User login
For MD-IQ use only
Neurofibromatosis: What Affects Quality of Life Most?
TOPLINE:
Mobile images may be reliable for assessing cutaneous neurofibroma (cNF) features in patients with neurofibromatosis type 1 (NF1), according to a crowd-sourced .
METHODOLOGY:
- To learn more about the association of cNFs with QoL, pain, and itch in patients with this rare disease, researchers enrolled 1016 individuals aged 40 years and older with NF1 who had at least one cNF, from May 2021 to December 2023, after reaching out to patient-led or NF1 advocacy organizations in 13 countries, including the United States.
- Participants provided demographic data, detailed photographs, and saliva samples for genetic sequencing, with 583 participants (mean age, 51.7 years; 65.9% women) submitting high-quality photographs from seven body regions at the time of the study analysis.
- A subset of 50 participants also underwent whole-body imaging.
- Four researchers independently rated the photographs for various cNF features, including general severity, number, size, facial severity, and subtypes.
TAKEAWAY:
- Based on evaluations by NF1 specialists, the agreement between mobile and whole-body images was “substantial” (74%-88% agreement) for the number of cNFs, general severity, and facial severity. Agreement between self-reported numbers of cNFs and investigator-rated numbers based on photographs was “minimal to fair.”
- Female sex, the number of cNFs, severity of cNFs on the face, and globular cNFs were associated with worse QoL (based on Skindex scores); severity of cNFs on the face had the strongest impact on overall QoL (P < .001).
- An increasing number of cNFs and worsening facial severity were strongly correlated with higher emotion subdomain scores.
- A higher number of cNFs, more severe cNFs on the face, and larger cNFs were all slightly associated with increased itch and pain (P < .01).
IN PRACTICE:
“To develop effective therapeutics, meaningful clinical outcomes that are tied with improvement in QoL for persons with NF1 must be clearly defined,” the authors wrote. The results of this study, they added, “suggested the benefit of this crowd-sourced resource by identifying the features of cNFs with the greatest association with QoL and symptoms of pain and itch in persons with NF1, highlighting new intervention strategies and features to target to most improve QoL in NF1.”
SOURCE:
The study was led by Michelle Jade Lin, BS, Stanford University School of Medicine, Redwood City, California, and was published online in JAMA Dermatology.
LIMITATIONS:
The study included only a small number of individuals from racial and ethnic minority groups and did not capture ethnicity information, which could have provided further insights into disease impact across different demographics.
DISCLOSURES:
This study was supported by Johns Hopkins University, Baltimore, and the Bloomberg Family Foundation. Ms. Lin reported support from the Stanford Medical Scholars Research Program. Three authors reported personal fees or grants outside this work. Other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Mobile images may be reliable for assessing cutaneous neurofibroma (cNF) features in patients with neurofibromatosis type 1 (NF1), according to a crowd-sourced .
METHODOLOGY:
- To learn more about the association of cNFs with QoL, pain, and itch in patients with this rare disease, researchers enrolled 1016 individuals aged 40 years and older with NF1 who had at least one cNF, from May 2021 to December 2023, after reaching out to patient-led or NF1 advocacy organizations in 13 countries, including the United States.
- Participants provided demographic data, detailed photographs, and saliva samples for genetic sequencing, with 583 participants (mean age, 51.7 years; 65.9% women) submitting high-quality photographs from seven body regions at the time of the study analysis.
- A subset of 50 participants also underwent whole-body imaging.
- Four researchers independently rated the photographs for various cNF features, including general severity, number, size, facial severity, and subtypes.
TAKEAWAY:
- Based on evaluations by NF1 specialists, the agreement between mobile and whole-body images was “substantial” (74%-88% agreement) for the number of cNFs, general severity, and facial severity. Agreement between self-reported numbers of cNFs and investigator-rated numbers based on photographs was “minimal to fair.”
- Female sex, the number of cNFs, severity of cNFs on the face, and globular cNFs were associated with worse QoL (based on Skindex scores); severity of cNFs on the face had the strongest impact on overall QoL (P < .001).
- An increasing number of cNFs and worsening facial severity were strongly correlated with higher emotion subdomain scores.
- A higher number of cNFs, more severe cNFs on the face, and larger cNFs were all slightly associated with increased itch and pain (P < .01).
IN PRACTICE:
“To develop effective therapeutics, meaningful clinical outcomes that are tied with improvement in QoL for persons with NF1 must be clearly defined,” the authors wrote. The results of this study, they added, “suggested the benefit of this crowd-sourced resource by identifying the features of cNFs with the greatest association with QoL and symptoms of pain and itch in persons with NF1, highlighting new intervention strategies and features to target to most improve QoL in NF1.”
SOURCE:
The study was led by Michelle Jade Lin, BS, Stanford University School of Medicine, Redwood City, California, and was published online in JAMA Dermatology.
LIMITATIONS:
The study included only a small number of individuals from racial and ethnic minority groups and did not capture ethnicity information, which could have provided further insights into disease impact across different demographics.
DISCLOSURES:
This study was supported by Johns Hopkins University, Baltimore, and the Bloomberg Family Foundation. Ms. Lin reported support from the Stanford Medical Scholars Research Program. Three authors reported personal fees or grants outside this work. Other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Mobile images may be reliable for assessing cutaneous neurofibroma (cNF) features in patients with neurofibromatosis type 1 (NF1), according to a crowd-sourced .
METHODOLOGY:
- To learn more about the association of cNFs with QoL, pain, and itch in patients with this rare disease, researchers enrolled 1016 individuals aged 40 years and older with NF1 who had at least one cNF, from May 2021 to December 2023, after reaching out to patient-led or NF1 advocacy organizations in 13 countries, including the United States.
- Participants provided demographic data, detailed photographs, and saliva samples for genetic sequencing, with 583 participants (mean age, 51.7 years; 65.9% women) submitting high-quality photographs from seven body regions at the time of the study analysis.
- A subset of 50 participants also underwent whole-body imaging.
- Four researchers independently rated the photographs for various cNF features, including general severity, number, size, facial severity, and subtypes.
TAKEAWAY:
- Based on evaluations by NF1 specialists, the agreement between mobile and whole-body images was “substantial” (74%-88% agreement) for the number of cNFs, general severity, and facial severity. Agreement between self-reported numbers of cNFs and investigator-rated numbers based on photographs was “minimal to fair.”
- Female sex, the number of cNFs, severity of cNFs on the face, and globular cNFs were associated with worse QoL (based on Skindex scores); severity of cNFs on the face had the strongest impact on overall QoL (P < .001).
- An increasing number of cNFs and worsening facial severity were strongly correlated with higher emotion subdomain scores.
- A higher number of cNFs, more severe cNFs on the face, and larger cNFs were all slightly associated with increased itch and pain (P < .01).
IN PRACTICE:
“To develop effective therapeutics, meaningful clinical outcomes that are tied with improvement in QoL for persons with NF1 must be clearly defined,” the authors wrote. The results of this study, they added, “suggested the benefit of this crowd-sourced resource by identifying the features of cNFs with the greatest association with QoL and symptoms of pain and itch in persons with NF1, highlighting new intervention strategies and features to target to most improve QoL in NF1.”
SOURCE:
The study was led by Michelle Jade Lin, BS, Stanford University School of Medicine, Redwood City, California, and was published online in JAMA Dermatology.
LIMITATIONS:
The study included only a small number of individuals from racial and ethnic minority groups and did not capture ethnicity information, which could have provided further insights into disease impact across different demographics.
DISCLOSURES:
This study was supported by Johns Hopkins University, Baltimore, and the Bloomberg Family Foundation. Ms. Lin reported support from the Stanford Medical Scholars Research Program. Three authors reported personal fees or grants outside this work. Other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A New Era of Obesity Medicine
Obesity has now reached epidemic proportions, with global prevalence of the condition increasing more than threefold between 1975 and 2022. In the United States alone, roughly two in five adults have obesity. As healthcare providers are intimately aware, obesity is linked to many serious health conditions, including type 2 diabetes, cardiovascular disease, and metabolic-associated steatotic liver disease, as well as some forms of cancer. As such, it presents a major challenge to chronic disease prevention and overall health.
For many years, management of obesity was considered within the purview of primary care as part of chronic disease management. However, as obesity has become more common, our understanding of the underlying causes of obesity has improved, and optimal strategies to manage and treat obesity have evolved. From glucagon-like peptide 1 agonists to an expanding armamentarium of bariatric procedures, emerging therapeutics have revolutionized treatment of patients with obesity and related health conditions.
In this month’s Member Spotlight, we introduce you to gastroenterologist Dr. Janese Laster, who has built a successful career with a primary focus on obesity medicine. She shares her passionate perspective on why gastroenterologists should play a more prominent role in management of this complex, chronic disease. We also include a summary of obesity-related content presented as part of this spring’s AGA Post-Graduate Course, with helpful clinical pearls from experts Dr. Andres Acosta, Dr. Violeta Popov, Dr. Sonali Paul, and Dr. Pooja Singhal.
Also in our September issue, we highlight a recent, practice-changing randomized controlled trial from Clinical Gastroenterology and Hepatology supporting use of snare tip soft coagulation as the preferred thermal margin treatment to reduce recurrence rates following colorectal endoscopic mucosal resection. In our quarterly Perspectives column, Dr. Maggie Ham and Dr. Petr Protiva offer their insights into a pressing question on many of our minds — whether to take the 10-year “high-stakes” exam or opt for the Longitudinal Knowledge Assessment to maintain American Board of Internal Medicine certification. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Obesity has now reached epidemic proportions, with global prevalence of the condition increasing more than threefold between 1975 and 2022. In the United States alone, roughly two in five adults have obesity. As healthcare providers are intimately aware, obesity is linked to many serious health conditions, including type 2 diabetes, cardiovascular disease, and metabolic-associated steatotic liver disease, as well as some forms of cancer. As such, it presents a major challenge to chronic disease prevention and overall health.
For many years, management of obesity was considered within the purview of primary care as part of chronic disease management. However, as obesity has become more common, our understanding of the underlying causes of obesity has improved, and optimal strategies to manage and treat obesity have evolved. From glucagon-like peptide 1 agonists to an expanding armamentarium of bariatric procedures, emerging therapeutics have revolutionized treatment of patients with obesity and related health conditions.
In this month’s Member Spotlight, we introduce you to gastroenterologist Dr. Janese Laster, who has built a successful career with a primary focus on obesity medicine. She shares her passionate perspective on why gastroenterologists should play a more prominent role in management of this complex, chronic disease. We also include a summary of obesity-related content presented as part of this spring’s AGA Post-Graduate Course, with helpful clinical pearls from experts Dr. Andres Acosta, Dr. Violeta Popov, Dr. Sonali Paul, and Dr. Pooja Singhal.
Also in our September issue, we highlight a recent, practice-changing randomized controlled trial from Clinical Gastroenterology and Hepatology supporting use of snare tip soft coagulation as the preferred thermal margin treatment to reduce recurrence rates following colorectal endoscopic mucosal resection. In our quarterly Perspectives column, Dr. Maggie Ham and Dr. Petr Protiva offer their insights into a pressing question on many of our minds — whether to take the 10-year “high-stakes” exam or opt for the Longitudinal Knowledge Assessment to maintain American Board of Internal Medicine certification. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Obesity has now reached epidemic proportions, with global prevalence of the condition increasing more than threefold between 1975 and 2022. In the United States alone, roughly two in five adults have obesity. As healthcare providers are intimately aware, obesity is linked to many serious health conditions, including type 2 diabetes, cardiovascular disease, and metabolic-associated steatotic liver disease, as well as some forms of cancer. As such, it presents a major challenge to chronic disease prevention and overall health.
For many years, management of obesity was considered within the purview of primary care as part of chronic disease management. However, as obesity has become more common, our understanding of the underlying causes of obesity has improved, and optimal strategies to manage and treat obesity have evolved. From glucagon-like peptide 1 agonists to an expanding armamentarium of bariatric procedures, emerging therapeutics have revolutionized treatment of patients with obesity and related health conditions.
In this month’s Member Spotlight, we introduce you to gastroenterologist Dr. Janese Laster, who has built a successful career with a primary focus on obesity medicine. She shares her passionate perspective on why gastroenterologists should play a more prominent role in management of this complex, chronic disease. We also include a summary of obesity-related content presented as part of this spring’s AGA Post-Graduate Course, with helpful clinical pearls from experts Dr. Andres Acosta, Dr. Violeta Popov, Dr. Sonali Paul, and Dr. Pooja Singhal.
Also in our September issue, we highlight a recent, practice-changing randomized controlled trial from Clinical Gastroenterology and Hepatology supporting use of snare tip soft coagulation as the preferred thermal margin treatment to reduce recurrence rates following colorectal endoscopic mucosal resection. In our quarterly Perspectives column, Dr. Maggie Ham and Dr. Petr Protiva offer their insights into a pressing question on many of our minds — whether to take the 10-year “high-stakes” exam or opt for the Longitudinal Knowledge Assessment to maintain American Board of Internal Medicine certification. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
SBRT vs Surgery in CRC Lung Metastases: Which Is Better?
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cancer Cases, Deaths in Men Predicted to Surge by 2050
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Prurigo Nodularis Mechanisms and Current Management Options
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26
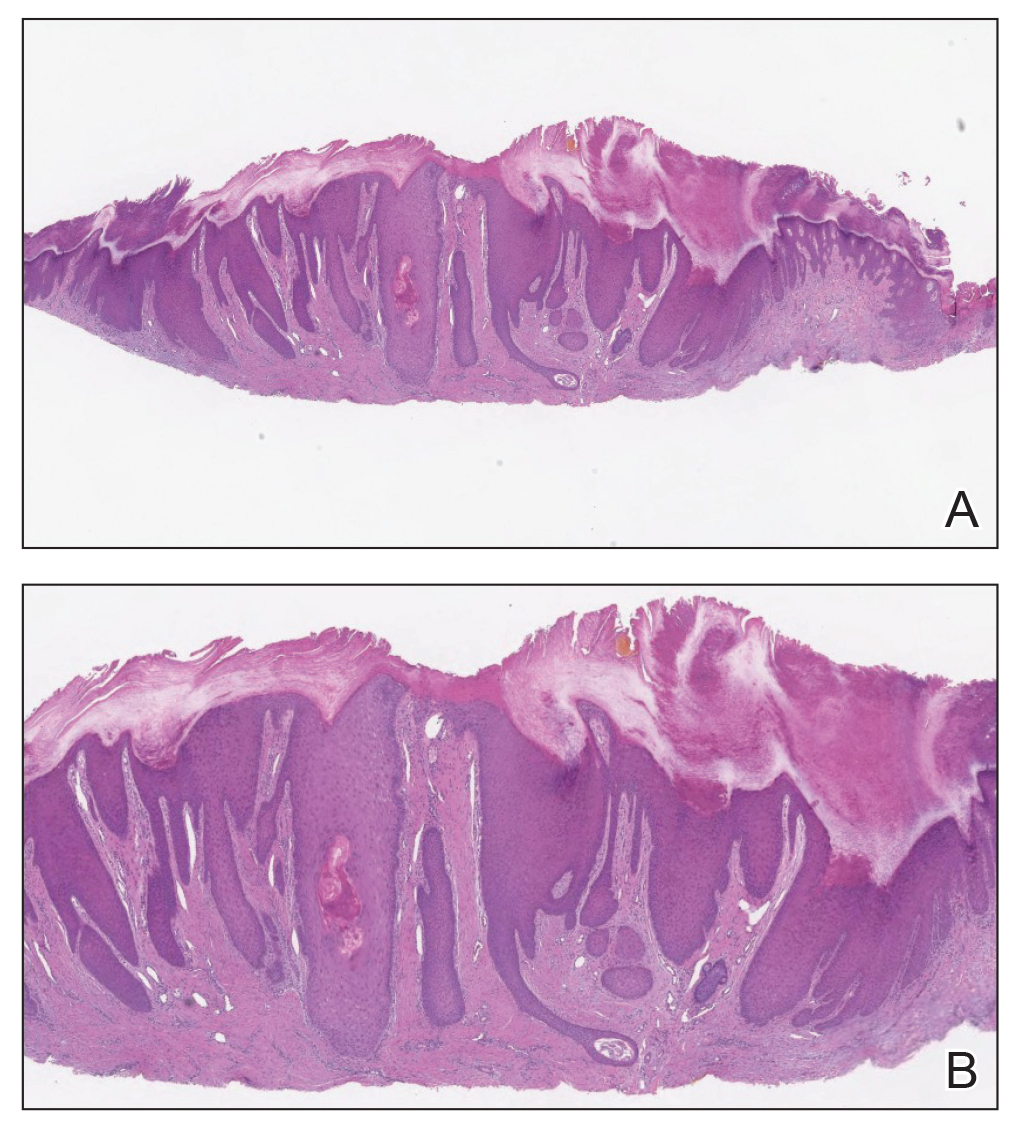
Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31
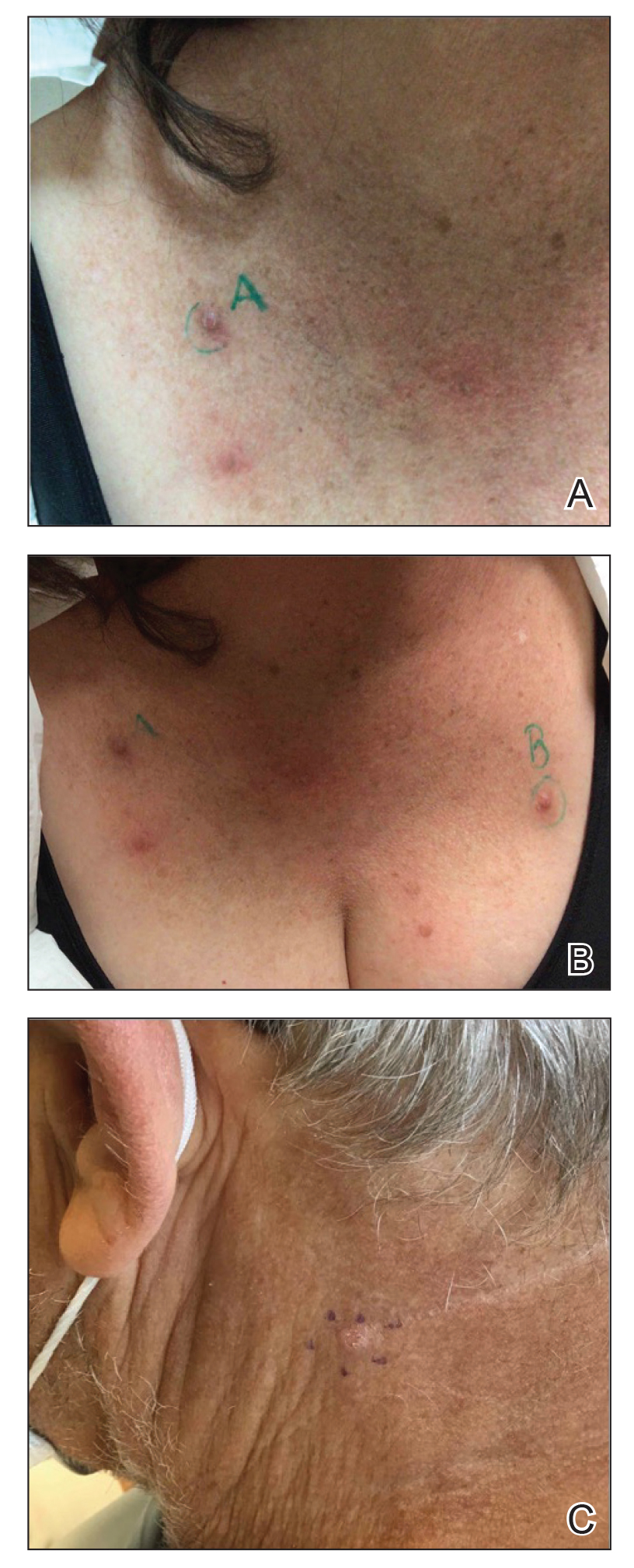
Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43
Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53
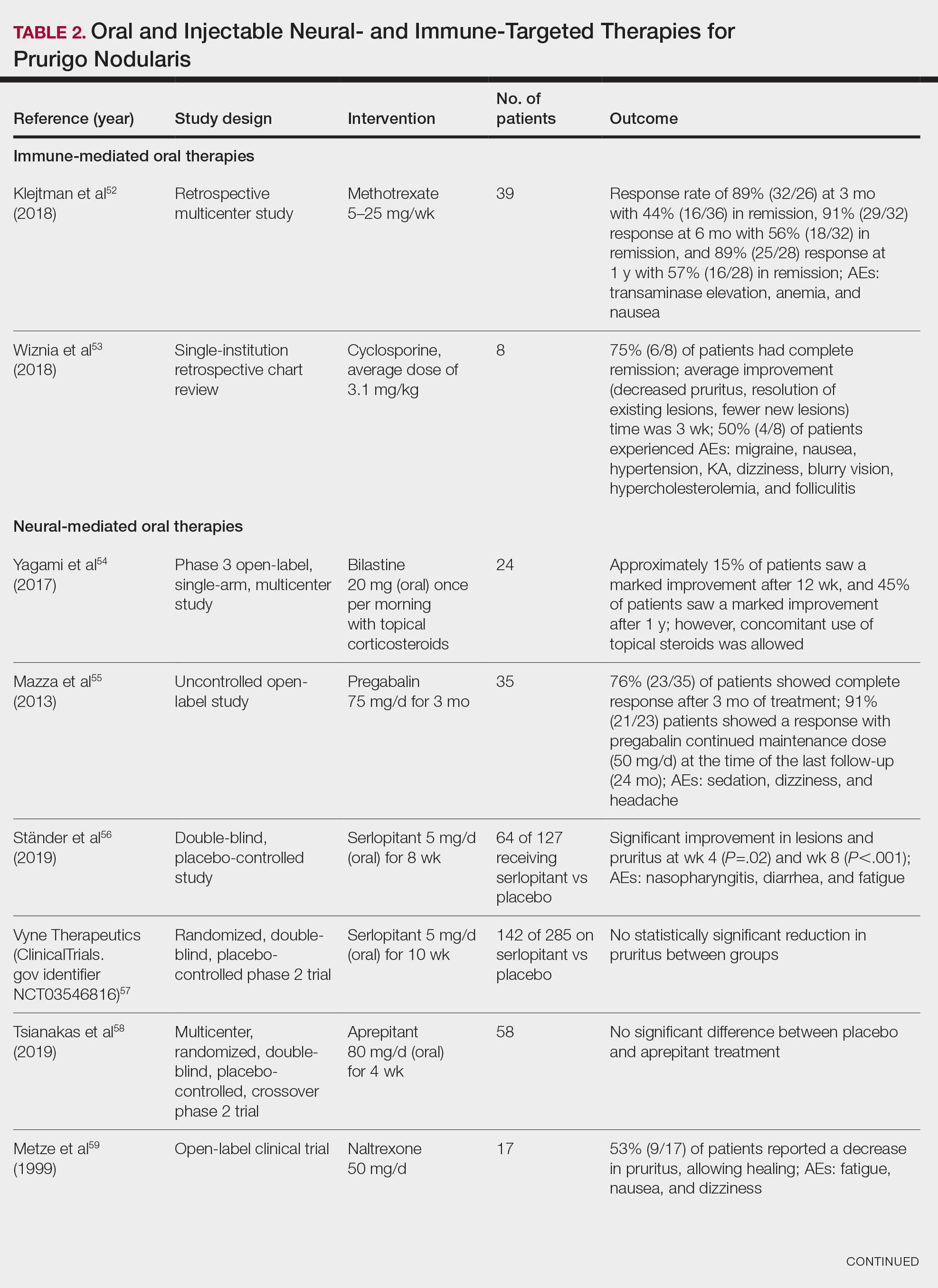
Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26

Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31

Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43

Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53


Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26

Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31

Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43

Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53


Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
Practice Points
- Clinically, prurigo nodularis can mimic an array of dermatologic skin conditions and may be diagnosed more frequently in patients with comorbidities.
- Dupilumab is the first and only treatment for prurigo nodularis approved by the US Food and Drug Administration; however, many topical treatments are currently used as first-line therapies.
A Roadmap to Research Opportunities for Dermatology Residents
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
Resident Pearls
- Establishing a strong relationship with a research mentor is crucial for success in resident research. If your program lacks the necessary infrastructure, take the initiative to network at society meetings or apply for formal mentorship programs.
- For residents facing limited access to patient cohorts and large datasets or those without access to a robust research infrastructure, conducting a systematic review is a valuable and feasible research option, allowing for meaningful contributions to the medical literature.
Blaschkolinear Lupus Erythematosus: Strategies for Early Detection and Management
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).
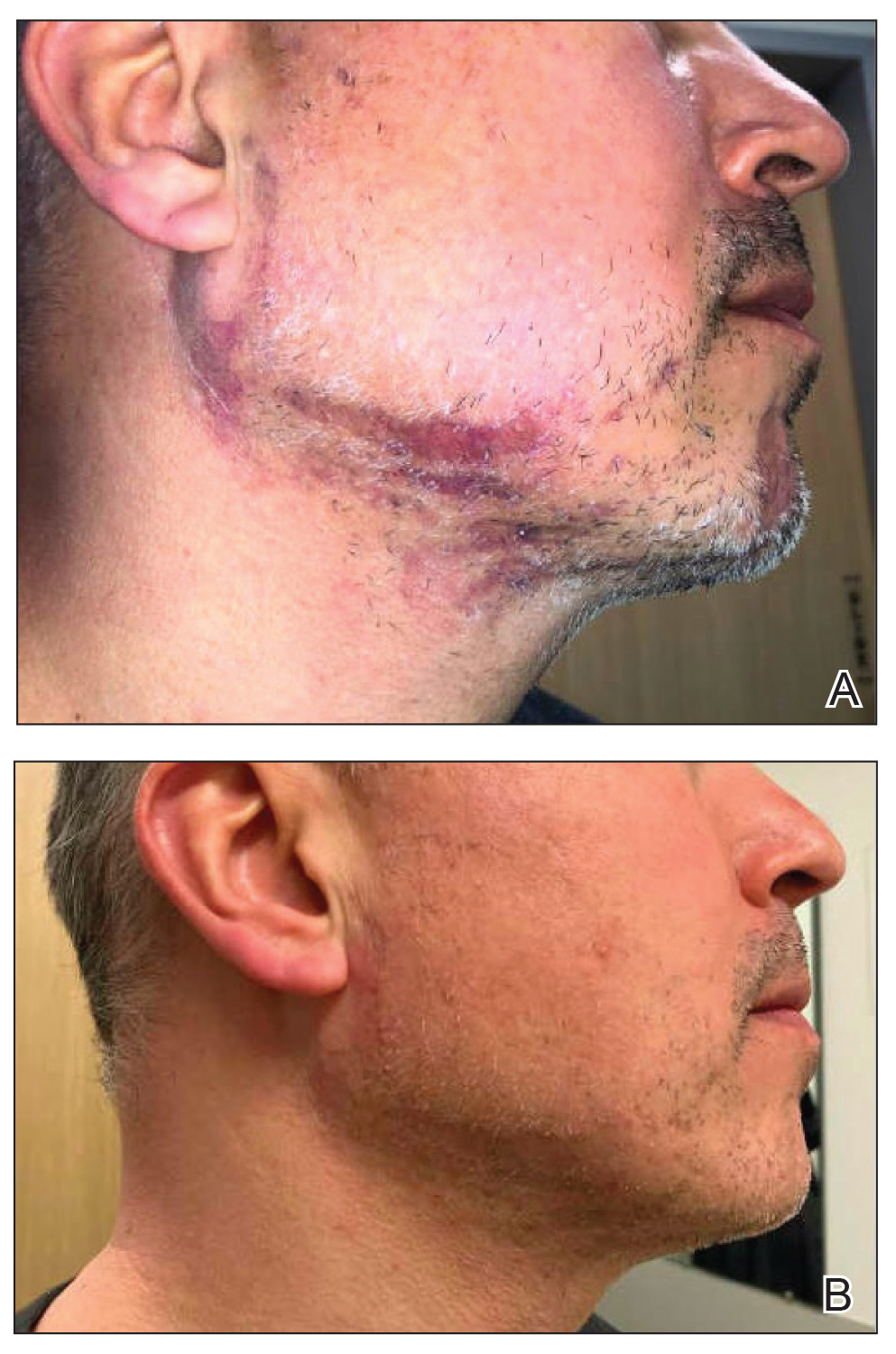
Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
Practice Points
- Blaschkolinear lupus erythematosus (BLE), an exceedingly rare subtype of chronic cutaneous lupus erythematosus, usually presents during childhood as linear plaques along the lines of Blaschko.
- It is important to consider linear lichen planus in patients with a blaschkolinear eruption, as the clinical manifestations are similar but there are differences in histopathology, management, prognosis, and long-term follow-up.
- Serial monitoring is indicated in patients with BLE given the potential for progression to systemic lupus erythematosus, which may be delayed with early use of hydroxychloroquine.
Evidence Growing for Inflammation’s Role in Elevating Risk for Psychiatric Illness
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.