User login
Male breast cancer risk linked with infertility
, according to new research funded by the charity Breast Cancer Now and published in Breast Cancer Research. The study is one of the largest ever into male breast cancer, enabling the team to show a highly statistically significant association.
A link with infertility had been suspected, since parity markedly reduces the risk of female breast cancer; there are known genetic links in both sexes, and a high risk of both breast cancer and infertility among men with Klinefelter syndrome, suggesting some sex hormone-related involvement. However, the rarity of breast cancer in men – with an annual incidence of about 370 cases and 80 deaths per year in the United Kingdom – meant that past studies were necessarily small and yielded mixed results.
“Compared with previous studies, our study of male breast cancer is large,” said study coauthor Michael Jones, PhD, of the division of genetics and epidemiology at the Institute of Cancer Research (ICR) in London. “It was carried out nationwide across England and Wales and was set in motion more than 15 years ago. Because of how rare male breast cancer is, it took us over 12 years to identify and interview the nearly 2,000 men with breast cancer who were part of this study.”
The latest research is part of the wider Breast Cancer Now Male Breast Cancer Study, launched by the charity in 2007. For the new study, the ICR team interviewed 1,998 males living in England and Wales who had been diagnosed with breast cancer between 2005 and 2017. All were aged under 80 but most 60 or older at diagnosis; 92% of their tumors were invasive, and almost all were estrogen receptor positive (98.5% of those with known status).
Their responses were compared with those of a control group of 1,597 men without breast cancer, matched by age at diagnosis and geographic region, recruited from male non-blood relatives of cases and from husbands of women participating in the Generations cohort study of breast cancer etiology.
Raised risk with history of male infertility
Overall, 112 cases (5.6%) and 80 controls (5.0%) reported that they had had infertility problems for which they or their partner had consulted a doctor or infertility clinic. This represented a raised odds ratio of 1.29 (95% confidence interval, 0.94-1.77), which was statistically not significant. However, when analyzed by outcome of the infertility consultation, there was a significant and more than doubled risk of breast cancer among men who were diagnosed as the source of the couple’s infertility (OR = 2.03 [1.18-3.49]), whereas this was not the case among men whose partner was the source (OR = 0.86 [0.51-1.45]) or for whom no source was identified (OR = 1.26 [0.71-2.24]).
In addition, proportionately fewer cases (1,615, or 80.8%) compared with controls (1,423, or 89.1%) had fathered any children, also giving a statistically significantly raised risk of breast cancer for men with no biological children (OR = 1.50 [1.21-1.86], P < .001), “congruent with infertility as a risk factor,” the authors said. The risk was statistically significant for invasive tumors but not for the much smaller number of in situ tumors.
Analysis by number of children showed a decreasing risk with increasing numbers of children, with a highly significant (P < .001) inverse trend where zero was included as a value, but a borderline significant trend (P = .04) if it was not. The team noted that number of children beyond one is difficult to interpret as an indicator of male fertility, since it may more reflect social and cultural factors than fertility per se.
Baseline demographic factors were adjusted for in the risk analyses, and results were not materially changed by sensitivity analyses adjusting additionally for alcohol consumption, smoking, liver disease, and family history of breast cancer. The association also largely remained after exclusion of patients with other preexisting potential confounders including severe obesity and testicular abnormalities, and was consistent irrespective of HER-2 status (there were too few ER-negative tumors to analyze results by ER status).
Potential underlying factors
“The causes of breast cancer in men are largely unknown, partly because it is rare and partly because previous studies have been small,” Dr. Jones said. “The evidence presented in our study suggests that the association of infertility and breast cancer should be confirmed with further research, and future investigations are needed into the potential underlying factors, such as hormone imbalances.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, told this news organization: “Overall, there isn’t strong evidence that infertility is a risk factor for male breast cancer. This study helps to shed light onto a cancer type that is sadly still not very well understood, but much more research is needed to say that infertility is a risk factor for male breast cancer.”
She added that although male breast cancer is a rare condition, it’s still important for men to be aware of what looks and feels normal for them, and to be encouraged to seek medical advice if something is not quite right.
A spokesperson for Breast Cancer UK told this news organization: “[We] believe it’s important to understand what leads to breast cancer in men as well as women and that high quality, long-term studies such as this will help with this understanding.
The findings are consistent with an earlier study that found that U.S. men who have never fathered children are at higher risk of breast cancer. This new long-term U.K. study provides strong evidence, which supports this finding.
“As the authors note, the biological reasons are unclear, but may be associated with altered hormone levels. The ratio of circulating levels of estrogen and androgens (e.g. testosterone) is crucial in healthy functioning of breast tissue. Disruption to this, for example as a result of damage to testes, may affect both fertility and breast cancer risk.
“It is also possible that external factors, such as exposure to certain endocrine (hormone) disrupting chemicals (EDCs), which affect sex hormones, may also affect both fertility and breast cancer risk.
“More studies into breast cancer in men are needed to help us understand better all the risk factors associated with this disease including both hormonal factors and chemical exposures.”
Simon Vincent, PhD, director of research, support, and influencing at Breast Cancer Now, said: “Research has discovered different treatments directed at some features of breast cancer in women; however, breast cancer is not as well understood for men. This is why Breast Cancer Now funds the Male Breast Cancer Study, which looks at what might cause the disease in men. Discovering a link between infertility and male breast cancer is a step towards us understanding male breast cancer and how we could find more ways to diagnose and treat men – and possibly women – with this devastating disease.”
A version of this article first appeared on Medscape UK.
, according to new research funded by the charity Breast Cancer Now and published in Breast Cancer Research. The study is one of the largest ever into male breast cancer, enabling the team to show a highly statistically significant association.
A link with infertility had been suspected, since parity markedly reduces the risk of female breast cancer; there are known genetic links in both sexes, and a high risk of both breast cancer and infertility among men with Klinefelter syndrome, suggesting some sex hormone-related involvement. However, the rarity of breast cancer in men – with an annual incidence of about 370 cases and 80 deaths per year in the United Kingdom – meant that past studies were necessarily small and yielded mixed results.
“Compared with previous studies, our study of male breast cancer is large,” said study coauthor Michael Jones, PhD, of the division of genetics and epidemiology at the Institute of Cancer Research (ICR) in London. “It was carried out nationwide across England and Wales and was set in motion more than 15 years ago. Because of how rare male breast cancer is, it took us over 12 years to identify and interview the nearly 2,000 men with breast cancer who were part of this study.”
The latest research is part of the wider Breast Cancer Now Male Breast Cancer Study, launched by the charity in 2007. For the new study, the ICR team interviewed 1,998 males living in England and Wales who had been diagnosed with breast cancer between 2005 and 2017. All were aged under 80 but most 60 or older at diagnosis; 92% of their tumors were invasive, and almost all were estrogen receptor positive (98.5% of those with known status).
Their responses were compared with those of a control group of 1,597 men without breast cancer, matched by age at diagnosis and geographic region, recruited from male non-blood relatives of cases and from husbands of women participating in the Generations cohort study of breast cancer etiology.
Raised risk with history of male infertility
Overall, 112 cases (5.6%) and 80 controls (5.0%) reported that they had had infertility problems for which they or their partner had consulted a doctor or infertility clinic. This represented a raised odds ratio of 1.29 (95% confidence interval, 0.94-1.77), which was statistically not significant. However, when analyzed by outcome of the infertility consultation, there was a significant and more than doubled risk of breast cancer among men who were diagnosed as the source of the couple’s infertility (OR = 2.03 [1.18-3.49]), whereas this was not the case among men whose partner was the source (OR = 0.86 [0.51-1.45]) or for whom no source was identified (OR = 1.26 [0.71-2.24]).
In addition, proportionately fewer cases (1,615, or 80.8%) compared with controls (1,423, or 89.1%) had fathered any children, also giving a statistically significantly raised risk of breast cancer for men with no biological children (OR = 1.50 [1.21-1.86], P < .001), “congruent with infertility as a risk factor,” the authors said. The risk was statistically significant for invasive tumors but not for the much smaller number of in situ tumors.
Analysis by number of children showed a decreasing risk with increasing numbers of children, with a highly significant (P < .001) inverse trend where zero was included as a value, but a borderline significant trend (P = .04) if it was not. The team noted that number of children beyond one is difficult to interpret as an indicator of male fertility, since it may more reflect social and cultural factors than fertility per se.
Baseline demographic factors were adjusted for in the risk analyses, and results were not materially changed by sensitivity analyses adjusting additionally for alcohol consumption, smoking, liver disease, and family history of breast cancer. The association also largely remained after exclusion of patients with other preexisting potential confounders including severe obesity and testicular abnormalities, and was consistent irrespective of HER-2 status (there were too few ER-negative tumors to analyze results by ER status).
Potential underlying factors
“The causes of breast cancer in men are largely unknown, partly because it is rare and partly because previous studies have been small,” Dr. Jones said. “The evidence presented in our study suggests that the association of infertility and breast cancer should be confirmed with further research, and future investigations are needed into the potential underlying factors, such as hormone imbalances.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, told this news organization: “Overall, there isn’t strong evidence that infertility is a risk factor for male breast cancer. This study helps to shed light onto a cancer type that is sadly still not very well understood, but much more research is needed to say that infertility is a risk factor for male breast cancer.”
She added that although male breast cancer is a rare condition, it’s still important for men to be aware of what looks and feels normal for them, and to be encouraged to seek medical advice if something is not quite right.
A spokesperson for Breast Cancer UK told this news organization: “[We] believe it’s important to understand what leads to breast cancer in men as well as women and that high quality, long-term studies such as this will help with this understanding.
The findings are consistent with an earlier study that found that U.S. men who have never fathered children are at higher risk of breast cancer. This new long-term U.K. study provides strong evidence, which supports this finding.
“As the authors note, the biological reasons are unclear, but may be associated with altered hormone levels. The ratio of circulating levels of estrogen and androgens (e.g. testosterone) is crucial in healthy functioning of breast tissue. Disruption to this, for example as a result of damage to testes, may affect both fertility and breast cancer risk.
“It is also possible that external factors, such as exposure to certain endocrine (hormone) disrupting chemicals (EDCs), which affect sex hormones, may also affect both fertility and breast cancer risk.
“More studies into breast cancer in men are needed to help us understand better all the risk factors associated with this disease including both hormonal factors and chemical exposures.”
Simon Vincent, PhD, director of research, support, and influencing at Breast Cancer Now, said: “Research has discovered different treatments directed at some features of breast cancer in women; however, breast cancer is not as well understood for men. This is why Breast Cancer Now funds the Male Breast Cancer Study, which looks at what might cause the disease in men. Discovering a link between infertility and male breast cancer is a step towards us understanding male breast cancer and how we could find more ways to diagnose and treat men – and possibly women – with this devastating disease.”
A version of this article first appeared on Medscape UK.
, according to new research funded by the charity Breast Cancer Now and published in Breast Cancer Research. The study is one of the largest ever into male breast cancer, enabling the team to show a highly statistically significant association.
A link with infertility had been suspected, since parity markedly reduces the risk of female breast cancer; there are known genetic links in both sexes, and a high risk of both breast cancer and infertility among men with Klinefelter syndrome, suggesting some sex hormone-related involvement. However, the rarity of breast cancer in men – with an annual incidence of about 370 cases and 80 deaths per year in the United Kingdom – meant that past studies were necessarily small and yielded mixed results.
“Compared with previous studies, our study of male breast cancer is large,” said study coauthor Michael Jones, PhD, of the division of genetics and epidemiology at the Institute of Cancer Research (ICR) in London. “It was carried out nationwide across England and Wales and was set in motion more than 15 years ago. Because of how rare male breast cancer is, it took us over 12 years to identify and interview the nearly 2,000 men with breast cancer who were part of this study.”
The latest research is part of the wider Breast Cancer Now Male Breast Cancer Study, launched by the charity in 2007. For the new study, the ICR team interviewed 1,998 males living in England and Wales who had been diagnosed with breast cancer between 2005 and 2017. All were aged under 80 but most 60 or older at diagnosis; 92% of their tumors were invasive, and almost all were estrogen receptor positive (98.5% of those with known status).
Their responses were compared with those of a control group of 1,597 men without breast cancer, matched by age at diagnosis and geographic region, recruited from male non-blood relatives of cases and from husbands of women participating in the Generations cohort study of breast cancer etiology.
Raised risk with history of male infertility
Overall, 112 cases (5.6%) and 80 controls (5.0%) reported that they had had infertility problems for which they or their partner had consulted a doctor or infertility clinic. This represented a raised odds ratio of 1.29 (95% confidence interval, 0.94-1.77), which was statistically not significant. However, when analyzed by outcome of the infertility consultation, there was a significant and more than doubled risk of breast cancer among men who were diagnosed as the source of the couple’s infertility (OR = 2.03 [1.18-3.49]), whereas this was not the case among men whose partner was the source (OR = 0.86 [0.51-1.45]) or for whom no source was identified (OR = 1.26 [0.71-2.24]).
In addition, proportionately fewer cases (1,615, or 80.8%) compared with controls (1,423, or 89.1%) had fathered any children, also giving a statistically significantly raised risk of breast cancer for men with no biological children (OR = 1.50 [1.21-1.86], P < .001), “congruent with infertility as a risk factor,” the authors said. The risk was statistically significant for invasive tumors but not for the much smaller number of in situ tumors.
Analysis by number of children showed a decreasing risk with increasing numbers of children, with a highly significant (P < .001) inverse trend where zero was included as a value, but a borderline significant trend (P = .04) if it was not. The team noted that number of children beyond one is difficult to interpret as an indicator of male fertility, since it may more reflect social and cultural factors than fertility per se.
Baseline demographic factors were adjusted for in the risk analyses, and results were not materially changed by sensitivity analyses adjusting additionally for alcohol consumption, smoking, liver disease, and family history of breast cancer. The association also largely remained after exclusion of patients with other preexisting potential confounders including severe obesity and testicular abnormalities, and was consistent irrespective of HER-2 status (there were too few ER-negative tumors to analyze results by ER status).
Potential underlying factors
“The causes of breast cancer in men are largely unknown, partly because it is rare and partly because previous studies have been small,” Dr. Jones said. “The evidence presented in our study suggests that the association of infertility and breast cancer should be confirmed with further research, and future investigations are needed into the potential underlying factors, such as hormone imbalances.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, told this news organization: “Overall, there isn’t strong evidence that infertility is a risk factor for male breast cancer. This study helps to shed light onto a cancer type that is sadly still not very well understood, but much more research is needed to say that infertility is a risk factor for male breast cancer.”
She added that although male breast cancer is a rare condition, it’s still important for men to be aware of what looks and feels normal for them, and to be encouraged to seek medical advice if something is not quite right.
A spokesperson for Breast Cancer UK told this news organization: “[We] believe it’s important to understand what leads to breast cancer in men as well as women and that high quality, long-term studies such as this will help with this understanding.
The findings are consistent with an earlier study that found that U.S. men who have never fathered children are at higher risk of breast cancer. This new long-term U.K. study provides strong evidence, which supports this finding.
“As the authors note, the biological reasons are unclear, but may be associated with altered hormone levels. The ratio of circulating levels of estrogen and androgens (e.g. testosterone) is crucial in healthy functioning of breast tissue. Disruption to this, for example as a result of damage to testes, may affect both fertility and breast cancer risk.
“It is also possible that external factors, such as exposure to certain endocrine (hormone) disrupting chemicals (EDCs), which affect sex hormones, may also affect both fertility and breast cancer risk.
“More studies into breast cancer in men are needed to help us understand better all the risk factors associated with this disease including both hormonal factors and chemical exposures.”
Simon Vincent, PhD, director of research, support, and influencing at Breast Cancer Now, said: “Research has discovered different treatments directed at some features of breast cancer in women; however, breast cancer is not as well understood for men. This is why Breast Cancer Now funds the Male Breast Cancer Study, which looks at what might cause the disease in men. Discovering a link between infertility and male breast cancer is a step towards us understanding male breast cancer and how we could find more ways to diagnose and treat men – and possibly women – with this devastating disease.”
A version of this article first appeared on Medscape UK.
FROM BREAST CANCER RESEARCH
New guideline gives active surveillance a boost
Experts hailed the new guidelines, released May 10 by the American Urological Association (AUA) and the American Society for Radiation Oncology (ASTRO) as a boon for patients with low-risk to favorable intermediate-risk prostate cancers.
“The guideline is unequivocal that AS is the preferred management option for the majority of men with low-risk prostate cancer,” panel chair James A. Eastham, MD, Peter T. Scardino Chair in Oncology and chief of urology at Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The new guideline is the first guideline for localized prostate cancer since 2017.
In the new document, guideline writers merged low-risk patients and very-low-risk patients into a single category of “low-risk.” Dr. Eastham said a distinction between very-low-risk and low-risk is inconsequential since the treatment for the two groups of patients is identical.
The 2022 guideline for the first time makes AS the recommended treatment for select patients with favorable intermediate-risk Gleason 3+4 prostate cancer, he said. The document also provides guidance on how such patients should be selected for AS.
Most research suggests that as many as 40% of patients newly diagnosed with prostate cancer have low-risk disease. Favorable intermediate-risk cancer represents 10%-15% of newly diagnosed patients, said Todd Morgan, MD, the Jack Lapides, MD, Research Professor and chief of urologic oncology at Michigan Medicine, Ann Arbor.
Dr. Morgan, who was not on the AUA/ASTRO panel, called the new recommendations “a very strong update compared to the guideline from 5 years ago.”
The guideline has been pared back some from 2017 to include fewer statements, but it covers several key clinical trials that have appeared over the past 6 years to strengthen the evidence base for the document, he said.
“I would say that we still have to acknowledge that many statements are based on ‘expert opinion’ rather than high-level evidence, which highlights the continued need for well-conducted studies that prove or disprove some of these statements,” Dr. Morgan added.
Patients weighed in
This year, AUA’s advocacy group urged patients to comment on the proposed guideline.
Rick Davis, founder of the AnCan Foundation, a virtual support network for prostate cancer and other diseases, thanked the groups for acknowledging the value of peer support and virtual support groups.
“AnCan congratulates the AUA/ASTRO on endorsing the proper role for the Active Surveillance protocol to manage early low-risk and favorable intermediate-risk prostate cancer and also their qualified and well-supported warnings against focal therapy,” Mr. Davis, who reviewed the guideline, said in an email. “We are, however, disappointed at the lack of a recommendation to provide comprehensive counseling when hormone therapy is prescribed.”
James Schraidt, another patient reviewer for AnCan, said that on balance, the 2022 guideline was an improvement over 2017 and will benefit patients.
He praised AUA/ASTRO for, at the urging of patient reviewers, introducing the “cribriform” and “intraductal” pathology patterns into the guideline for the first time as risk factors.
But he criticized the doctor groups for “a less than fulsome and orderly discussion of the use of MRI. It is not mentioned as a tool that should be used prior to initial biopsy, leaving the door wide open to random biopsies. The recommended role of MRI in AS monitoring was unclear.” He also said the panel should have reviewed micro-ultrasound, an emerging technology, that can be used by itself or to complement MRIs.
Many of the AUA/ASTRO guideline changes involve semantic issues – but which experts said nevertheless were important nuances.
Dr. Eastham said the AUA/ASTRO panel debated and finally settled on the word “preferred” for AS rather than “recommended” or “strongly recommended.”
“This is a very strong statement from the AUA/ASTRO,” Dr. Morgan said. “The semantics are definitely important, but ... ’preferred’ is actually a strong word. For the AUA, what’s really important is the ‘strong recommendation’ and Grade A level of evidence.”
Dr. Morgan also observed that the AS recommendations for patients with low-risk prostate cancer are stronger in the new AUA guideline than those in the latest recommendations from the National Comprehensive Cancer Network (NCCN), which he helped write.
The AUA/ASTRO guideline states that AS is preferred for patients with low-risk cancer, whereas in the NCCN guideline the language is: “preferred for most patients with low-risk disease cancer,” Dr. Morgan said.
“All of these statements ultimately acknowledge what I think that the vast majority of experts agree on – a small proportion of patients with low-risk prostate cancer may appropriately be recommended to undergo primary therapy,” he said.
Dr. Eastham said the goal of the guideline is to persuade surgeons to emphasize that AS is the best choice for most patients with low-risk prostate cancer: “The hope is that surgeons read the guideline. The guideline is definitive in recommending AS in low-risk prostate cancer.”
Dr. Eastham said the new guideline also does the following:
- Further endorses shared decisionmaking, with the understanding that for a decision to be made, both patient and physician need appropriate information regarding the risk posed by the cancer and the risk posed by treatment;
- Endorses selective use of somatic genetic testing when the data are needed for shared decisionmaking;
- Updates a section on genetic testing in patients considered to be at high risk for a germline mutation;
- Updates pretreatment evaluation for patients opting for treatment, primarily the role of imaging and how the evolution of next-generation imaging – such as , a new type of nuclear medicine procedure, in clinically localized prostate cancer;
- Addresses aspects of both radiotherapy and surgery, including nerve sparing, pelvic lymph node dissection, and adjuvant/neoadjuvant therapy, such as chemotherapy or hormone therapy delivered before or after the primary treatment. Dr. Eastham said the “significant evolution” in how best to provide radiotherapy resulted in several changes to this section.
No relevant financial relationships have been reported.
A version of this article first appeared on Medscape.com.
Experts hailed the new guidelines, released May 10 by the American Urological Association (AUA) and the American Society for Radiation Oncology (ASTRO) as a boon for patients with low-risk to favorable intermediate-risk prostate cancers.
“The guideline is unequivocal that AS is the preferred management option for the majority of men with low-risk prostate cancer,” panel chair James A. Eastham, MD, Peter T. Scardino Chair in Oncology and chief of urology at Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The new guideline is the first guideline for localized prostate cancer since 2017.
In the new document, guideline writers merged low-risk patients and very-low-risk patients into a single category of “low-risk.” Dr. Eastham said a distinction between very-low-risk and low-risk is inconsequential since the treatment for the two groups of patients is identical.
The 2022 guideline for the first time makes AS the recommended treatment for select patients with favorable intermediate-risk Gleason 3+4 prostate cancer, he said. The document also provides guidance on how such patients should be selected for AS.
Most research suggests that as many as 40% of patients newly diagnosed with prostate cancer have low-risk disease. Favorable intermediate-risk cancer represents 10%-15% of newly diagnosed patients, said Todd Morgan, MD, the Jack Lapides, MD, Research Professor and chief of urologic oncology at Michigan Medicine, Ann Arbor.
Dr. Morgan, who was not on the AUA/ASTRO panel, called the new recommendations “a very strong update compared to the guideline from 5 years ago.”
The guideline has been pared back some from 2017 to include fewer statements, but it covers several key clinical trials that have appeared over the past 6 years to strengthen the evidence base for the document, he said.
“I would say that we still have to acknowledge that many statements are based on ‘expert opinion’ rather than high-level evidence, which highlights the continued need for well-conducted studies that prove or disprove some of these statements,” Dr. Morgan added.
Patients weighed in
This year, AUA’s advocacy group urged patients to comment on the proposed guideline.
Rick Davis, founder of the AnCan Foundation, a virtual support network for prostate cancer and other diseases, thanked the groups for acknowledging the value of peer support and virtual support groups.
“AnCan congratulates the AUA/ASTRO on endorsing the proper role for the Active Surveillance protocol to manage early low-risk and favorable intermediate-risk prostate cancer and also their qualified and well-supported warnings against focal therapy,” Mr. Davis, who reviewed the guideline, said in an email. “We are, however, disappointed at the lack of a recommendation to provide comprehensive counseling when hormone therapy is prescribed.”
James Schraidt, another patient reviewer for AnCan, said that on balance, the 2022 guideline was an improvement over 2017 and will benefit patients.
He praised AUA/ASTRO for, at the urging of patient reviewers, introducing the “cribriform” and “intraductal” pathology patterns into the guideline for the first time as risk factors.
But he criticized the doctor groups for “a less than fulsome and orderly discussion of the use of MRI. It is not mentioned as a tool that should be used prior to initial biopsy, leaving the door wide open to random biopsies. The recommended role of MRI in AS monitoring was unclear.” He also said the panel should have reviewed micro-ultrasound, an emerging technology, that can be used by itself or to complement MRIs.
Many of the AUA/ASTRO guideline changes involve semantic issues – but which experts said nevertheless were important nuances.
Dr. Eastham said the AUA/ASTRO panel debated and finally settled on the word “preferred” for AS rather than “recommended” or “strongly recommended.”
“This is a very strong statement from the AUA/ASTRO,” Dr. Morgan said. “The semantics are definitely important, but ... ’preferred’ is actually a strong word. For the AUA, what’s really important is the ‘strong recommendation’ and Grade A level of evidence.”
Dr. Morgan also observed that the AS recommendations for patients with low-risk prostate cancer are stronger in the new AUA guideline than those in the latest recommendations from the National Comprehensive Cancer Network (NCCN), which he helped write.
The AUA/ASTRO guideline states that AS is preferred for patients with low-risk cancer, whereas in the NCCN guideline the language is: “preferred for most patients with low-risk disease cancer,” Dr. Morgan said.
“All of these statements ultimately acknowledge what I think that the vast majority of experts agree on – a small proportion of patients with low-risk prostate cancer may appropriately be recommended to undergo primary therapy,” he said.
Dr. Eastham said the goal of the guideline is to persuade surgeons to emphasize that AS is the best choice for most patients with low-risk prostate cancer: “The hope is that surgeons read the guideline. The guideline is definitive in recommending AS in low-risk prostate cancer.”
Dr. Eastham said the new guideline also does the following:
- Further endorses shared decisionmaking, with the understanding that for a decision to be made, both patient and physician need appropriate information regarding the risk posed by the cancer and the risk posed by treatment;
- Endorses selective use of somatic genetic testing when the data are needed for shared decisionmaking;
- Updates a section on genetic testing in patients considered to be at high risk for a germline mutation;
- Updates pretreatment evaluation for patients opting for treatment, primarily the role of imaging and how the evolution of next-generation imaging – such as , a new type of nuclear medicine procedure, in clinically localized prostate cancer;
- Addresses aspects of both radiotherapy and surgery, including nerve sparing, pelvic lymph node dissection, and adjuvant/neoadjuvant therapy, such as chemotherapy or hormone therapy delivered before or after the primary treatment. Dr. Eastham said the “significant evolution” in how best to provide radiotherapy resulted in several changes to this section.
No relevant financial relationships have been reported.
A version of this article first appeared on Medscape.com.
Experts hailed the new guidelines, released May 10 by the American Urological Association (AUA) and the American Society for Radiation Oncology (ASTRO) as a boon for patients with low-risk to favorable intermediate-risk prostate cancers.
“The guideline is unequivocal that AS is the preferred management option for the majority of men with low-risk prostate cancer,” panel chair James A. Eastham, MD, Peter T. Scardino Chair in Oncology and chief of urology at Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The new guideline is the first guideline for localized prostate cancer since 2017.
In the new document, guideline writers merged low-risk patients and very-low-risk patients into a single category of “low-risk.” Dr. Eastham said a distinction between very-low-risk and low-risk is inconsequential since the treatment for the two groups of patients is identical.
The 2022 guideline for the first time makes AS the recommended treatment for select patients with favorable intermediate-risk Gleason 3+4 prostate cancer, he said. The document also provides guidance on how such patients should be selected for AS.
Most research suggests that as many as 40% of patients newly diagnosed with prostate cancer have low-risk disease. Favorable intermediate-risk cancer represents 10%-15% of newly diagnosed patients, said Todd Morgan, MD, the Jack Lapides, MD, Research Professor and chief of urologic oncology at Michigan Medicine, Ann Arbor.
Dr. Morgan, who was not on the AUA/ASTRO panel, called the new recommendations “a very strong update compared to the guideline from 5 years ago.”
The guideline has been pared back some from 2017 to include fewer statements, but it covers several key clinical trials that have appeared over the past 6 years to strengthen the evidence base for the document, he said.
“I would say that we still have to acknowledge that many statements are based on ‘expert opinion’ rather than high-level evidence, which highlights the continued need for well-conducted studies that prove or disprove some of these statements,” Dr. Morgan added.
Patients weighed in
This year, AUA’s advocacy group urged patients to comment on the proposed guideline.
Rick Davis, founder of the AnCan Foundation, a virtual support network for prostate cancer and other diseases, thanked the groups for acknowledging the value of peer support and virtual support groups.
“AnCan congratulates the AUA/ASTRO on endorsing the proper role for the Active Surveillance protocol to manage early low-risk and favorable intermediate-risk prostate cancer and also their qualified and well-supported warnings against focal therapy,” Mr. Davis, who reviewed the guideline, said in an email. “We are, however, disappointed at the lack of a recommendation to provide comprehensive counseling when hormone therapy is prescribed.”
James Schraidt, another patient reviewer for AnCan, said that on balance, the 2022 guideline was an improvement over 2017 and will benefit patients.
He praised AUA/ASTRO for, at the urging of patient reviewers, introducing the “cribriform” and “intraductal” pathology patterns into the guideline for the first time as risk factors.
But he criticized the doctor groups for “a less than fulsome and orderly discussion of the use of MRI. It is not mentioned as a tool that should be used prior to initial biopsy, leaving the door wide open to random biopsies. The recommended role of MRI in AS monitoring was unclear.” He also said the panel should have reviewed micro-ultrasound, an emerging technology, that can be used by itself or to complement MRIs.
Many of the AUA/ASTRO guideline changes involve semantic issues – but which experts said nevertheless were important nuances.
Dr. Eastham said the AUA/ASTRO panel debated and finally settled on the word “preferred” for AS rather than “recommended” or “strongly recommended.”
“This is a very strong statement from the AUA/ASTRO,” Dr. Morgan said. “The semantics are definitely important, but ... ’preferred’ is actually a strong word. For the AUA, what’s really important is the ‘strong recommendation’ and Grade A level of evidence.”
Dr. Morgan also observed that the AS recommendations for patients with low-risk prostate cancer are stronger in the new AUA guideline than those in the latest recommendations from the National Comprehensive Cancer Network (NCCN), which he helped write.
The AUA/ASTRO guideline states that AS is preferred for patients with low-risk cancer, whereas in the NCCN guideline the language is: “preferred for most patients with low-risk disease cancer,” Dr. Morgan said.
“All of these statements ultimately acknowledge what I think that the vast majority of experts agree on – a small proportion of patients with low-risk prostate cancer may appropriately be recommended to undergo primary therapy,” he said.
Dr. Eastham said the goal of the guideline is to persuade surgeons to emphasize that AS is the best choice for most patients with low-risk prostate cancer: “The hope is that surgeons read the guideline. The guideline is definitive in recommending AS in low-risk prostate cancer.”
Dr. Eastham said the new guideline also does the following:
- Further endorses shared decisionmaking, with the understanding that for a decision to be made, both patient and physician need appropriate information regarding the risk posed by the cancer and the risk posed by treatment;
- Endorses selective use of somatic genetic testing when the data are needed for shared decisionmaking;
- Updates a section on genetic testing in patients considered to be at high risk for a germline mutation;
- Updates pretreatment evaluation for patients opting for treatment, primarily the role of imaging and how the evolution of next-generation imaging – such as , a new type of nuclear medicine procedure, in clinically localized prostate cancer;
- Addresses aspects of both radiotherapy and surgery, including nerve sparing, pelvic lymph node dissection, and adjuvant/neoadjuvant therapy, such as chemotherapy or hormone therapy delivered before or after the primary treatment. Dr. Eastham said the “significant evolution” in how best to provide radiotherapy resulted in several changes to this section.
No relevant financial relationships have been reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF UROLOGY
Most men with low-risk prostate tumors now forgoing treatment
, according to experts who say the dramatic increase reflects a growing understanding among both researchers and patients that low-grade prostate tumors can be safely watched for years without requiring treatment.
Roughly 60% of men eligible for AS chose that approach in 2021, up from 27% in 2014 and less than 10% in 2010, according to panel member Matthew Cooperberg, MD, MPH, of University of California, San Francisco. He presented the data for a panel of the American Urological Association (AUA) at the group’s annual meeting in New Orleans.
Dr. Cooperberg attributed the hike in AS rates in the United States to the growing scientific literature and guidelines supportive of the approach, which calls for periodic assessments of low-risk tumors but no surgery, radiation, or other therapies. In Canada and parts of Europe, approximately 80%-90% of men who are eligible for AS choose that approach, experts said.
Earlier this month, the AUA and the American Society for Radiation Oncology released the strongest guidelines to date supporting AS for low-risk patients, and, for the first time, for select patients with favorable intermediate-risk prostate cancer.
In 2012, the U.S. Preventative Services Task Force (USPSTF) recommended against screening for prostate-specific antigen (PSA), concluding that the benefits of the test did not outweigh the risks, such as overdiagnosis and overtreatment of low-risk prostate cancer.
Urologists blamed the USPSTF policy for a decline in PSA screening and an uptick in the diagnosis of advanced prostate cancer.
Dr. Cooperberg said the shift served as “a bit of a wake-up call for at least a segment of the urology community that if we didn’t fix the overtreatment problem, we would never retake the chunks of the conversation about screening and early detection.”
In 2018, following protests by urologists and patient advocates, the USPSTF revised its statements to include shared decisionmaking for PSA testing in men aged 55-69 years, reflecting emerging evidence of longer-term benefits and widespread adoption of active surveillance after detection of low-risk disease.
Laurence Klotz, MD, the University of Toronto researcher who named and helped develop AS 30 years ago, and who was not on the AUA panel, said other factors also help to explain the growing interest in AS. These include an increasing consensus among experts on the value of the strategy, mounting public awareness of its benefits, the efforts of support and advocacy groups, and the arrival of more sophisticated imaging and biomarkers that help further refine risk.
“We’re shrinking the gray zone,” Dr. Klotz said. “Remaining resistance to AS is due to legitimate concerns about missing significant cancer and losing a patient to metastatic disease, and perhaps financial drivers, particularly with less invasive technologies like radiation and focal therapy.”
The national rate for AS increased from 26.5% in 2014, when data were first reported through the AUA’s AQUA data registry. AQUA’s data comes from electronic health records and included 27,289 patients with newly diagnosed low-risk prostate cancer.
In 2014, radical prostatectomy was the leading treatment in the low-risk population, with 29.7% of these patients overall opting for surgery, edging out external beam radiotherapy (EBRT) and AS, at 28.2% and 26.5% respectively.
In 2015, AS and EBRT overtook surgery, and by 2021, 59.6% of low-risk patients had chosen AS, followed by 20.9% for EBRT and 15.8% for prostatectomy.
Aiming higher
William Catalona, MD, a panel member from Northwestern University Feinberg School of Medicine, Chicago, said the AUA’s Prostate Cancer Active Surveillance Project has set a goal of 80% uptake of AS in patients with low-risk prostate cancer. Dr. Catalona, an early critic of AS, called that figure “optimal and realistic,” something that should happen “as soon as possible.”
Dr. Catalona said the 80% benchmark matches acceptance of AS within the U.S. Department of Veterans Affairs hospitals.
However, Dr. Klotz said the American culture of treatment, which is driven at least in part by financial incentives on the part of physicians, may prevent the growth of AS above 80% in this country.
Dr. Cooperberg said financial incentives are real. “I think it’s a small minority of docs that are heavily driven by the financial incentive, but it certainly exists,” he told this news organization. When you look at the extreme variation of active surveillance rates, there is no question that factors like reimbursement are going to play a role.”
Dr. Catalona, who through the first decade of the 2000s regularly debated Dr. Klotz about the concept of AS, said he today recommends AS when appropriate.
“The variability of AS adoption among practices and physicians varies from 0% to 100%. Therefore, some are too ‘tight’ in recommending AS and some are ‘too loose.’ I do not attempt to steer [patients] into treatment unless I believe that would be their best option. Nevertheless, some opt for surveillance when I believe they are making a mistake, and some opt for treatment when I believe surveillance would have been a rational choice.”
Dr. Cooperberg agreed that a personalized approach is important and that both physicians and patients should be flexible in their decisionmaking. “There will always be some men with low-grade disease who should get immediate treatment. For example, a young man with very high-volume disease, even if it’s Gleason 3+3,” he said. “If it is clearly inevitable that he’s going to need treatment, he could reasonably make a decision to get immediate treatment.”
Dr. Cooperberg, Dr. Klotz, and Dr. Catalona have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to experts who say the dramatic increase reflects a growing understanding among both researchers and patients that low-grade prostate tumors can be safely watched for years without requiring treatment.
Roughly 60% of men eligible for AS chose that approach in 2021, up from 27% in 2014 and less than 10% in 2010, according to panel member Matthew Cooperberg, MD, MPH, of University of California, San Francisco. He presented the data for a panel of the American Urological Association (AUA) at the group’s annual meeting in New Orleans.
Dr. Cooperberg attributed the hike in AS rates in the United States to the growing scientific literature and guidelines supportive of the approach, which calls for periodic assessments of low-risk tumors but no surgery, radiation, or other therapies. In Canada and parts of Europe, approximately 80%-90% of men who are eligible for AS choose that approach, experts said.
Earlier this month, the AUA and the American Society for Radiation Oncology released the strongest guidelines to date supporting AS for low-risk patients, and, for the first time, for select patients with favorable intermediate-risk prostate cancer.
In 2012, the U.S. Preventative Services Task Force (USPSTF) recommended against screening for prostate-specific antigen (PSA), concluding that the benefits of the test did not outweigh the risks, such as overdiagnosis and overtreatment of low-risk prostate cancer.
Urologists blamed the USPSTF policy for a decline in PSA screening and an uptick in the diagnosis of advanced prostate cancer.
Dr. Cooperberg said the shift served as “a bit of a wake-up call for at least a segment of the urology community that if we didn’t fix the overtreatment problem, we would never retake the chunks of the conversation about screening and early detection.”
In 2018, following protests by urologists and patient advocates, the USPSTF revised its statements to include shared decisionmaking for PSA testing in men aged 55-69 years, reflecting emerging evidence of longer-term benefits and widespread adoption of active surveillance after detection of low-risk disease.
Laurence Klotz, MD, the University of Toronto researcher who named and helped develop AS 30 years ago, and who was not on the AUA panel, said other factors also help to explain the growing interest in AS. These include an increasing consensus among experts on the value of the strategy, mounting public awareness of its benefits, the efforts of support and advocacy groups, and the arrival of more sophisticated imaging and biomarkers that help further refine risk.
“We’re shrinking the gray zone,” Dr. Klotz said. “Remaining resistance to AS is due to legitimate concerns about missing significant cancer and losing a patient to metastatic disease, and perhaps financial drivers, particularly with less invasive technologies like radiation and focal therapy.”
The national rate for AS increased from 26.5% in 2014, when data were first reported through the AUA’s AQUA data registry. AQUA’s data comes from electronic health records and included 27,289 patients with newly diagnosed low-risk prostate cancer.
In 2014, radical prostatectomy was the leading treatment in the low-risk population, with 29.7% of these patients overall opting for surgery, edging out external beam radiotherapy (EBRT) and AS, at 28.2% and 26.5% respectively.
In 2015, AS and EBRT overtook surgery, and by 2021, 59.6% of low-risk patients had chosen AS, followed by 20.9% for EBRT and 15.8% for prostatectomy.
Aiming higher
William Catalona, MD, a panel member from Northwestern University Feinberg School of Medicine, Chicago, said the AUA’s Prostate Cancer Active Surveillance Project has set a goal of 80% uptake of AS in patients with low-risk prostate cancer. Dr. Catalona, an early critic of AS, called that figure “optimal and realistic,” something that should happen “as soon as possible.”
Dr. Catalona said the 80% benchmark matches acceptance of AS within the U.S. Department of Veterans Affairs hospitals.
However, Dr. Klotz said the American culture of treatment, which is driven at least in part by financial incentives on the part of physicians, may prevent the growth of AS above 80% in this country.
Dr. Cooperberg said financial incentives are real. “I think it’s a small minority of docs that are heavily driven by the financial incentive, but it certainly exists,” he told this news organization. When you look at the extreme variation of active surveillance rates, there is no question that factors like reimbursement are going to play a role.”
Dr. Catalona, who through the first decade of the 2000s regularly debated Dr. Klotz about the concept of AS, said he today recommends AS when appropriate.
“The variability of AS adoption among practices and physicians varies from 0% to 100%. Therefore, some are too ‘tight’ in recommending AS and some are ‘too loose.’ I do not attempt to steer [patients] into treatment unless I believe that would be their best option. Nevertheless, some opt for surveillance when I believe they are making a mistake, and some opt for treatment when I believe surveillance would have been a rational choice.”
Dr. Cooperberg agreed that a personalized approach is important and that both physicians and patients should be flexible in their decisionmaking. “There will always be some men with low-grade disease who should get immediate treatment. For example, a young man with very high-volume disease, even if it’s Gleason 3+3,” he said. “If it is clearly inevitable that he’s going to need treatment, he could reasonably make a decision to get immediate treatment.”
Dr. Cooperberg, Dr. Klotz, and Dr. Catalona have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to experts who say the dramatic increase reflects a growing understanding among both researchers and patients that low-grade prostate tumors can be safely watched for years without requiring treatment.
Roughly 60% of men eligible for AS chose that approach in 2021, up from 27% in 2014 and less than 10% in 2010, according to panel member Matthew Cooperberg, MD, MPH, of University of California, San Francisco. He presented the data for a panel of the American Urological Association (AUA) at the group’s annual meeting in New Orleans.
Dr. Cooperberg attributed the hike in AS rates in the United States to the growing scientific literature and guidelines supportive of the approach, which calls for periodic assessments of low-risk tumors but no surgery, radiation, or other therapies. In Canada and parts of Europe, approximately 80%-90% of men who are eligible for AS choose that approach, experts said.
Earlier this month, the AUA and the American Society for Radiation Oncology released the strongest guidelines to date supporting AS for low-risk patients, and, for the first time, for select patients with favorable intermediate-risk prostate cancer.
In 2012, the U.S. Preventative Services Task Force (USPSTF) recommended against screening for prostate-specific antigen (PSA), concluding that the benefits of the test did not outweigh the risks, such as overdiagnosis and overtreatment of low-risk prostate cancer.
Urologists blamed the USPSTF policy for a decline in PSA screening and an uptick in the diagnosis of advanced prostate cancer.
Dr. Cooperberg said the shift served as “a bit of a wake-up call for at least a segment of the urology community that if we didn’t fix the overtreatment problem, we would never retake the chunks of the conversation about screening and early detection.”
In 2018, following protests by urologists and patient advocates, the USPSTF revised its statements to include shared decisionmaking for PSA testing in men aged 55-69 years, reflecting emerging evidence of longer-term benefits and widespread adoption of active surveillance after detection of low-risk disease.
Laurence Klotz, MD, the University of Toronto researcher who named and helped develop AS 30 years ago, and who was not on the AUA panel, said other factors also help to explain the growing interest in AS. These include an increasing consensus among experts on the value of the strategy, mounting public awareness of its benefits, the efforts of support and advocacy groups, and the arrival of more sophisticated imaging and biomarkers that help further refine risk.
“We’re shrinking the gray zone,” Dr. Klotz said. “Remaining resistance to AS is due to legitimate concerns about missing significant cancer and losing a patient to metastatic disease, and perhaps financial drivers, particularly with less invasive technologies like radiation and focal therapy.”
The national rate for AS increased from 26.5% in 2014, when data were first reported through the AUA’s AQUA data registry. AQUA’s data comes from electronic health records and included 27,289 patients with newly diagnosed low-risk prostate cancer.
In 2014, radical prostatectomy was the leading treatment in the low-risk population, with 29.7% of these patients overall opting for surgery, edging out external beam radiotherapy (EBRT) and AS, at 28.2% and 26.5% respectively.
In 2015, AS and EBRT overtook surgery, and by 2021, 59.6% of low-risk patients had chosen AS, followed by 20.9% for EBRT and 15.8% for prostatectomy.
Aiming higher
William Catalona, MD, a panel member from Northwestern University Feinberg School of Medicine, Chicago, said the AUA’s Prostate Cancer Active Surveillance Project has set a goal of 80% uptake of AS in patients with low-risk prostate cancer. Dr. Catalona, an early critic of AS, called that figure “optimal and realistic,” something that should happen “as soon as possible.”
Dr. Catalona said the 80% benchmark matches acceptance of AS within the U.S. Department of Veterans Affairs hospitals.
However, Dr. Klotz said the American culture of treatment, which is driven at least in part by financial incentives on the part of physicians, may prevent the growth of AS above 80% in this country.
Dr. Cooperberg said financial incentives are real. “I think it’s a small minority of docs that are heavily driven by the financial incentive, but it certainly exists,” he told this news organization. When you look at the extreme variation of active surveillance rates, there is no question that factors like reimbursement are going to play a role.”
Dr. Catalona, who through the first decade of the 2000s regularly debated Dr. Klotz about the concept of AS, said he today recommends AS when appropriate.
“The variability of AS adoption among practices and physicians varies from 0% to 100%. Therefore, some are too ‘tight’ in recommending AS and some are ‘too loose.’ I do not attempt to steer [patients] into treatment unless I believe that would be their best option. Nevertheless, some opt for surveillance when I believe they are making a mistake, and some opt for treatment when I believe surveillance would have been a rational choice.”
Dr. Cooperberg agreed that a personalized approach is important and that both physicians and patients should be flexible in their decisionmaking. “There will always be some men with low-grade disease who should get immediate treatment. For example, a young man with very high-volume disease, even if it’s Gleason 3+3,” he said. “If it is clearly inevitable that he’s going to need treatment, he could reasonably make a decision to get immediate treatment.”
Dr. Cooperberg, Dr. Klotz, and Dr. Catalona have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AUA ANNUAL MEETING
Abaloparatide works in ‘ignored population’: Men with osteoporosis
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
AT AACE 2022
Colorado law would lift veil of secrecy on sperm donations
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Is it time to remove ‘cancer’ label from low-risk prostate tumors?
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
You want me to tan my WHAT, Tucker Carlson?
Did you hear the one about the TV host suggesting men get their testicles tanned?
The nutty idea dropped into the lexicon last weekend thanks to Fox News commentator Tucker Carlson.
He aired a promo for a show about an alleged decline of manhood. It featured shirtless, muscled men doing macho things like shooting automatic rifles and wrestling, and a naked man rather triumphantly exposing his crotch to a red-light device made to look like some sort of charging station.
The guest also said he’s heard of something he called “bromeopathy” for people who are suspicious of “mainstream” information. Yes, it’s a combination of the slang term “bro” and the practice of homeopathic medicine.
So, men of America, do you really need to start zapping your privates like Mr. Carlson seems to suggest?
Doctors say the answer is simple: Absolutely not.
‘No legitimate evidence’
“There is no legitimate evidence that this type of treatment is effective in improving testosterone levels,” says Petar Bajic MD, a urologist at the Cleveland Clinic who specializes in men’s health and testosterone.
The red light wouldn’t even be able to penetrate the body deep enough to reach the, uhm, targets, he said, citing “no scientific basis” for Mr. Tucker’s claims that we should be “open minded” about this kind of thing.
“It’s not only a waste of time but also a waste of money,” Dr. Bajic says. “There is a large amount of research and high-quality studies” into treating low testosterone, which is produced primarily in the testicles. “We have very effective and proven treatments available, and this is simply not one of them.”
Testosterone is an important hormone that contributes to masculine physical characteristics, “such as muscle mass and strength, and growth of facial and body hair,” according to the Mayo Clinic. It’s important for bone density, sperm production, erectile function, and more.
As men age, testosterone levels often drop, lowering energy and sexual function while causing weight gain and muscle loss.
If men experience some of these symptoms or become curious about their testosterone levels, they shouldn’t self-diagnose or rely on two guys promoting a TV show, Dr. Bajic says.
Instead, they should see their primary care doctor for a simple blood test, he says. Patient and doctor can decide on treatments, which commonly include:
- Topical gels
- Arm patches
- Injections into the muscle of the leg or the fatty tissue of the belly
- Pellets placed under the skin
Diet, exercise, sleep, and other factors play a role.
‘So much misinformation’
The men’s health consumer market is bloated with products promising to raise testosterone levels and help men boost their bedroom performance, among other claims.
But they’re usually based on nothing more than marketing, and erectile disfunction is more commonly caused by reduced blood flow than a lack of testosterone, Dr. Bajic says.
“It all comes down to looking at all of these as a consumer and as a patient ... with a critical eye. There’s always a new ‘cure all’ for whatever your ailment is,” he says.
Testosterone levels change throughout the day. It’s thought to be produced during REM sleep, which can be diminished by alcohol use and other factors.
“All these things are related,” Dr. Bajic said, so there’s no reason to flash a light where it’s usually not seen – especially since neither the safety nor efficacy of testicle tanning has been established.
Oregon urologist Ashley Winter, MD, got into the Twitter fray about Carlson’s comments.
“Also, by definition you CANNOT have data on testicle tanning because you cannot TAN an internal organ,” she said on the social media network. “Tanning your scrotal sack and calling it ‘testicle tanning,’ is like tanning your abdominal skin and calling it ‘liver tanning.’”
What advocates say
What do proponents of red light therapy say? A Men’s Health article claims red light “works to stimulate ATP production, increase energy available to the cell and in particular, increase the activity of the Leydig cells in your testes, which are the cells responsible for testosterone production.”
The article also helpfully points out: “It’s important to note that there are currently no light therapy devices on the market cleared by FDA for the enhanced production of testosterone LED-based therapy.” And many lamps sold for red light therapy can get so hot that they damage the skin.
The author ordered a Joovv device, which Mr. Carlson’s “fitness professional” guest name-dropped. They range from $600 to almost $10,000. He liked the way it felt and said it seemed to improve his sexual performance.
Still a hard sell
Atlanta dermatologist Emily de Golian, MD, says tanning genitalia can be dangerous to the skin.
“There is no such thing as a safe tan, all tanning is indicative of sun damage in the skin and is the body’s effort to shield the DNA from further damage, and tanning increases the risk of skin cancer,” she says. “Scrotal skin is particularly delicate and sensitive to sun exposure, and the risk of sunburn, which further increases the risk of skin cancer, is high.”
Mat Rezaei, founder and CEO of UPGUYS, which provides erectile disfunction medicine, says, “UV light has no negative or positive response to balancing testosterone deficiency.”
Even frequent Fox guest Kid Rock wasn’t buying into the idea.
“Dude, stop! Testicle tanning? Come on,” Mr. Rock said to Mr. Carlson. “I mean, I haven’t heard anything that good in a long time.”
“Open your mind,” said Mr. Carlson as he laughed along with the musician.
Kid Rock replied, “I’m starting a punk rock band and it’s called Testicle Tanning. That’s the end of it.”
A version of this article first appeared on WebMD.com.
Did you hear the one about the TV host suggesting men get their testicles tanned?
The nutty idea dropped into the lexicon last weekend thanks to Fox News commentator Tucker Carlson.
He aired a promo for a show about an alleged decline of manhood. It featured shirtless, muscled men doing macho things like shooting automatic rifles and wrestling, and a naked man rather triumphantly exposing his crotch to a red-light device made to look like some sort of charging station.
The guest also said he’s heard of something he called “bromeopathy” for people who are suspicious of “mainstream” information. Yes, it’s a combination of the slang term “bro” and the practice of homeopathic medicine.
So, men of America, do you really need to start zapping your privates like Mr. Carlson seems to suggest?
Doctors say the answer is simple: Absolutely not.
‘No legitimate evidence’
“There is no legitimate evidence that this type of treatment is effective in improving testosterone levels,” says Petar Bajic MD, a urologist at the Cleveland Clinic who specializes in men’s health and testosterone.
The red light wouldn’t even be able to penetrate the body deep enough to reach the, uhm, targets, he said, citing “no scientific basis” for Mr. Tucker’s claims that we should be “open minded” about this kind of thing.
“It’s not only a waste of time but also a waste of money,” Dr. Bajic says. “There is a large amount of research and high-quality studies” into treating low testosterone, which is produced primarily in the testicles. “We have very effective and proven treatments available, and this is simply not one of them.”
Testosterone is an important hormone that contributes to masculine physical characteristics, “such as muscle mass and strength, and growth of facial and body hair,” according to the Mayo Clinic. It’s important for bone density, sperm production, erectile function, and more.
As men age, testosterone levels often drop, lowering energy and sexual function while causing weight gain and muscle loss.
If men experience some of these symptoms or become curious about their testosterone levels, they shouldn’t self-diagnose or rely on two guys promoting a TV show, Dr. Bajic says.
Instead, they should see their primary care doctor for a simple blood test, he says. Patient and doctor can decide on treatments, which commonly include:
- Topical gels
- Arm patches
- Injections into the muscle of the leg or the fatty tissue of the belly
- Pellets placed under the skin
Diet, exercise, sleep, and other factors play a role.
‘So much misinformation’
The men’s health consumer market is bloated with products promising to raise testosterone levels and help men boost their bedroom performance, among other claims.
But they’re usually based on nothing more than marketing, and erectile disfunction is more commonly caused by reduced blood flow than a lack of testosterone, Dr. Bajic says.
“It all comes down to looking at all of these as a consumer and as a patient ... with a critical eye. There’s always a new ‘cure all’ for whatever your ailment is,” he says.
Testosterone levels change throughout the day. It’s thought to be produced during REM sleep, which can be diminished by alcohol use and other factors.
“All these things are related,” Dr. Bajic said, so there’s no reason to flash a light where it’s usually not seen – especially since neither the safety nor efficacy of testicle tanning has been established.
Oregon urologist Ashley Winter, MD, got into the Twitter fray about Carlson’s comments.
“Also, by definition you CANNOT have data on testicle tanning because you cannot TAN an internal organ,” she said on the social media network. “Tanning your scrotal sack and calling it ‘testicle tanning,’ is like tanning your abdominal skin and calling it ‘liver tanning.’”
What advocates say
What do proponents of red light therapy say? A Men’s Health article claims red light “works to stimulate ATP production, increase energy available to the cell and in particular, increase the activity of the Leydig cells in your testes, which are the cells responsible for testosterone production.”
The article also helpfully points out: “It’s important to note that there are currently no light therapy devices on the market cleared by FDA for the enhanced production of testosterone LED-based therapy.” And many lamps sold for red light therapy can get so hot that they damage the skin.
The author ordered a Joovv device, which Mr. Carlson’s “fitness professional” guest name-dropped. They range from $600 to almost $10,000. He liked the way it felt and said it seemed to improve his sexual performance.
Still a hard sell
Atlanta dermatologist Emily de Golian, MD, says tanning genitalia can be dangerous to the skin.
“There is no such thing as a safe tan, all tanning is indicative of sun damage in the skin and is the body’s effort to shield the DNA from further damage, and tanning increases the risk of skin cancer,” she says. “Scrotal skin is particularly delicate and sensitive to sun exposure, and the risk of sunburn, which further increases the risk of skin cancer, is high.”
Mat Rezaei, founder and CEO of UPGUYS, which provides erectile disfunction medicine, says, “UV light has no negative or positive response to balancing testosterone deficiency.”
Even frequent Fox guest Kid Rock wasn’t buying into the idea.
“Dude, stop! Testicle tanning? Come on,” Mr. Rock said to Mr. Carlson. “I mean, I haven’t heard anything that good in a long time.”
“Open your mind,” said Mr. Carlson as he laughed along with the musician.
Kid Rock replied, “I’m starting a punk rock band and it’s called Testicle Tanning. That’s the end of it.”
A version of this article first appeared on WebMD.com.
Did you hear the one about the TV host suggesting men get their testicles tanned?
The nutty idea dropped into the lexicon last weekend thanks to Fox News commentator Tucker Carlson.
He aired a promo for a show about an alleged decline of manhood. It featured shirtless, muscled men doing macho things like shooting automatic rifles and wrestling, and a naked man rather triumphantly exposing his crotch to a red-light device made to look like some sort of charging station.
The guest also said he’s heard of something he called “bromeopathy” for people who are suspicious of “mainstream” information. Yes, it’s a combination of the slang term “bro” and the practice of homeopathic medicine.
So, men of America, do you really need to start zapping your privates like Mr. Carlson seems to suggest?
Doctors say the answer is simple: Absolutely not.
‘No legitimate evidence’
“There is no legitimate evidence that this type of treatment is effective in improving testosterone levels,” says Petar Bajic MD, a urologist at the Cleveland Clinic who specializes in men’s health and testosterone.
The red light wouldn’t even be able to penetrate the body deep enough to reach the, uhm, targets, he said, citing “no scientific basis” for Mr. Tucker’s claims that we should be “open minded” about this kind of thing.
“It’s not only a waste of time but also a waste of money,” Dr. Bajic says. “There is a large amount of research and high-quality studies” into treating low testosterone, which is produced primarily in the testicles. “We have very effective and proven treatments available, and this is simply not one of them.”
Testosterone is an important hormone that contributes to masculine physical characteristics, “such as muscle mass and strength, and growth of facial and body hair,” according to the Mayo Clinic. It’s important for bone density, sperm production, erectile function, and more.
As men age, testosterone levels often drop, lowering energy and sexual function while causing weight gain and muscle loss.
If men experience some of these symptoms or become curious about their testosterone levels, they shouldn’t self-diagnose or rely on two guys promoting a TV show, Dr. Bajic says.
Instead, they should see their primary care doctor for a simple blood test, he says. Patient and doctor can decide on treatments, which commonly include:
- Topical gels
- Arm patches
- Injections into the muscle of the leg or the fatty tissue of the belly
- Pellets placed under the skin
Diet, exercise, sleep, and other factors play a role.
‘So much misinformation’
The men’s health consumer market is bloated with products promising to raise testosterone levels and help men boost their bedroom performance, among other claims.
But they’re usually based on nothing more than marketing, and erectile disfunction is more commonly caused by reduced blood flow than a lack of testosterone, Dr. Bajic says.
“It all comes down to looking at all of these as a consumer and as a patient ... with a critical eye. There’s always a new ‘cure all’ for whatever your ailment is,” he says.
Testosterone levels change throughout the day. It’s thought to be produced during REM sleep, which can be diminished by alcohol use and other factors.
“All these things are related,” Dr. Bajic said, so there’s no reason to flash a light where it’s usually not seen – especially since neither the safety nor efficacy of testicle tanning has been established.
Oregon urologist Ashley Winter, MD, got into the Twitter fray about Carlson’s comments.
“Also, by definition you CANNOT have data on testicle tanning because you cannot TAN an internal organ,” she said on the social media network. “Tanning your scrotal sack and calling it ‘testicle tanning,’ is like tanning your abdominal skin and calling it ‘liver tanning.’”
What advocates say
What do proponents of red light therapy say? A Men’s Health article claims red light “works to stimulate ATP production, increase energy available to the cell and in particular, increase the activity of the Leydig cells in your testes, which are the cells responsible for testosterone production.”
The article also helpfully points out: “It’s important to note that there are currently no light therapy devices on the market cleared by FDA for the enhanced production of testosterone LED-based therapy.” And many lamps sold for red light therapy can get so hot that they damage the skin.
The author ordered a Joovv device, which Mr. Carlson’s “fitness professional” guest name-dropped. They range from $600 to almost $10,000. He liked the way it felt and said it seemed to improve his sexual performance.
Still a hard sell
Atlanta dermatologist Emily de Golian, MD, says tanning genitalia can be dangerous to the skin.
“There is no such thing as a safe tan, all tanning is indicative of sun damage in the skin and is the body’s effort to shield the DNA from further damage, and tanning increases the risk of skin cancer,” she says. “Scrotal skin is particularly delicate and sensitive to sun exposure, and the risk of sunburn, which further increases the risk of skin cancer, is high.”
Mat Rezaei, founder and CEO of UPGUYS, which provides erectile disfunction medicine, says, “UV light has no negative or positive response to balancing testosterone deficiency.”
Even frequent Fox guest Kid Rock wasn’t buying into the idea.
“Dude, stop! Testicle tanning? Come on,” Mr. Rock said to Mr. Carlson. “I mean, I haven’t heard anything that good in a long time.”
“Open your mind,” said Mr. Carlson as he laughed along with the musician.
Kid Rock replied, “I’m starting a punk rock band and it’s called Testicle Tanning. That’s the end of it.”
A version of this article first appeared on WebMD.com.
Real-world data suggest coprescribing PDE5 inhibitors and nitrates may be safe
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
FROM ANNALS OF INTERNAL MEDICINE
Erectile dysfunction drugs linked to ocular conditions
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).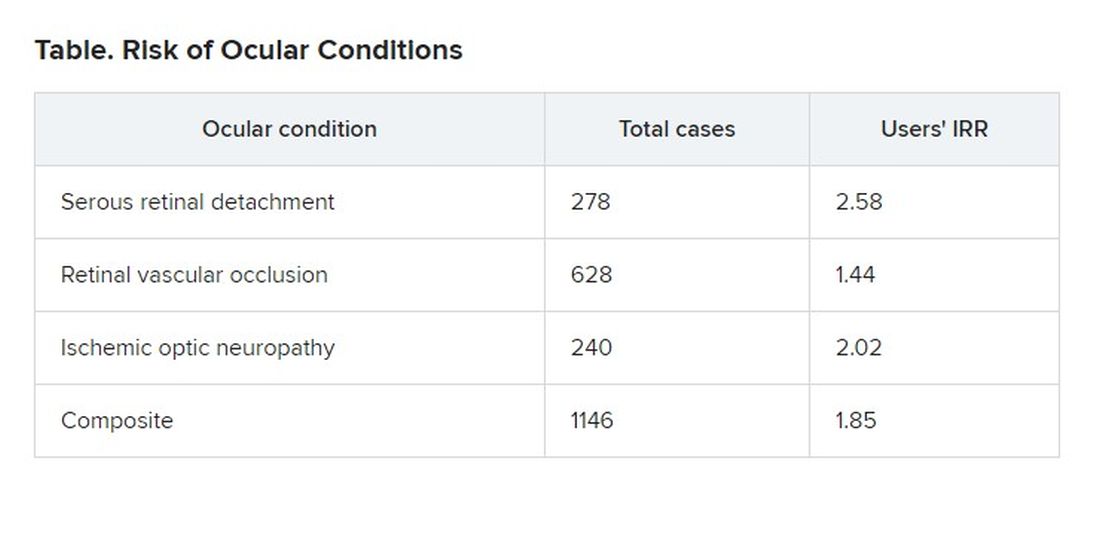
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
Sex differences in COPD slow to be recognized, treated
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.