels greater than or equal to 10 mg/L predicted poorer survival and higher rates of recurrence.
For a subset of 115 patients, data from sequential blood draws showed a correlation between increasing CRP levels in an individual and disease progression. Changes in CRP levels were obtained at a median of 17.12 months apart, and increased levels were associated with poorer response to treatment (P < .001), progression of disease (P < .001), increase in cancer stage (P = .0065), and increase vs. no increase in number of metastases (P = .0013).
Dr. Fang and his associates suggest further studies on potential benefits of reducing inflammation and/or CRP on melanoma patients. Statins reduce CRP levels, but their effect on cancer prevention is unclear.
“Although there is as yet no defined role for clinical use of statins in cancer treatment, our data suggest that preclinical evaluation of statin therapy in melanoma models is reasonable to pursue,” they wrote.
Among patients with early or advanced melanoma, elevated blood levels of C-reactive protein (CRP) predicted disease recurrence and poorer survival. In a subset of patients who had sequential blood draws, CRP levels indicated melanoma disease progression, according to a study published online March 16 in the Journal of Clinical Oncology.
CRP measurements for 1,144 patients demonstrated that elevated CRP was associated with increased overall risk of death (hazard ratio, 1.44/U increase of logarithmic CRP; 95% confidence interval, 1.30-1.59; P < .001), reported Dr. Shenying Fang of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
“These data provide strong evidence that CRP is an independent prognostic biomarker in patients with melanoma, including those with early-stage disease as well as those with advanced-stage disease. A markedly elevated CRP level in particular seems to identify a subgroup of patients at high risk for disease recurrence and death,” they said (J. Clin. Onc. 2015 March 16 [doi: 10.1200/JCO.2014.58.0209]).
Investigators demonstrated a dose effect by dividing CRP levels into quintiles and showing patients in the highest quintile had significantly poorer overall survival than did those in the lowest quintile (HR, 4.14; 2.58-6.64), and significant trends across quintiles. Recursive partitioning indicated the best CRP cutoff value was 10.94 mg/L, close to the commonly used clinical cutoff of 10 mg/L. CRP levels greater than or equal to 10 mg/L predicted poorer survival and higher rates of recurrence.
For a subset of 115 patients, data from sequential blood draws showed a correlation between increasing CRP levels in an individual and disease progression. Changes in CRP levels were obtained at a median of 17.12 months apart, and increased levels were associated with poorer response to treatment (P < .001), progression of disease (P < .001), increase in cancer stage (P = .0065), and increase vs. no increase in number of metastases (P = .0013).
Dr. Fang and his associates suggest further studies on potential benefits of reducing inflammation and/or CRP on melanoma patients. Statins reduce CRP levels, but their effect on cancer prevention is unclear.
“Although there is as yet no defined role for clinical use of statins in cancer treatment, our data suggest that preclinical evaluation of statin therapy in melanoma models is reasonable to pursue,” they wrote.
Among patients with early or advanced melanoma, elevated blood levels of C-reactive protein (CRP) predicted disease recurrence and poorer survival. In a subset of patients who had sequential blood draws, CRP levels indicated melanoma disease progression, according to a study published online March 16 in the Journal of Clinical Oncology.
CRP measurements for 1,144 patients demonstrated that elevated CRP was associated with increased overall risk of death (hazard ratio, 1.44/U increase of logarithmic CRP; 95% confidence interval, 1.30-1.59; P < .001), reported Dr. Shenying Fang of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
“These data provide strong evidence that CRP is an independent prognostic biomarker in patients with melanoma, including those with early-stage disease as well as those with advanced-stage disease. A markedly elevated CRP level in particular seems to identify a subgroup of patients at high risk for disease recurrence and death,” they said (J. Clin. Onc. 2015 March 16 [doi: 10.1200/JCO.2014.58.0209]).
Investigators demonstrated a dose effect by dividing CRP levels into quintiles and showing patients in the highest quintile had significantly poorer overall survival than did those in the lowest quintile (HR, 4.14; 2.58-6.64), and significant trends across quintiles. Recursive partitioning indicated the best CRP cutoff value was 10.94 mg/L, close to the commonly used clinical cutoff of 10 mg/L. CRP levels greater than or equal to 10 mg/L predicted poorer survival and higher rates of recurrence.
For a subset of 115 patients, data from sequential blood draws showed a correlation between increasing CRP levels in an individual and disease progression. Changes in CRP levels were obtained at a median of 17.12 months apart, and increased levels were associated with poorer response to treatment (P < .001), progression of disease (P < .001), increase in cancer stage (P = .0065), and increase vs. no increase in number of metastases (P = .0013).
Dr. Fang and his associates suggest further studies on potential benefits of reducing inflammation and/or CRP on melanoma patients. Statins reduce CRP levels, but their effect on cancer prevention is unclear.
“Although there is as yet no defined role for clinical use of statins in cancer treatment, our data suggest that preclinical evaluation of statin therapy in melanoma models is reasonable to pursue,” they wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In patients with melanoma at any stage, elevated C-reactive protein (CRP) was associated with poorer survival.
Major finding: Overall risk of death was increased by a factor of 1.44/unit increase of logarithmic CRP; CRP ≥ to 10 mg/L was associated with poorer outcomes.
Data source: Plasma from 1,144 patients with all stages of invasive cutaneous melanoma were tested for CRP and outcomes assessed after a median follow-up of 6.23 years.
Disclosures: Dr. Shenying Fang reported having no financial disclosures; several coauthors reported ties to numerous industry sources.
Nail Biopsy: 6 Techniques to Biopsy the Nail Matrix
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
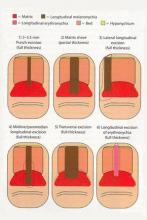
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Laser-enhanced 5-FU scores with squamous cell, basal cell patients
Ablative fractional laser–assisted delivery of topical fluorouracil resulted in 100% histologic clearance in patients with squamous cell carcinoma in situ and 71% in patients with superficial basal cell carcinoma, based on data from 28 patients (mean age 71 years). Each patient underwent one pass with an ablative fractional laser, followed by one application of topical 5-FU 5% under occlusion for 7 days.
Histologic clearance and patient satisfaction were assessed 4-8 weeks after treatment; no serious adverse events were reported, and all patients said they would recommend the treatment to others.
“This treatment modality may be particularly useful for older patients, tumors located on lower extremities or back, and multiple tumors scattered on different areas of the body,” although controlled studies in diverse populations with longer follow-up times are needed, wrote Dr. Bichchau T. Nguyen of Tufts University, Boston, and colleagues (JAAD 2015; 72:558-60).
Read the full article from the Journal of the American Academy of Dermatology here.
Ablative fractional laser–assisted delivery of topical fluorouracil resulted in 100% histologic clearance in patients with squamous cell carcinoma in situ and 71% in patients with superficial basal cell carcinoma, based on data from 28 patients (mean age 71 years). Each patient underwent one pass with an ablative fractional laser, followed by one application of topical 5-FU 5% under occlusion for 7 days.
Histologic clearance and patient satisfaction were assessed 4-8 weeks after treatment; no serious adverse events were reported, and all patients said they would recommend the treatment to others.
“This treatment modality may be particularly useful for older patients, tumors located on lower extremities or back, and multiple tumors scattered on different areas of the body,” although controlled studies in diverse populations with longer follow-up times are needed, wrote Dr. Bichchau T. Nguyen of Tufts University, Boston, and colleagues (JAAD 2015; 72:558-60).
Read the full article from the Journal of the American Academy of Dermatology here.
Ablative fractional laser–assisted delivery of topical fluorouracil resulted in 100% histologic clearance in patients with squamous cell carcinoma in situ and 71% in patients with superficial basal cell carcinoma, based on data from 28 patients (mean age 71 years). Each patient underwent one pass with an ablative fractional laser, followed by one application of topical 5-FU 5% under occlusion for 7 days.
Histologic clearance and patient satisfaction were assessed 4-8 weeks after treatment; no serious adverse events were reported, and all patients said they would recommend the treatment to others.
“This treatment modality may be particularly useful for older patients, tumors located on lower extremities or back, and multiple tumors scattered on different areas of the body,” although controlled studies in diverse populations with longer follow-up times are needed, wrote Dr. Bichchau T. Nguyen of Tufts University, Boston, and colleagues (JAAD 2015; 72:558-60).
Read the full article from the Journal of the American Academy of Dermatology here.
NRAS mutations predict immunotherapy outcomes in melanoma patients
Patients with advanced melanoma who were treated with immunotherapy responded better if they harbored mutations in the NRAS gene, according to a study published March 3 in Cancer Immunology Research.
Out of 229 cases retrospectively analyzed, 26% had mutations in NRASG12/G13/Q61, 23% had BRAFV600, and 51% were wild type for NRAS and BRAF. Patients received first-line therapy with high-dose IL-2 (25%), ipilimumab (62%), or anti-PD-1/PD-L1 (12%), investigators reported (Cancer Immunol. Res. 2015 March 3).
Complete or partial responses were found in 32% of patients with NRAS-mutant melanomas, compared with 20% of those without NRAS mutations (P = .07). Clinical benefit (defined as complete response, partial response, or stable disease for 24 weeks or longer) was observed in 50% of the NRAS mutant group vs. 30% of the non–mutant NRAS group (P < .01), reported Dr. Douglas B. Johnson of Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center, Nashville, Tenn., and associates.
Although the numbers for individual agents were small, the NRAS-mutant benefit was most pronounced for immune checkpoint inhibitors, especially anti-PD-1/PD-L1 therapy, where clinical benefit was observed in 8 of 11 NRAS-mutant patients vs. 13 of 37 patients with wild-type NRAS.
“This finding could have implications for molecular testing and treatment decision making, and it provides early insights into the complex relationship between tumor genetics and the immune response,” Dr. Johnson and associates wrote.
Patients with NRAS-mutant melanoma account for 15%-20% of all melanomas, and the mutation is associated with a poorer prognosis. The authors speculate that elevated PD-1 expression may be a factor in inferior prognosis of NRAS-mutant phenotypes as well as the observed improved response to anti-PD-1.
“We studied a small group of patients, but the results were quite suggestive. Our findings need to be confirmed in a prospective study. This study highlights the need to find predictive markers that can help us understand which patients will respond to therapy. Our study will hopefully lead to understanding the biological mechanisms that explain why NRAS mutations predict response,” Dr. Johnson and his associates said.
Patients with advanced melanoma who were treated with immunotherapy responded better if they harbored mutations in the NRAS gene, according to a study published March 3 in Cancer Immunology Research.
Out of 229 cases retrospectively analyzed, 26% had mutations in NRASG12/G13/Q61, 23% had BRAFV600, and 51% were wild type for NRAS and BRAF. Patients received first-line therapy with high-dose IL-2 (25%), ipilimumab (62%), or anti-PD-1/PD-L1 (12%), investigators reported (Cancer Immunol. Res. 2015 March 3).
Complete or partial responses were found in 32% of patients with NRAS-mutant melanomas, compared with 20% of those without NRAS mutations (P = .07). Clinical benefit (defined as complete response, partial response, or stable disease for 24 weeks or longer) was observed in 50% of the NRAS mutant group vs. 30% of the non–mutant NRAS group (P < .01), reported Dr. Douglas B. Johnson of Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center, Nashville, Tenn., and associates.
Although the numbers for individual agents were small, the NRAS-mutant benefit was most pronounced for immune checkpoint inhibitors, especially anti-PD-1/PD-L1 therapy, where clinical benefit was observed in 8 of 11 NRAS-mutant patients vs. 13 of 37 patients with wild-type NRAS.
“This finding could have implications for molecular testing and treatment decision making, and it provides early insights into the complex relationship between tumor genetics and the immune response,” Dr. Johnson and associates wrote.
Patients with NRAS-mutant melanoma account for 15%-20% of all melanomas, and the mutation is associated with a poorer prognosis. The authors speculate that elevated PD-1 expression may be a factor in inferior prognosis of NRAS-mutant phenotypes as well as the observed improved response to anti-PD-1.
“We studied a small group of patients, but the results were quite suggestive. Our findings need to be confirmed in a prospective study. This study highlights the need to find predictive markers that can help us understand which patients will respond to therapy. Our study will hopefully lead to understanding the biological mechanisms that explain why NRAS mutations predict response,” Dr. Johnson and his associates said.
Patients with advanced melanoma who were treated with immunotherapy responded better if they harbored mutations in the NRAS gene, according to a study published March 3 in Cancer Immunology Research.
Out of 229 cases retrospectively analyzed, 26% had mutations in NRASG12/G13/Q61, 23% had BRAFV600, and 51% were wild type for NRAS and BRAF. Patients received first-line therapy with high-dose IL-2 (25%), ipilimumab (62%), or anti-PD-1/PD-L1 (12%), investigators reported (Cancer Immunol. Res. 2015 March 3).
Complete or partial responses were found in 32% of patients with NRAS-mutant melanomas, compared with 20% of those without NRAS mutations (P = .07). Clinical benefit (defined as complete response, partial response, or stable disease for 24 weeks or longer) was observed in 50% of the NRAS mutant group vs. 30% of the non–mutant NRAS group (P < .01), reported Dr. Douglas B. Johnson of Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center, Nashville, Tenn., and associates.
Although the numbers for individual agents were small, the NRAS-mutant benefit was most pronounced for immune checkpoint inhibitors, especially anti-PD-1/PD-L1 therapy, where clinical benefit was observed in 8 of 11 NRAS-mutant patients vs. 13 of 37 patients with wild-type NRAS.
“This finding could have implications for molecular testing and treatment decision making, and it provides early insights into the complex relationship between tumor genetics and the immune response,” Dr. Johnson and associates wrote.
Patients with NRAS-mutant melanoma account for 15%-20% of all melanomas, and the mutation is associated with a poorer prognosis. The authors speculate that elevated PD-1 expression may be a factor in inferior prognosis of NRAS-mutant phenotypes as well as the observed improved response to anti-PD-1.
“We studied a small group of patients, but the results were quite suggestive. Our findings need to be confirmed in a prospective study. This study highlights the need to find predictive markers that can help us understand which patients will respond to therapy. Our study will hopefully lead to understanding the biological mechanisms that explain why NRAS mutations predict response,” Dr. Johnson and his associates said.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Patients with advanced melanoma and mutations in the NRAS gene had better responses to immunotherapy than did those without NRAS mutations.
Major finding: Of the patients with mutated NRAS, 28% had complete or partial responses to first-line immunotherapy, compared with 16% of patients without NRAS mutations.
Data source: The retrospective study evaluated medical records from 229 patients with advanced melanoma who were treated with ipilimumab, IL-2, or anti-PD-1/PD-L1.
Disclosures: Dr. Johnson had no disclosures. Dr. Lovly received grants from AstraZeneca and Novartis. Dr. Iafrate has ownership in ArcherDx and an advisory role with BioReference Labs.
Ipilimumab’s survival benefit extends to 5 years
Among patients with advanced melanoma, twice as many survived to 5 years if they received ipilimumab plus dacarbazine than if they received placebo plus dacarbazine as part of a phase III randomized clinical trial, according to a report published online Feb. 23 in the Journal of Clinical Oncology.
The investigators described their analysis as the first examination of long-term survival after a course of ipilimumab therapy, as well as the first analysis of safety outcomes in a small subset of patients who continued to receive ipilimumab as maintenance therapy. They performed this milestone survival analysis “to confirm prior reports of long-term survival in a proportion of patients from nonrandomized phase II studies,” and their findings do in fact confirm those reports, said Dr. Michele Maio of University Hospital of Siena (Italy) and her associates.
The industry-sponsored study (CA184-024) involved 502 adults with treatment-naive unresectable stage III or IV melanoma who were randomly assigned to receive ipilimumab plus dacarbazine (250 patients) or placebo plus dacarbazine (252 patients) for 10 weeks. Those who showed stable disease, a partial response, or a complete response at week 24 were permitted to continue on ipilimumab or placebo as maintenance therapy every 12 weeks thereafter.
At a minimum follow-up of 5 years, 40 patients (18.2%) in the ipilimumab group and 20 (8.8%) in the placebo group were still alive. Five-year survival with ipilimumab was not only double that with placebo, it also was roughly double the historical survival rate of patients with stage IV melanoma, which is approximately 10%, Dr. Maio and her associates reported (J. Clin. Oncol. 2015 Feb. 23 [doi:10.1200/JCO.2014.56.6018]).
With ipilimumab, 3 long-term survivors (7.5%) achieved a complete response, 17 (42.5%) had a partial response, and 11 (27.5%) achieved stable disease. In contrast, with placebo no long-term survivors achieved a complete response, 7 (35%) had a partial response, and 8 (40%) had stable disease.
There were no grade 5 adverse events. Grade 3-4 adverse events were confined to the skin and comprised pruritus and rash. Low-grade adverse events included rash, vitiligo, pruritus, GI problems, and an increased ALT or AST level.
The subset of study participants who used ipilimumab maintenance therapy was small (seven patients) but “provides some insight into the safety profile of ipilimumab during extended treatment.” The only high-grade adverse events were pruritus and rash, which affected only one patient.
“This analysis demonstrates that a proportion of treatment-naive patients with advanced melanoma can achieve durable, long-term survival with ipilimumab therapy,” Dr. Maio and her associates wrote.
Among patients with advanced melanoma, twice as many survived to 5 years if they received ipilimumab plus dacarbazine than if they received placebo plus dacarbazine as part of a phase III randomized clinical trial, according to a report published online Feb. 23 in the Journal of Clinical Oncology.
The investigators described their analysis as the first examination of long-term survival after a course of ipilimumab therapy, as well as the first analysis of safety outcomes in a small subset of patients who continued to receive ipilimumab as maintenance therapy. They performed this milestone survival analysis “to confirm prior reports of long-term survival in a proportion of patients from nonrandomized phase II studies,” and their findings do in fact confirm those reports, said Dr. Michele Maio of University Hospital of Siena (Italy) and her associates.
The industry-sponsored study (CA184-024) involved 502 adults with treatment-naive unresectable stage III or IV melanoma who were randomly assigned to receive ipilimumab plus dacarbazine (250 patients) or placebo plus dacarbazine (252 patients) for 10 weeks. Those who showed stable disease, a partial response, or a complete response at week 24 were permitted to continue on ipilimumab or placebo as maintenance therapy every 12 weeks thereafter.
At a minimum follow-up of 5 years, 40 patients (18.2%) in the ipilimumab group and 20 (8.8%) in the placebo group were still alive. Five-year survival with ipilimumab was not only double that with placebo, it also was roughly double the historical survival rate of patients with stage IV melanoma, which is approximately 10%, Dr. Maio and her associates reported (J. Clin. Oncol. 2015 Feb. 23 [doi:10.1200/JCO.2014.56.6018]).
With ipilimumab, 3 long-term survivors (7.5%) achieved a complete response, 17 (42.5%) had a partial response, and 11 (27.5%) achieved stable disease. In contrast, with placebo no long-term survivors achieved a complete response, 7 (35%) had a partial response, and 8 (40%) had stable disease.
There were no grade 5 adverse events. Grade 3-4 adverse events were confined to the skin and comprised pruritus and rash. Low-grade adverse events included rash, vitiligo, pruritus, GI problems, and an increased ALT or AST level.
The subset of study participants who used ipilimumab maintenance therapy was small (seven patients) but “provides some insight into the safety profile of ipilimumab during extended treatment.” The only high-grade adverse events were pruritus and rash, which affected only one patient.
“This analysis demonstrates that a proportion of treatment-naive patients with advanced melanoma can achieve durable, long-term survival with ipilimumab therapy,” Dr. Maio and her associates wrote.
Among patients with advanced melanoma, twice as many survived to 5 years if they received ipilimumab plus dacarbazine than if they received placebo plus dacarbazine as part of a phase III randomized clinical trial, according to a report published online Feb. 23 in the Journal of Clinical Oncology.
The investigators described their analysis as the first examination of long-term survival after a course of ipilimumab therapy, as well as the first analysis of safety outcomes in a small subset of patients who continued to receive ipilimumab as maintenance therapy. They performed this milestone survival analysis “to confirm prior reports of long-term survival in a proportion of patients from nonrandomized phase II studies,” and their findings do in fact confirm those reports, said Dr. Michele Maio of University Hospital of Siena (Italy) and her associates.
The industry-sponsored study (CA184-024) involved 502 adults with treatment-naive unresectable stage III or IV melanoma who were randomly assigned to receive ipilimumab plus dacarbazine (250 patients) or placebo plus dacarbazine (252 patients) for 10 weeks. Those who showed stable disease, a partial response, or a complete response at week 24 were permitted to continue on ipilimumab or placebo as maintenance therapy every 12 weeks thereafter.
At a minimum follow-up of 5 years, 40 patients (18.2%) in the ipilimumab group and 20 (8.8%) in the placebo group were still alive. Five-year survival with ipilimumab was not only double that with placebo, it also was roughly double the historical survival rate of patients with stage IV melanoma, which is approximately 10%, Dr. Maio and her associates reported (J. Clin. Oncol. 2015 Feb. 23 [doi:10.1200/JCO.2014.56.6018]).
With ipilimumab, 3 long-term survivors (7.5%) achieved a complete response, 17 (42.5%) had a partial response, and 11 (27.5%) achieved stable disease. In contrast, with placebo no long-term survivors achieved a complete response, 7 (35%) had a partial response, and 8 (40%) had stable disease.
There were no grade 5 adverse events. Grade 3-4 adverse events were confined to the skin and comprised pruritus and rash. Low-grade adverse events included rash, vitiligo, pruritus, GI problems, and an increased ALT or AST level.
The subset of study participants who used ipilimumab maintenance therapy was small (seven patients) but “provides some insight into the safety profile of ipilimumab during extended treatment.” The only high-grade adverse events were pruritus and rash, which affected only one patient.
“This analysis demonstrates that a proportion of treatment-naive patients with advanced melanoma can achieve durable, long-term survival with ipilimumab therapy,” Dr. Maio and her associates wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The short-term survival benefit conferred by ipilimumab therapy in advanced melanoma extends to at least 5 years.
Major finding: Five-year survival with ipilimumab (18.2%) was double that with placebo (8.8%) and roughly double the historical survival rate of patients with stage IV melanoma (approximately 10%).
Data source: An industry-sponsored “milestone survival” analysis to capture 5-year mortality data for 502 adults with advanced melanoma who participated in a phase III clinical trial comparing ipilimumab with placebo.
Disclosures: This study (NCT 00324155) was sponsored by Bristol-Myers Squibb. Dr. Maio reported receiving honoraria, research funding, and other compensation and serving as a consultant/adviser to Bristol-Myers Squibb, GlaxoSmithKline, and Roche. Her associates reported ties to numerous industry sources.
UVA damage to skin DNA continues for hours after exposure
DNA damage to skin continues for hours after UVA exposure ceases, and melanin is a key culprit in the process, based on the results of cell cultures and studies in mice.
“What is extraordinary about this study is that it implicates melanin as a potential source of oxidative stress, [which can] lead to DNA damage and ultimately accelerate skin aging and skin cancer,” said Dr. Adam Friedman, director of dermatologic research at Albert Einstein College of Medicine, New York, who was not involved in the research. “For years, melanin was thought of as only a UV radiation protectant and antioxidant. Could this be a missing element in our fight to prevent skin cancer? The emphasis has always been on preventing the fire, not necessarily putting it out.”
The findings suggest that antioxidant-rich lotions might one day help prevent damage if users apply the “quenchers” soon after sun exposure, said lead author Sanjay Premi, Ph.D. at Yale University in New Haven, Conn., and his associates. Vitamin E and ethyl sorbate are two possible candidates for such “evening after” sunscreens, the researchers said (Science 2015 Feb. 20;347:842-7). For their study, the researchers exposed mouse fibroblasts and melanocytes to UVA, the main type of cancer-causing radiation from sunlight and tanning beds. Then they measured the accumulation of cyclobutane pyrimidine dimers (CPDs), a hallmark of UV-induced DNA damage. Levels of CPDs peaked immediately after UVA exposure in skin cells from albino mice, but kept rising for at least another 3 hours in melanocytes from pigmented mice, the investigators reported. Furthermore, 2 hours after live mice were exposed to UVA, their CPD levels were three times greater than just after UVA exposure, they said. “If the same holds for human skin, this would mean that past measurements of CPDs immediately after UV exposure have underestimated the consequences of UV exposure,” the researchers concluded.
They found similar results for human melanocytes, except that CPD levels varied more, probably because of genetic differences among human skin donors, Dr. Premi and his associates wrote. Further testing of melanin fragments revealed the process by which DNA damage accumulates, even after UVA exposures ends: Reactive oxygen and nitrogen species together excited an electron that in turn damaged DNA in the same way that UV radiation did. “A consequence of these events is that melanin may be carcinogenic as well as protective against cancer,” the researchers emphasized. “This double nature would explain the apparent cancer-facilitating effects of melanin seen in mice and in human epidemiology.”
Although “it is difficult to translate cell assays and mouse models to the human system,” the findings “no doubt raise important, paradigm-shifting ideas in terms of our understanding of UV-induced DNA damage,” said Dr. Friedman. For example, studies have shown that skin cells incur DNA damage even when individuals wear sunscreen as directed, he noted. “Are these cellular changes a result of sunscreen inefficiency, or is it delayed damage due to persistent melanin radicals?” he asked. “We see patients all the time who say, ‘I used sunscreen but I still got burned.’ Granted, there are likely many confounding factors, such as amount of sunscreen applied, frequency, clothing choice, and water exposure. However, maybe we can add progressive and persistent oxidative stress to the list.”
Developing effective “evening-after” lotions to halt UVA damage after exposure will take work, Dr. Friedman added. “The reality is that many, if not most, antioxidants are highly unstable, whether they oxidize easily when exposed to air, or are photo-labile, meaning they degrade upon exposure to sunlight,” he said. One solution is to encapsulate antioxidants within liposomes, solid lipid nanoparticles, or polymeric nanoparticles, but “skin penetration is not the only issue,” he added. “These antioxidants need to actually get into the cells to be effective.”
For that reason, researchers have explored systemic administration of agents such as polypodium leucotomos, which has antioxidant and anti-inflammatory properties, Dr. Friedman noted. “The clinical data available looks at using this natural ingredient to increase minimal erythemal dose, rather then postirradiation recovery,” he said. “Could postirradiation intake be effective? Possibly.”
The research was funded by the U.S. Department of Defense, the National Institutes of Health, the University of Veterinary Medicine in Vienna, Fundação de Amparo à Pesquisa do Estado de São Paulo, and INCT–Processos Redox em Biomedicina. The investigators declared having no conflicts of interest.
DNA damage to skin continues for hours after UVA exposure ceases, and melanin is a key culprit in the process, based on the results of cell cultures and studies in mice.
“What is extraordinary about this study is that it implicates melanin as a potential source of oxidative stress, [which can] lead to DNA damage and ultimately accelerate skin aging and skin cancer,” said Dr. Adam Friedman, director of dermatologic research at Albert Einstein College of Medicine, New York, who was not involved in the research. “For years, melanin was thought of as only a UV radiation protectant and antioxidant. Could this be a missing element in our fight to prevent skin cancer? The emphasis has always been on preventing the fire, not necessarily putting it out.”
The findings suggest that antioxidant-rich lotions might one day help prevent damage if users apply the “quenchers” soon after sun exposure, said lead author Sanjay Premi, Ph.D. at Yale University in New Haven, Conn., and his associates. Vitamin E and ethyl sorbate are two possible candidates for such “evening after” sunscreens, the researchers said (Science 2015 Feb. 20;347:842-7). For their study, the researchers exposed mouse fibroblasts and melanocytes to UVA, the main type of cancer-causing radiation from sunlight and tanning beds. Then they measured the accumulation of cyclobutane pyrimidine dimers (CPDs), a hallmark of UV-induced DNA damage. Levels of CPDs peaked immediately after UVA exposure in skin cells from albino mice, but kept rising for at least another 3 hours in melanocytes from pigmented mice, the investigators reported. Furthermore, 2 hours after live mice were exposed to UVA, their CPD levels were three times greater than just after UVA exposure, they said. “If the same holds for human skin, this would mean that past measurements of CPDs immediately after UV exposure have underestimated the consequences of UV exposure,” the researchers concluded.
They found similar results for human melanocytes, except that CPD levels varied more, probably because of genetic differences among human skin donors, Dr. Premi and his associates wrote. Further testing of melanin fragments revealed the process by which DNA damage accumulates, even after UVA exposures ends: Reactive oxygen and nitrogen species together excited an electron that in turn damaged DNA in the same way that UV radiation did. “A consequence of these events is that melanin may be carcinogenic as well as protective against cancer,” the researchers emphasized. “This double nature would explain the apparent cancer-facilitating effects of melanin seen in mice and in human epidemiology.”
Although “it is difficult to translate cell assays and mouse models to the human system,” the findings “no doubt raise important, paradigm-shifting ideas in terms of our understanding of UV-induced DNA damage,” said Dr. Friedman. For example, studies have shown that skin cells incur DNA damage even when individuals wear sunscreen as directed, he noted. “Are these cellular changes a result of sunscreen inefficiency, or is it delayed damage due to persistent melanin radicals?” he asked. “We see patients all the time who say, ‘I used sunscreen but I still got burned.’ Granted, there are likely many confounding factors, such as amount of sunscreen applied, frequency, clothing choice, and water exposure. However, maybe we can add progressive and persistent oxidative stress to the list.”
Developing effective “evening-after” lotions to halt UVA damage after exposure will take work, Dr. Friedman added. “The reality is that many, if not most, antioxidants are highly unstable, whether they oxidize easily when exposed to air, or are photo-labile, meaning they degrade upon exposure to sunlight,” he said. One solution is to encapsulate antioxidants within liposomes, solid lipid nanoparticles, or polymeric nanoparticles, but “skin penetration is not the only issue,” he added. “These antioxidants need to actually get into the cells to be effective.”
For that reason, researchers have explored systemic administration of agents such as polypodium leucotomos, which has antioxidant and anti-inflammatory properties, Dr. Friedman noted. “The clinical data available looks at using this natural ingredient to increase minimal erythemal dose, rather then postirradiation recovery,” he said. “Could postirradiation intake be effective? Possibly.”
The research was funded by the U.S. Department of Defense, the National Institutes of Health, the University of Veterinary Medicine in Vienna, Fundação de Amparo à Pesquisa do Estado de São Paulo, and INCT–Processos Redox em Biomedicina. The investigators declared having no conflicts of interest.
DNA damage to skin continues for hours after UVA exposure ceases, and melanin is a key culprit in the process, based on the results of cell cultures and studies in mice.
“What is extraordinary about this study is that it implicates melanin as a potential source of oxidative stress, [which can] lead to DNA damage and ultimately accelerate skin aging and skin cancer,” said Dr. Adam Friedman, director of dermatologic research at Albert Einstein College of Medicine, New York, who was not involved in the research. “For years, melanin was thought of as only a UV radiation protectant and antioxidant. Could this be a missing element in our fight to prevent skin cancer? The emphasis has always been on preventing the fire, not necessarily putting it out.”
The findings suggest that antioxidant-rich lotions might one day help prevent damage if users apply the “quenchers” soon after sun exposure, said lead author Sanjay Premi, Ph.D. at Yale University in New Haven, Conn., and his associates. Vitamin E and ethyl sorbate are two possible candidates for such “evening after” sunscreens, the researchers said (Science 2015 Feb. 20;347:842-7). For their study, the researchers exposed mouse fibroblasts and melanocytes to UVA, the main type of cancer-causing radiation from sunlight and tanning beds. Then they measured the accumulation of cyclobutane pyrimidine dimers (CPDs), a hallmark of UV-induced DNA damage. Levels of CPDs peaked immediately after UVA exposure in skin cells from albino mice, but kept rising for at least another 3 hours in melanocytes from pigmented mice, the investigators reported. Furthermore, 2 hours after live mice were exposed to UVA, their CPD levels were three times greater than just after UVA exposure, they said. “If the same holds for human skin, this would mean that past measurements of CPDs immediately after UV exposure have underestimated the consequences of UV exposure,” the researchers concluded.
They found similar results for human melanocytes, except that CPD levels varied more, probably because of genetic differences among human skin donors, Dr. Premi and his associates wrote. Further testing of melanin fragments revealed the process by which DNA damage accumulates, even after UVA exposures ends: Reactive oxygen and nitrogen species together excited an electron that in turn damaged DNA in the same way that UV radiation did. “A consequence of these events is that melanin may be carcinogenic as well as protective against cancer,” the researchers emphasized. “This double nature would explain the apparent cancer-facilitating effects of melanin seen in mice and in human epidemiology.”
Although “it is difficult to translate cell assays and mouse models to the human system,” the findings “no doubt raise important, paradigm-shifting ideas in terms of our understanding of UV-induced DNA damage,” said Dr. Friedman. For example, studies have shown that skin cells incur DNA damage even when individuals wear sunscreen as directed, he noted. “Are these cellular changes a result of sunscreen inefficiency, or is it delayed damage due to persistent melanin radicals?” he asked. “We see patients all the time who say, ‘I used sunscreen but I still got burned.’ Granted, there are likely many confounding factors, such as amount of sunscreen applied, frequency, clothing choice, and water exposure. However, maybe we can add progressive and persistent oxidative stress to the list.”
Developing effective “evening-after” lotions to halt UVA damage after exposure will take work, Dr. Friedman added. “The reality is that many, if not most, antioxidants are highly unstable, whether they oxidize easily when exposed to air, or are photo-labile, meaning they degrade upon exposure to sunlight,” he said. One solution is to encapsulate antioxidants within liposomes, solid lipid nanoparticles, or polymeric nanoparticles, but “skin penetration is not the only issue,” he added. “These antioxidants need to actually get into the cells to be effective.”
For that reason, researchers have explored systemic administration of agents such as polypodium leucotomos, which has antioxidant and anti-inflammatory properties, Dr. Friedman noted. “The clinical data available looks at using this natural ingredient to increase minimal erythemal dose, rather then postirradiation recovery,” he said. “Could postirradiation intake be effective? Possibly.”
The research was funded by the U.S. Department of Defense, the National Institutes of Health, the University of Veterinary Medicine in Vienna, Fundação de Amparo à Pesquisa do Estado de São Paulo, and INCT–Processos Redox em Biomedicina. The investigators declared having no conflicts of interest.
FROM SCIENCE
Key clinical point: Melanin played a key role in DNA damage to skin cells hours after UVA exposure.
Major finding: Melanocytes continued to form cyclobutane pyrimidine dimers for at least 3 hours after exposure to UVA radiation.
Data source: Histologic study of UV-exposed skin cells from mice and humans, and of in vivo mice.
Disclosures: The research was funded by the U.S. Department of Defense, the National Institutes of Health, the University of Veterinary Medicine in Vienna, Fundação de Amparo à Pesquisa do Estado de São Paulo, and INCT–Processos Redox em Biomedicina. The investigators declared having no conflicts of interest.
Diagnose Pediatric Melanoma Using the CUP Criteria
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
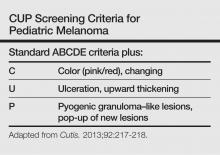
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
VIDEO: Biologics slowly taming metastatic melanoma
MAUI, HAWAII – Though costly, newer biologics increase 1-year survival with metastatic melanoma from perhaps 40% to more than 70%.
Among the latest are nivolumab and pembrolizumab, antibodies against PD-1 (programmed cell death) cell surface receptors that were approved in 2014 for unresectable melanoma no longer responding to other drugs; another anti-PD-1 is in development.
In this video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. George Martin of the Dermatology and Laser Center of Maui explains the latest developments and shares his excitement about them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – Though costly, newer biologics increase 1-year survival with metastatic melanoma from perhaps 40% to more than 70%.
Among the latest are nivolumab and pembrolizumab, antibodies against PD-1 (programmed cell death) cell surface receptors that were approved in 2014 for unresectable melanoma no longer responding to other drugs; another anti-PD-1 is in development.
In this video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. George Martin of the Dermatology and Laser Center of Maui explains the latest developments and shares his excitement about them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – Though costly, newer biologics increase 1-year survival with metastatic melanoma from perhaps 40% to more than 70%.
Among the latest are nivolumab and pembrolizumab, antibodies against PD-1 (programmed cell death) cell surface receptors that were approved in 2014 for unresectable melanoma no longer responding to other drugs; another anti-PD-1 is in development.
In this video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. George Martin of the Dermatology and Laser Center of Maui explains the latest developments and shares his excitement about them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT RWCS 2015
Melanoma incidence highest in Oregon, lowest in Texas in 2015
Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.
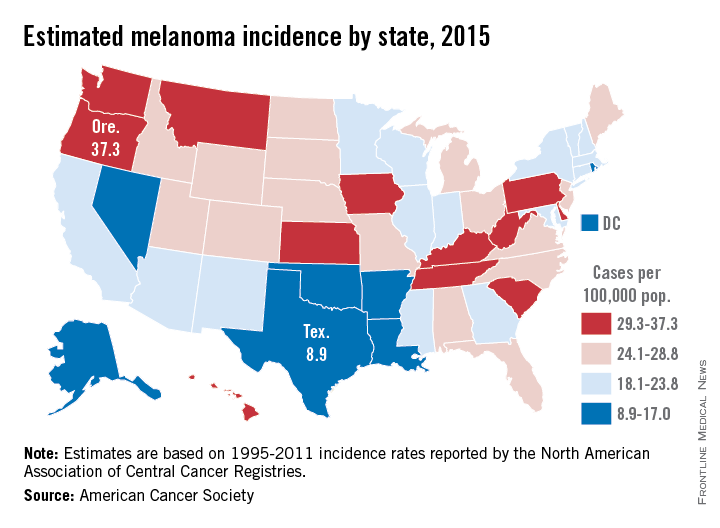
Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.

Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.