User login
Transvaginal mesh, native tissue repair have similar outcomes in 3-year trial
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
FROM OBSTETRICS & GYNECOLOGY
Unraveling primary ovarian insufficiency
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
‘Forever chemicals’ exposures may compound diabetes risk
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
SERMs revisited: Can they improve menopausal care?
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
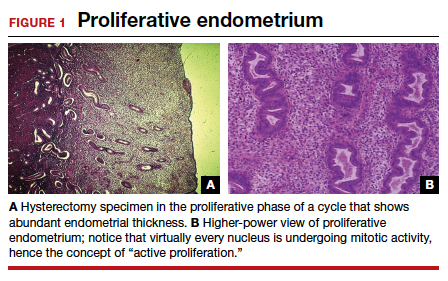
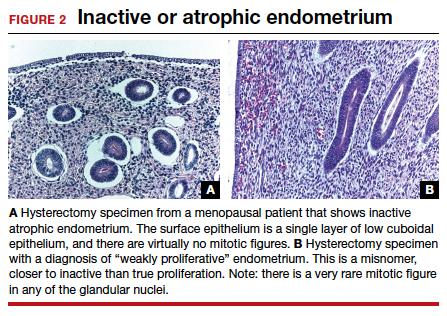
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32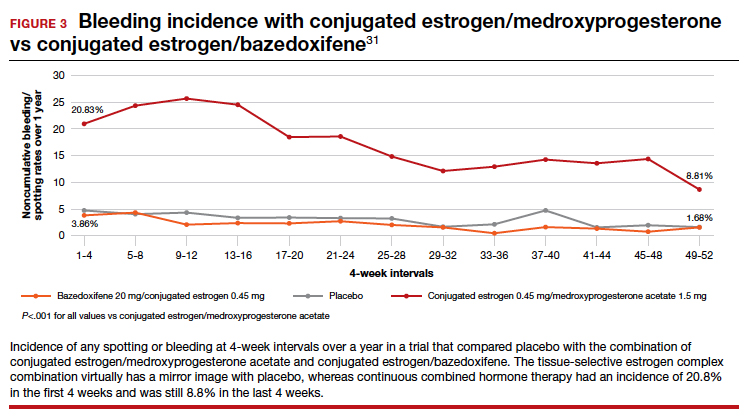
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Some reproductive factors linked with risk of dementia
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
FROM PLOS MEDICINE
Postmenopausal women may benefit from vaginal estradiol for treatment of symptoms
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
More questions than answers when managing HIV and menopause
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
Doctors treat osteoporosis with hormone therapy against guidelines
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
Big missed opportunities for BP control in premenopausal women
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hair loss affects more than half of postmenopausal women
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
FROM MENOPAUSE