User login
For MD-IQ use only
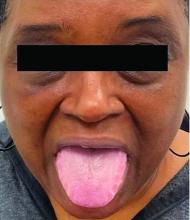
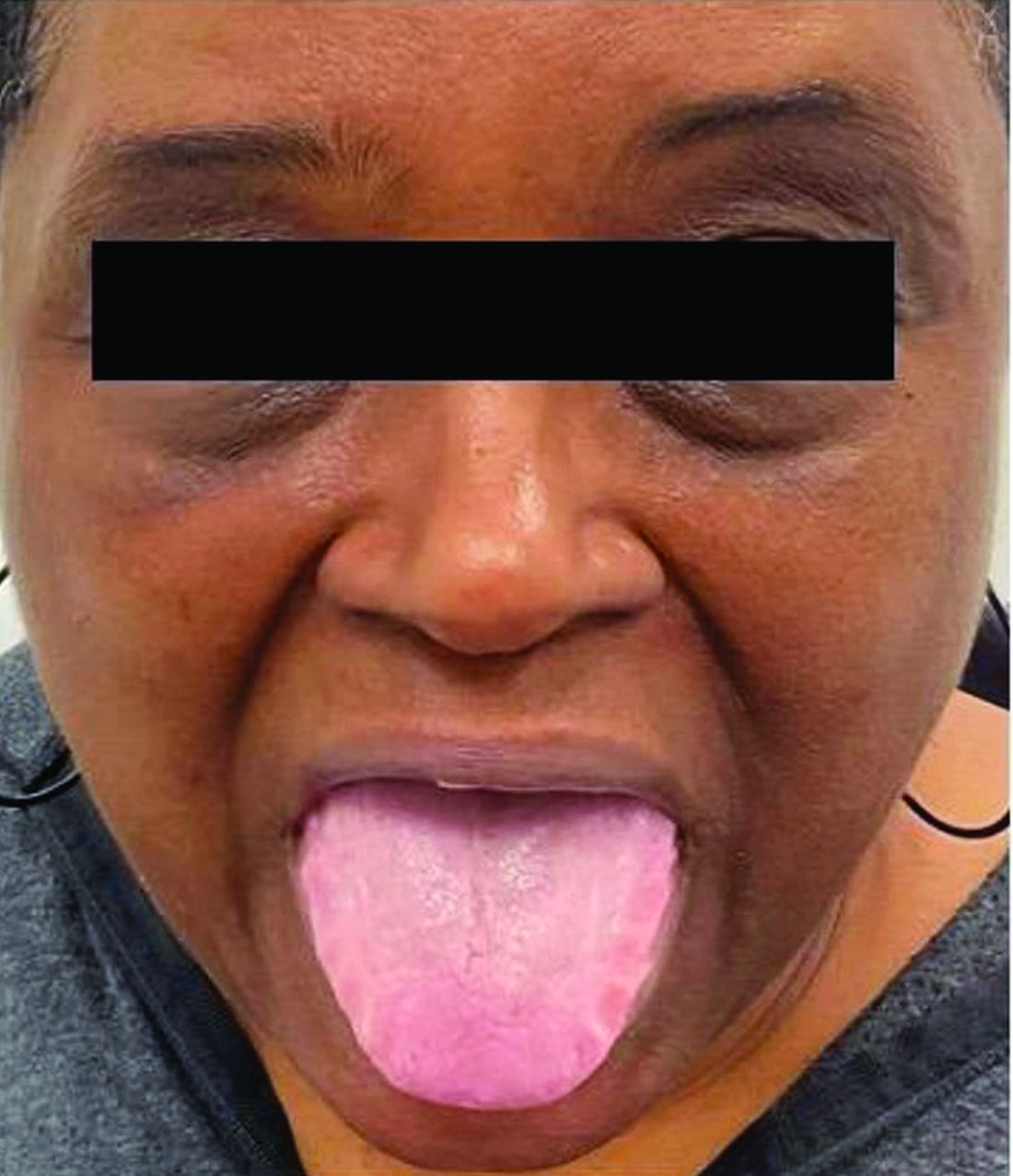
A 62-year-old Black female presented with an epidermal inclusion cyst on her left upper back
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Diagnosing, Treating Rashes In Patients on Immune Checkpoint Inhibitors
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Jeffrey Weber, MD, PhD, Giant of Cancer Care, Dies
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Applications for the CUTIS 2025 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Is There a Role for GLP-1s in Neurology and Psychiatry?
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Regularly Drinking Alcohol After Age 60 Linked to Early Death
That’s according to the findings of a new, large study that was published in JAMA Network Openand build upon numerous other recent studies concluding that any amount of alcohol consumption is linked to significant health risks. That’s a change from decades of public health messaging suggesting that moderate alcohol intake (one or two drinks per day) wasn’t dangerous. Recently, experts have uncovered flaws in how researchers came to those earlier conclusions.
In this latest study, researchers in Spain analyzed health data for more than 135,000 people, all of whom were at least 60 years old, lived in the United Kingdom, and provided their health information to the UK Biobank database. The average age of people at the start of the analysis period was 64.
The researchers compared 12 years of health outcomes for occasional drinkers with those who averaged drinking at least some alcohol on a daily basis. The greatest health risks were seen between occasional drinkers and those whom the researchers labeled “high risk.” Occasional drinkers had less than about two drinks per week. The high-risk group included men who averaged nearly three drinks per day or more, and women who averaged about a drink and a half per day or more. The analysis showed that, compared with occasional drinking, high-risk drinking was linked to a 33% increased risk of early death, a 39% increased risk of dying from cancer, and a 21% increased risk of dying from problems with the heart and blood vessels.
More moderate drinking habits were also linked to an increased risk of early death and dying from cancer, and even just averaging about one drink or less daily was associated with an 11% higher risk of dying from cancer. Low and moderate drinkers were most at risk if they also had health problems or experienced socioeconomic factors like living in less affluent neighborhoods.
The findings also suggested the potential that mostly drinking wine, or drinking mostly with meals, may be lower risk, but the researchers called for further study on those topics since “it may mostly reflect the effect of healthier lifestyles, slower alcohol absorption, or nonalcoholic components of beverages.”
A recent Gallup poll showed that overall, Americans’ attitudes toward the health impacts of alcohol are changing, with 65% of young adults (ages 18-34) saying that drinking can have negative health effects. But just 39% of adults age 55 or older agreed that drinking is bad for a person’s health. The gap in perspectives between younger and older adults about drinking is the largest on record, Gallup reported.
The study investigators reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
That’s according to the findings of a new, large study that was published in JAMA Network Openand build upon numerous other recent studies concluding that any amount of alcohol consumption is linked to significant health risks. That’s a change from decades of public health messaging suggesting that moderate alcohol intake (one or two drinks per day) wasn’t dangerous. Recently, experts have uncovered flaws in how researchers came to those earlier conclusions.
In this latest study, researchers in Spain analyzed health data for more than 135,000 people, all of whom were at least 60 years old, lived in the United Kingdom, and provided their health information to the UK Biobank database. The average age of people at the start of the analysis period was 64.
The researchers compared 12 years of health outcomes for occasional drinkers with those who averaged drinking at least some alcohol on a daily basis. The greatest health risks were seen between occasional drinkers and those whom the researchers labeled “high risk.” Occasional drinkers had less than about two drinks per week. The high-risk group included men who averaged nearly three drinks per day or more, and women who averaged about a drink and a half per day or more. The analysis showed that, compared with occasional drinking, high-risk drinking was linked to a 33% increased risk of early death, a 39% increased risk of dying from cancer, and a 21% increased risk of dying from problems with the heart and blood vessels.
More moderate drinking habits were also linked to an increased risk of early death and dying from cancer, and even just averaging about one drink or less daily was associated with an 11% higher risk of dying from cancer. Low and moderate drinkers were most at risk if they also had health problems or experienced socioeconomic factors like living in less affluent neighborhoods.
The findings also suggested the potential that mostly drinking wine, or drinking mostly with meals, may be lower risk, but the researchers called for further study on those topics since “it may mostly reflect the effect of healthier lifestyles, slower alcohol absorption, or nonalcoholic components of beverages.”
A recent Gallup poll showed that overall, Americans’ attitudes toward the health impacts of alcohol are changing, with 65% of young adults (ages 18-34) saying that drinking can have negative health effects. But just 39% of adults age 55 or older agreed that drinking is bad for a person’s health. The gap in perspectives between younger and older adults about drinking is the largest on record, Gallup reported.
The study investigators reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
That’s according to the findings of a new, large study that was published in JAMA Network Openand build upon numerous other recent studies concluding that any amount of alcohol consumption is linked to significant health risks. That’s a change from decades of public health messaging suggesting that moderate alcohol intake (one or two drinks per day) wasn’t dangerous. Recently, experts have uncovered flaws in how researchers came to those earlier conclusions.
In this latest study, researchers in Spain analyzed health data for more than 135,000 people, all of whom were at least 60 years old, lived in the United Kingdom, and provided their health information to the UK Biobank database. The average age of people at the start of the analysis period was 64.
The researchers compared 12 years of health outcomes for occasional drinkers with those who averaged drinking at least some alcohol on a daily basis. The greatest health risks were seen between occasional drinkers and those whom the researchers labeled “high risk.” Occasional drinkers had less than about two drinks per week. The high-risk group included men who averaged nearly three drinks per day or more, and women who averaged about a drink and a half per day or more. The analysis showed that, compared with occasional drinking, high-risk drinking was linked to a 33% increased risk of early death, a 39% increased risk of dying from cancer, and a 21% increased risk of dying from problems with the heart and blood vessels.
More moderate drinking habits were also linked to an increased risk of early death and dying from cancer, and even just averaging about one drink or less daily was associated with an 11% higher risk of dying from cancer. Low and moderate drinkers were most at risk if they also had health problems or experienced socioeconomic factors like living in less affluent neighborhoods.
The findings also suggested the potential that mostly drinking wine, or drinking mostly with meals, may be lower risk, but the researchers called for further study on those topics since “it may mostly reflect the effect of healthier lifestyles, slower alcohol absorption, or nonalcoholic components of beverages.”
A recent Gallup poll showed that overall, Americans’ attitudes toward the health impacts of alcohol are changing, with 65% of young adults (ages 18-34) saying that drinking can have negative health effects. But just 39% of adults age 55 or older agreed that drinking is bad for a person’s health. The gap in perspectives between younger and older adults about drinking is the largest on record, Gallup reported.
The study investigators reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Federal Health Care Data Trends 2024
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Study Identifies Oral Antibiotics Linked to Severe Cutaneous Reactions
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA
Association Between Pruritus and Fibromyalgia: Results of a Population-Based, Cross-Sectional Study
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.
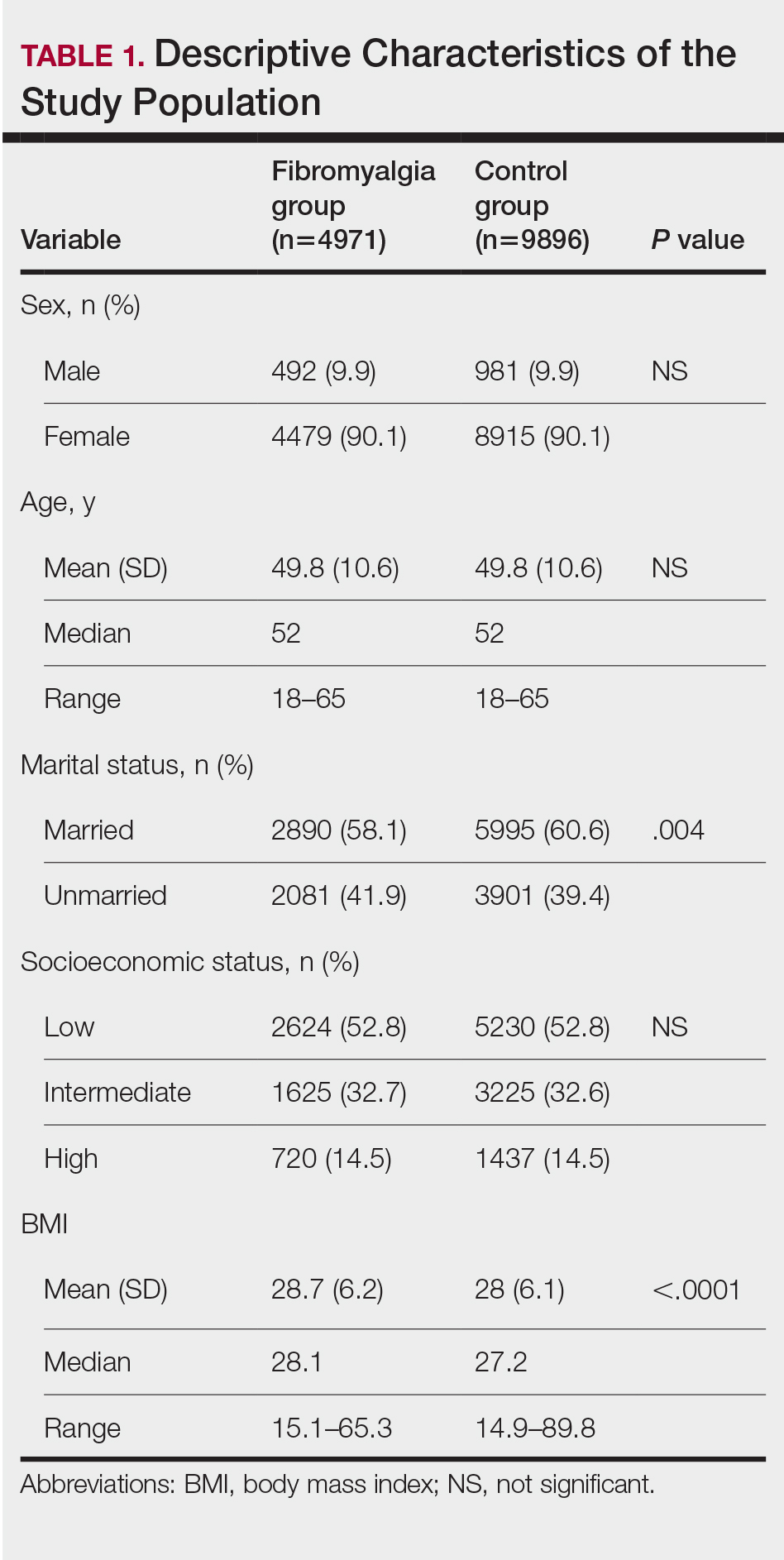
We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).
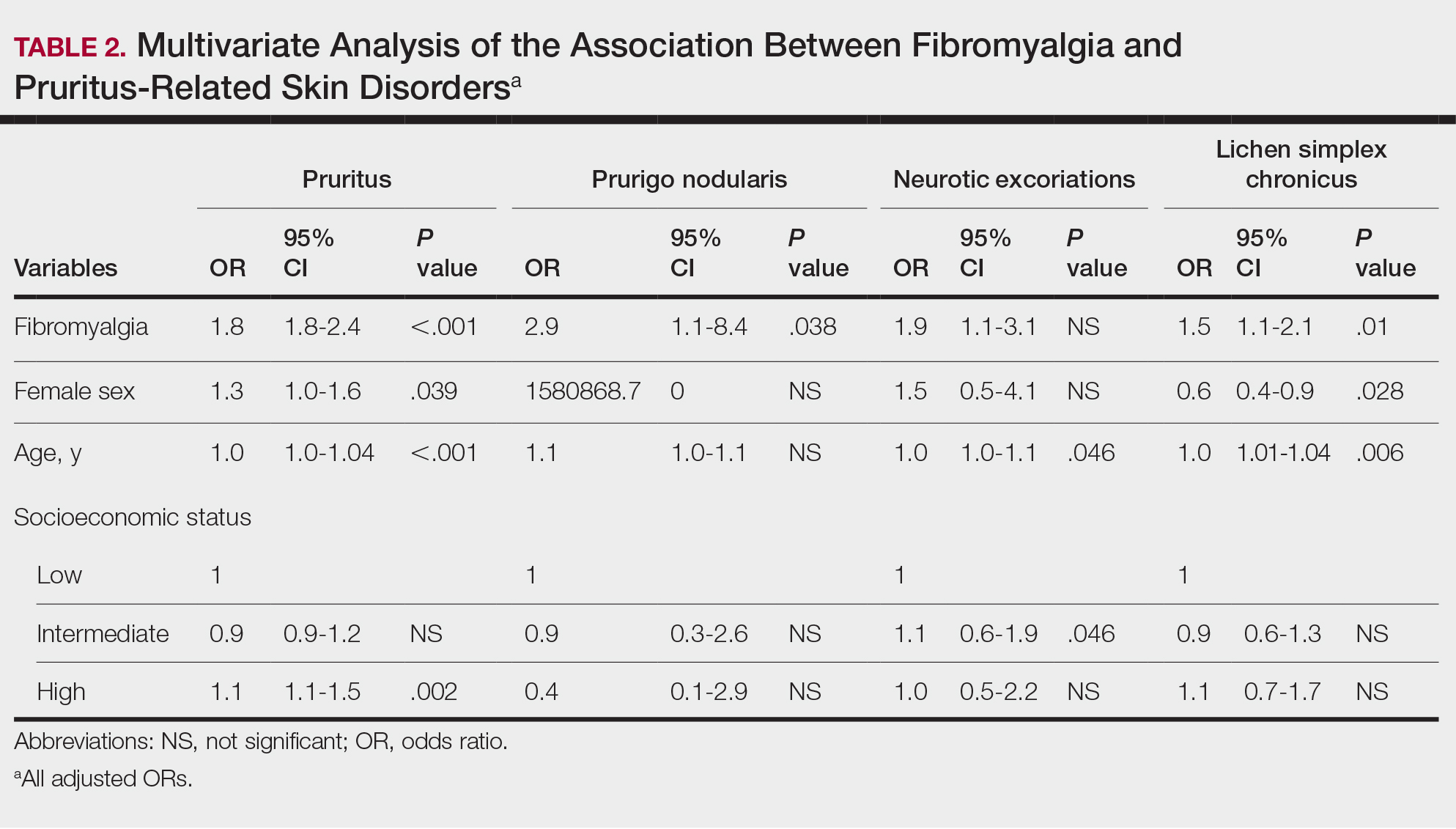
Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).
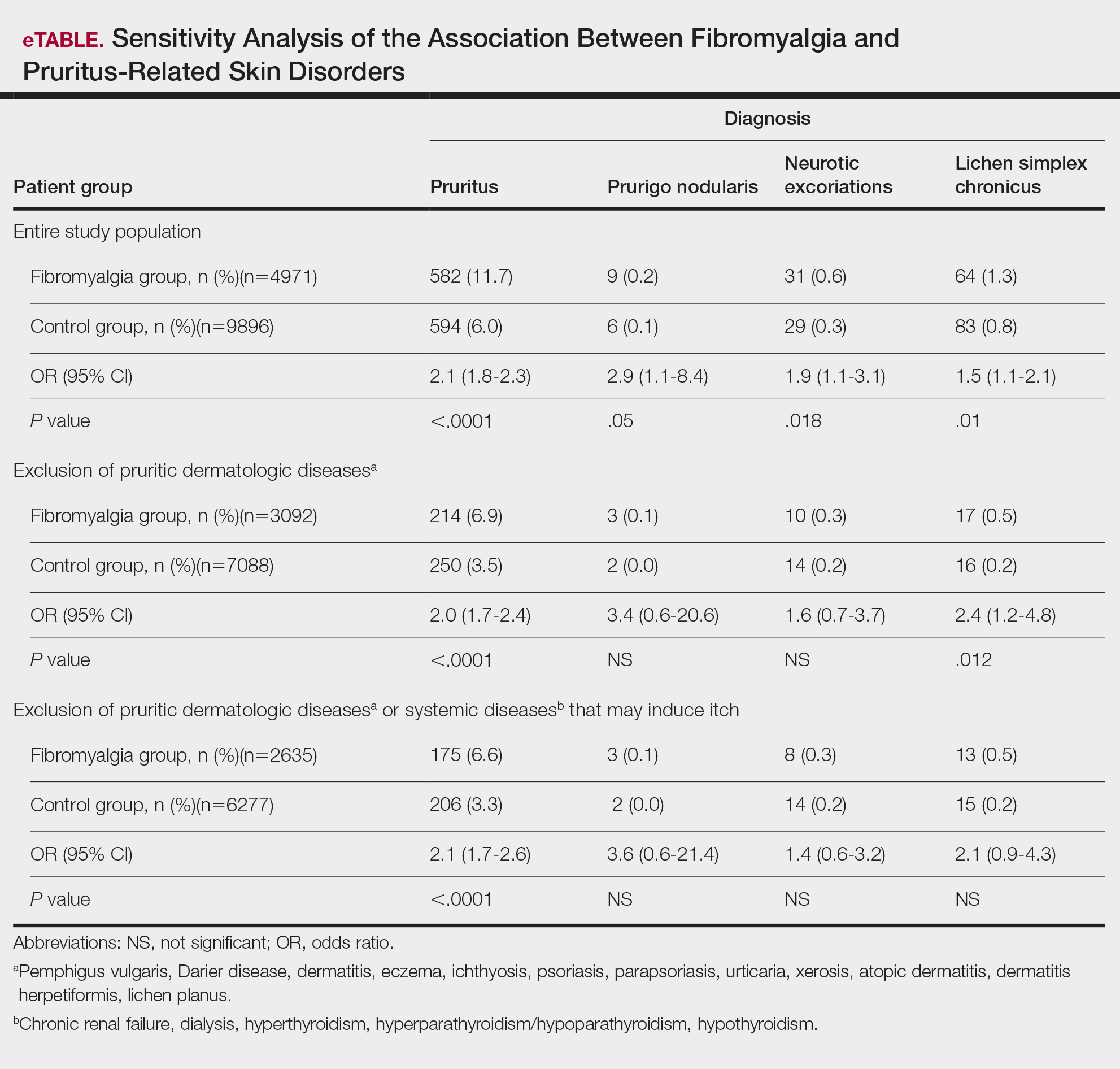
Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Practice Points
- Dermatologists should be aware of the connection between fibromyalgia, pruritus, and related conditions to improve patient care.
- The association between fibromyalgia and pruritus underscores the importance of employing multidisciplinary treatment strategies for managing these conditions.