User login
Three things hospitalists ‘do for no reason’... and should stop
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
FROM HOSPITAL MEDICINE 2016
FDA requires labeling changes to metformin-containing drugs
Metformin can be used safely in patients with mild impairment in kidney function and in some patients with moderate impairment in kidney function, according to the FDA’s recent review of several medical studies.
These findings have prompted the FDA to require manufacturers to change the labeling for metformin-containing drugs. These drugs’ labels now must include the results of the medical studies and new measures of kidney function for determining if a patient can use metformin, says a written statement from the FDA.
Metformin’s current labeling strongly recommends against its use in some patients with kidneys that do not work normally. The FDA is specifically requiring that new labels include the recommendation that the measure of kidney function used to determine whether a patient can receive metformin be changed from one based on a single laboratory parameter (blood creatinine concentration) to one that provides a better estimate of renal function (that is, the glomerular filtration rate estimating equation, eGFR).
The full labeling recommendations are available in the FDA’s written statement.
Additional information including a data summary and a list of metformin-containing drugs is available in the FDA Drug Safety Communication.
The FDA asks that healthcare professionals and patients report adverse events or side effects related to the use of metformin-containing drugs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Metformin can be used safely in patients with mild impairment in kidney function and in some patients with moderate impairment in kidney function, according to the FDA’s recent review of several medical studies.
These findings have prompted the FDA to require manufacturers to change the labeling for metformin-containing drugs. These drugs’ labels now must include the results of the medical studies and new measures of kidney function for determining if a patient can use metformin, says a written statement from the FDA.
Metformin’s current labeling strongly recommends against its use in some patients with kidneys that do not work normally. The FDA is specifically requiring that new labels include the recommendation that the measure of kidney function used to determine whether a patient can receive metformin be changed from one based on a single laboratory parameter (blood creatinine concentration) to one that provides a better estimate of renal function (that is, the glomerular filtration rate estimating equation, eGFR).
The full labeling recommendations are available in the FDA’s written statement.
Additional information including a data summary and a list of metformin-containing drugs is available in the FDA Drug Safety Communication.
The FDA asks that healthcare professionals and patients report adverse events or side effects related to the use of metformin-containing drugs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Metformin can be used safely in patients with mild impairment in kidney function and in some patients with moderate impairment in kidney function, according to the FDA’s recent review of several medical studies.
These findings have prompted the FDA to require manufacturers to change the labeling for metformin-containing drugs. These drugs’ labels now must include the results of the medical studies and new measures of kidney function for determining if a patient can use metformin, says a written statement from the FDA.
Metformin’s current labeling strongly recommends against its use in some patients with kidneys that do not work normally. The FDA is specifically requiring that new labels include the recommendation that the measure of kidney function used to determine whether a patient can receive metformin be changed from one based on a single laboratory parameter (blood creatinine concentration) to one that provides a better estimate of renal function (that is, the glomerular filtration rate estimating equation, eGFR).
The full labeling recommendations are available in the FDA’s written statement.
Additional information including a data summary and a list of metformin-containing drugs is available in the FDA Drug Safety Communication.
The FDA asks that healthcare professionals and patients report adverse events or side effects related to the use of metformin-containing drugs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
FDA adds safety warnings to certain type 2 diabetes medications
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Severe Psoriasis, Kidney Disease Linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Severe psoriasis, kidney disease linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Key clinical point: Severe psoriasis appears to increase the risk of both immunoglobulin A glomerulonephritis and glomerular disease.
Major finding: The risk of glomerulonephritis was five-fold higher and the risk of glomerular disease doubled in those with severe psoriasis.
Data source: A population based cohort study comprised about 1.2 million subjects.
Disclosures: Ms. Sungat Grewal had no financial disclosures.
Kidney Stones? It’s Time to Rethink Those Meds
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
Managing diabetes in hospitalized patients with chronic kidney disease
Managing glycemic control in hospitalized patients with chronic kidney disease (CKD) and diabetes mellitus is a challenge, with no published guidelines. In this setting, avoiding hypoglycemia takes precedence over meeting strict blood glucose targets. Optimal management is essential to reduce hypoglycemia and the risk of death from cardiovascular disease.1
This article reviews the evidence to guide diabetes management in hospitalized patients with CKD, focusing on blood glucose monitoring, insulin dosing, and concerns about other diabetic agents.
FOCUS ON AVOIDING HYPOGLYCEMIA
CKD is common, estimated to affect more than 50 million people worldwide.2 Diabetes mellitus is the primary cause of kidney failure in 45% of dialysis patients with CKD.
Tight control comes with a cost
Hyperglycemia in hospitalized patients is associated with a higher risk of death, a higher risk of infections, and a longer hospital stay.3,4 In 2001, Van den Berghe et al5 found that intensive insulin therapy reduced the mortality rate in critically ill patients in the surgical intensive care unit. But subsequent studies6,7 found that intensive insulin therapy to achieve tight glycemic control increased rates of morbidity and mortality without adding clinical benefit.
Randomized clinical trials in outpatients have shown that tight control of blood glucose levels reduces microvascular and macrovascular complications in patients with type 1 diabetes.8–10 In the Diabetes Control and Complications Trial,9 compared with conventional therapy, intensive insulin therapy reduced the incidence of retinopathy progression (4.7 vs 1.2 cases per 100 patient-years, number needed to treat [NNT] = 3 for 10 years) and clinical neuropathy (9.8 vs 3.1 per 100 patient-years, NNT = 1.5 for 10 years). The long-term likelihood of a cardiovascular event was also significantly lower in the intensive treatment group (0.38 vs 0.80 events per 100 patient-years).9
Similarly, in the Epidemiology of Diabetes Interventions and Complications follow-up study, the intensive therapy group had fewer cardiovascular deaths.11 On the other hand, the risk of severe hypoglycemia and subsequent coma or seizure was significantly higher in the intensive therapy group than in the conventional therapy group (16.3 vs 5.4 per 100 patient-years).8
CKD increases hypoglycemia risk
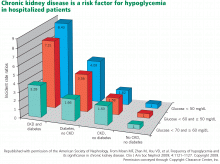
Moen et al12 found that the incidence of hypoglycemia was significantly higher in patients with CKD (estimated glomerular filtration rate [GFR] < 60 mL/min) with or without diabetes, and that patients with both conditions were at greatest risk (Figure 1). Multiple factors contribute to the increased risk of hypoglycemia: patients with advanced CKD tend to have poor nutrition, resulting in reduced glycogen stores, and a smaller renal mass reduces renal gluconeogenesis and decreases the elimination of insulin and oral antidiabetic agents.
After the onset of diabetic nephropathy, progression of renal complications and overall life expectancy are influenced by earlier glycemic control.8 Development of diabetic nephropathy is commonly accompanied by changes in metabolic control, particularly an increased risk of hypoglycemia.13 In addition, episodes of severe hypoglycemia constitute an independent cardiovascular risk factor.14
Aggressive glycemic control in hospitalized patients, particularly those with advanced CKD, is associated with a risk of hypoglycemia without overall improvement in outcomes.15 Elderly patients with type 2 diabetes are similar to patients with CKD in that they have a reduced GFR and are thus more sensitive to insulin. In both groups, intensifying glycemic control, especially in the hospital, is associated with more frequent episodes of severe hypoglycemia.16 The focus should be not only on maintaining optimal blood glucose concentration, but also on preventing hypoglycemia.
‘Burnt-out’ diabetes
Paradoxically, patients with end-stage renal disease and type 2 diabetes often experience altered glucose homeostasis with markedly improved glycemic control. They may attain normoglycemia and normalization of hemoglobin A1c, a condition known as “burnt-out” diabetes. Its precise mechanism is not understood and its significance remains unclear (Table 1).17
HEMOGLOBIN A1c CAN BE FALSELY HIGH OR FALSELY LOW
Hemoglobin A1c measurement is used to diagnose diabetes and to assess long-term glycemic control. It is a measure of the fraction of hemoglobin that has been glycated by exposure to glucose. Because the average lifespan of a red cell is 120 days, the hemoglobin A1c value reflects the mean blood glucose concentration over the preceding 3 months.
But hemoglobin A1c measurement has limitations: any condition that alters the lifespan of erythrocytes leads to higher or lower hemoglobin A1c levels. Hemoglobin A1c levels are also affected by kidney dysfunction, hemolysis, and acidosis.18
Falsely high hemoglobin A1c levels are associated with conditions that prolong the lifespan of erythrocytes, such as asplenia. Iron deficiency also increases the average age of circulating red cells because of reduced red cell production. For patients in whom blood glucose measurements do not correlate with hemoglobin A1c measurements, iron deficiency anemia should be considered before altering a treatment regimen.
Falsely low hemoglobin A1c levels are associated with conditions of more rapid erythrocyte turnover, such as autoimmune hemolytic anemia, hereditary spherocytosis, and acute blood loss anemia. In patients with CKD, recombinant erythropoietin treatment lowers hemoglobin A1c levels by increasing the number of immature red cells, which are less likely to glycosylate.19
Morgan et al20 compared the association between hemoglobin A1c and blood glucose levels in diabetic patients with moderate to severe CKD not requiring dialysis and in diabetic patients with normal renal function and found no difference between these two groups, suggesting that hemoglobin A1c is reliable in this setting. But study results conflict for patients on dialysis, making the usefulness of hemoglobin A1c testing for those patients less clear. In one study, hemoglobin A1c testing underestimated glycemic control,20 but other studies found that glycemic control was overestimated.21,22
Alternatives to hemoglobin A1c
Other measures of long-term glycemic control such as fructosamine and glycated albumin levels are sometimes used in conditions in which hemoglobin A1c may not be reliable.
Albumin also undergoes glycation when exposed to glucose. Glycated albumin appears to be a better measure of glycemic control in patients with CKD and diabetes than serum fructosamine,23 which has failed to show a significant correlation with blood glucose levels in patients with CKD.24 However, because serum albumin has a short half-life, glycated albumin reflects glycemic control in only the approximately 1 to 2 weeks before sampling,25 so monthly monitoring is required.
Glycated albumin levels may be reduced due to increased albumin turnover in patients with nephrotic-range proteinuria and in diabetic patients on peritoneal dialysis. Several issues remain unclear, such as the appropriate target level of glycated albumin and at what stage of CKD it should replace hemoglobin A1c testing. If an improved assay that is unaffected by changes in serum albumin becomes available, it may be appropriate to use glycated albumin measurements to assess long-term glycemic control for patients with CKD.
In general, therapeutic decisions to achieve optimum glycemic control in patients with diabetes and CKD should be based on hemoglobin A1c testing, multiple glucose measurements, and patient symptoms of hypoglycemia or hyperglycemia. The best measure for assessing glycemic control in hospitalized patients with CKD remains multiple blood glucose testing daily.
INSULIN THERAPY PREFERRED
Although several studies have evaluated inpatient glycemic control,26–29 no guidelines have been published for hospitalized patients with diabetes and CKD. Insulin therapy is preferred for achieving glycemic control in acutely ill or hospitalized patients with diabetes. Oral hypoglycemic agents should be discontinued.
Regardless of the form of insulin chosen to treat diabetes, caution is needed for patients with kidney disease. During hospitalization, clinical changes are expected owing to illness and differences in caloric intake and physical activity, resulting in altered insulin sensitivity. Insulin-treated hospitalized patients require individualized care, including multiple daily blood glucose tests and insulin therapy modifications for ideal glycemic control.
For surgical or medical intensive care patients on insulin therapy, the target blood glucose level before meals should be 140 mg/dL, and the target random level should be less than 180 mg/dL.15,26–29
Basal-bolus insulin
Sliding-scale therapy should be avoided as the only method for glycemic control. Instead, scheduled subcutaneous basal insulin once or twice daily combined with rapid- or short-acting insulin with meals is recommended.
Basal-bolus insulin therapy, one of the most advanced and flexible insulin replacement therapies, mimics endogenous insulin release and offers great advantages in diabetes care. Using mealtime bolus insulin permits variation in the amount of food eaten; more insulin can be taken with a larger meal and less with smaller meals. A bolus approach offers the flexibility of administering rapid-acting insulin immediately after meals when oral intake is variable.
Individualize insulin therapy
Optimizing glycemic control requires an understanding of the altered pharmacokinetics and pharmacodynamics of insulin in patients with diabetic nephropathy. Table 2 shows the pharmacokinetic profiles of insulin preparations in healthy people. Analogue insulins, which are manufactured by recombinant DNA technology, have conformational changes in the insulin molecule that alter their pharmacokinetics and pharmacodynamics. The rapid-acting analogue insulins are absorbed quickly, making them suitable for postprandial glucose control.
Changes in GFR are associated with altered pharmacokinetics and pharmacodynamics of insulin,30,31 but unlike for oral antidiabetic agents, these properties are not well characterized for insulin preparations in patients with renal insufficiency.13,32–36
CKD may reduce insulin clearance. Rave et al32 reported that the clearance of regular human insulin was reduced by 30% to 40% in patients with type 1 diabetes and a mean estimated GFR of 54 mL/min. They found that the metabolic activity of insulin lispro was more robust than that of short-acting regular human insulin in patients with diabetic nephropathy. In another study, patients with diabetes treated with insulin aspart did not show any significant change in the required insulin dosage in relation to the renal filtration rate.34 Biesenbach et al33 found a 38% reduction in insulin requirements in patients with type 1 diabetes as estimated GFR decreased from 80 mL/min to 10 mL/min. Further studies are required to better understand the safety of insulin in treating hospitalized patients with diabetes and renal insufficiency.
Few studies have compared the pharmacodynamics of long-acting insulins in relation to declining renal function. The long-acting analogue insulins have less of a peak than human insulin and thus better mimic endogenous insulin secretion. For insulin detemir, Lindholm and Jacobsen found no significant differences in the pharmacokinetics related to the stages of CKD.35 When using the long-acting insulins glargine or detemir, one should consider giving much lower doses (half the initial starting dosage) and titrating the dosage until target fasting glucose concentrations are reached to prevent hypoglycemia.
Table 3 summarizes recommended insulin dosage adjustments in CKD based on the literature and our clinical experience.
Considerations for dialysis patients
Subcutaneously administered insulin is eliminated renally, unlike endogenous insulin, which undergoes first-pass metabolism in the liver.13,37 As renal function declines, insulin clearance decreases and the insulin dosage must be reduced to prevent hypoglycemia.
Patients on hemodialysis or peritoneal dialysis pose a challenge for insulin dosing. Hemodialysis improves insulin sensitivity but also increases insulin clearance, making it difficult to determine insulin requirements. Sobngwi et al38 conducted a study in diabetic patients with end-stage renal disease on hemodialysis, using a 24-hour euglycemic clamp. They found that exogenous basal insulin requirements were 25% lower on the day after hemodialysis compared with the day before, but premeal insulin requirements stayed the same.
Peritoneal dialysis exposes patients to a high glucose load via the peritoneum, which can worsen insulin resistance. Intraperitoneal administration of insulin during peritoneal dialysis provides a more physiologic effect than subcutaneous administration: it prevents fluctuations of blood glucose and the formation of insulin antibodies. But insulin requirements are higher owing to a dilutional effect and to insulin binding to the plastic surface of the dialysis fluid reservoir.39
GLYCEMIC CONTROL FOR PROCEDURES
No guidelines have been established regarding the optimal blood glucose range for diabetic patients with CKD undergoing diagnostic or surgical procedures. Given the risk of hypoglycemia in such settings, less-stringent targets are reasonable, ie, premeal blood glucose levels of 140 mg/dL and random blood glucose levels of less than 180 mg/dL.
Before surgery, consideration should be given to the type of diabetes, surgical procedure, and metabolic control. Patients on insulin detemir or glargine as part of a basal-bolus regimen with rapid-acting insulin may safely be given the full dose of their basal insulin the night before or the morning of their procedure. However, patients on neutral protamine Hagedorn (NPH) insulin as a part of their basal-bolus regimen should receive half of their usual dose due to a difference in pharmacokinetic profile compared with insulin glargine or detemir.
In insulin-treated patients undergoing prolonged procedures (eg, coronary artery bypass grafting, transplant):
- Discontinue subcutaneous insulin and start an intravenous insulin infusion, titrated to maintain a blood glucose range of 140 to 180 mg/dL
- Subcutaneous insulin management may be acceptable for patients undergoing shorter outpatient procedures
- Supplemental subcutaneous doses of short- or rapid-acting insulin preparations can be given for blood glucose elevation greater than 180 mg/dL.
AVOID ORAL AGENTS AND NONINSULIN INJECTABLES
Oral antidiabetic agents and noninsulin injectables (Table 4) should generally be avoided in hospitalized patients, especially for those with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, or for those given intravenous contrast. Most oral medications used to treat diabetes are affected by reduced kidney function, resulting in prolonged drug exposure and increased risk of hypoglycemia in patients with moderate to severe CKD (stages 3–5).
Metformin, a biguanide, is contraindicated in patients with high serum creatinine levels (> 1.5 mg/dL in men, > 1.4 mg/dL in women) because of the theoretical risk of lactic acidosis.40
Sulfonylurea clearance depends on kidney function.41 Severe prolonged episodes of hypoglycemia have been reported in dialysis patients taking these drugs, except with glipizide, which carries a lower risk.41,42
Repaglinide, a nonsulfonylurea insulin secretagogue, can be used in CKD stages 3 to 4 without any dosage adjustment.43
Thiazolidinediones have been reported to slow the progression of diabetic kidney disease independent of glycemic control.44 Adverse effects include fluid retention, edema, and congestive heart failure. Thiazolidinediones should not be used in patients with New York Heart Association class 3 or 4 heart failure,45 and so should not be prescribed in the hospital except for patients who are clinically stable or ready for discharge.
Quick-release bromocriptine, a dopamine receptor agonist, has been shown to be effective in lowering fasting plasma glucose levels and hemoglobin A1c, and improving glucose tolerance in obese patients with type 2 diabetes, although its usefulness in hospitalized patients with diabetes is not known.46,47
Dipeptidyl peptidase inhibitors. Sitagliptin and saxagliptin have been shown to be safe and effective in hospitalized patients with type 2 diabetes.48 However, except for linagliptin, dose reduction is recommended in patients with CKD stage 3 and higher.49–52
GLP-1 receptor agonists. Drugs of this class are potent agents for the reduction of glucose in the outpatient setting but are relatively contraindicated if the GFR is less than 30 mL/min, and they are currently not used in the hospital.
BLOOD GLUCOSE MONITORING IN HOSPITALIZED PATIENTS
Bedside blood glucose monitoring is recommended for all hospitalized patients with known diabetes with or without CKD, those with newly recognized hyperglycemia, and those who receive therapy associated with high risk for hyperglycemia, such as glucocorticoid therapy and enteral and parenteral nutrition. For patients on scheduled diets, fingerstick blood glucose monitoring is recommended before meals and at bedtime. In patients with no oral intake or on continuous enteral or parenteral nutrition, blood glucose monitoring every 4 to 6 hours is recommended. More frequent monitoring (eg, adding a 3:00 am check) may be prudent in patients with CKD.
Continuous glucose monitoring systems use a sensor inserted under the skin and transmit information via radio to a wireless monitor. Such systems are more expensive than conventional glucose monitoring but may enable better glucose control by providing real-time glucose measurements, with levels displayed at 5-minute or 1-minute intervals. Marshall et al53 confirmed this technology’s accuracy and precision in uremic patients on dialysis.
Considerations for peritoneal dialysis
For patients on peritoneal dialysis, glucose in the dialysate exacerbates hyperglycemia. Dialysis solutions with the glucose polymer icodextrin as the osmotic agent instead of glucose have been suggested to reduce glucose exposure.
Glucose monitoring systems measure interstitial fluid glucose by the glucose oxidase reaction and therefore are not affected by icodextrin. However, icodextrin is converted to maltose, a disaccharide composed of two glucose molecules, which can cause spuriously high readings in devices that use test strips containing the enzymes glucose dehydrogenase pyrroloquinoline quinone or glucose dye oxidoreductase. Spurious hyperglycemia may lead to giving too much insulin, in turn leading to symptomatic hypoglycemia.
Clinicians caring for patients receiving icodextrin should ensure that the glucose monitoring system uses only test strips that contain glucose oxidase, glucose dehydrogenase-nicotinamide adenine dinucleotide, or glucose dehydrogenase-flavin adenine dinucleotide, which are not affected by icodextrin.54
IMPROVING QUALITY
Hospitalized patients face many barriers to optimal glycemic control. Less experienced practitioners tend to have insufficient knowledge of insulin preparations and appropriate insulin dosing. Also, diabetes is often listed as a secondary diagnosis and so may be overlooked by the inpatient care team.
Educational programs should be instituted to overcome these barriers and improve knowledge related to inpatient diabetes care. When necessary, the appropriate use of consultants is important in hospitalized settings to improve quality and make hospital care more efficient and cost-effective.
No national benchmarks currently exist for inpatient diabetes care, and they need to be developed to ensure best practices. Physicians should take the initiative to remedy this by collaborating with other healthcare providers, such as dedicated diabetes educators, nursing staff, pharmacists, registered dietitians, and physicians with expertise in diabetes management, with the aim of achieving optimum glycemic control and minimizing hypoglycemia.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305.
- Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess 2005; 9:iii–vi, xiii–163.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999; 22:1408–1414.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75:894–903.
- Nathan DM, Lachin J, Cleary P, et al; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348:2294–2303.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4:1121–1127.
- Iglesias P, Díez J. Insulin therapy in renal disease. Diabetes Obes Metab 2008; 10:811–823.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010; 23:148–156.
- De Marchi S, Cecchin E, Camurri C, et al. Origin of glycosylated hemoglobin A1 in chronic renal failure. Int J Artif Organs 1983; 6:77–82.
- Brown JN, Kemp DW, Brice KR. Class effect of erythropoietin therapy on hemoglobin A(1c) in a patient with diabetes mellitus and chronic kidney disease not undergoing hemodialysis. Pharmacotherapy 2009; 29:468–472.
- Morgan L, Marenah CB, Jeffcoate WJ, Morgan AG. Glycated proteins as indices of glycemic control in diabetic patients with chronic renal failure. Diabet Med 1996; 13:514–519.
- Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008; 73:1062–1068.
- Joy MS, Cefalu WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int 2010; 78(suppl 117):S41–S45.
- Alskar O, Korelli J, Duffull SB. A pharmacokinetic model for the glycation of albumin. J Pharmacokinet Pharmacodyn 2012; 39:273–282.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Bogun M, Inzucchi SE. Inpatient management of diabetes and hyperglycemia. Clin Ther 2013; 35:724–733.
- Miller DB. Glycemic targets in hospital and barriers to attaining them. Can J Diabetes 2014; 38:74–78.
- Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 1995; 38:565–572.
- Svensson M, Yu Z, Eriksson J. A small reduction in glomerular filtration is accompanied by insulin resistance in type I diabetes patients with diabetic nephropathy. Eur J Clin Invest 2002; 32:100–109.
- Rave K, Heise T, Pfutzner A, Heinemann L, Sawicki P. Impact of diabetic nephropathy on pharmacodynamics and pharmacokinetic properties of insulin in type I diabetic patients. Diabetes Care 2001; 24:886–890.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol 2005; 60:469–476.
- Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40:641–659.
- Bolli GB, Hahn AD, Schmidt R, et al. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care 2012; 35:2626–2630.
- Nielsen S. Time course and kinetics of proximal tubular processing of insulin. Am J Physiol 1992; 262:F813–F822.
- Sobngwi E, Enoru S, Ashuntantang G, et al. Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care 2010; 33:1409–1412.
- Quellhorst E. Insulin therapy during peritoneal dialysis: pros and cons of various forms of administration. J Am Soc Nephrol 2002; 13(suppl 1):S92–S96.
- Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med 1997; 102:99–110.
- Ahmed Z, Simon B, Choudhury D. Management of diabetes in patients with chronic kidney disease. Postgrad Med 2009; 121:52–60.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26(suppl 4):73–85.
- Hasslacher C; Multinational Repaglinide Renal Study Group. Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care 2003; 26:886–891.
- Iglesias P, Dies JJ. Peroxisome proliferator-activated receptor gamma agonists in renal disease. Eur J Endocrinol 2006; 154:613–621.
- Hollenberg NK. Considerations for management of fluid dynamic issues associated with thiazolidinediones. Am J Med 2003; 115(suppl. 8A) 111S–115S.
- Kamath V, Jones CN, Yip JC, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care 1997; 20:1697–1701.
- Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care 2000; 23:1154–1161.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10:545–555.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Onglyza package insert. www.azpicentral.com/onglyza/pi_onglyza.pdf. Accessed March 8, 2016.
- Gallwitz B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther Adv Endocrinol Metab 2013; 4:95–105.
- Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int 2003; 64:1480–1486.
- Schleis TG. Interference of maltose, icodextrin, galactose, or xylose with some blood glucose monitoring systems. Pharmacotherapy 2007; 27:1313–1321.
Managing glycemic control in hospitalized patients with chronic kidney disease (CKD) and diabetes mellitus is a challenge, with no published guidelines. In this setting, avoiding hypoglycemia takes precedence over meeting strict blood glucose targets. Optimal management is essential to reduce hypoglycemia and the risk of death from cardiovascular disease.1
This article reviews the evidence to guide diabetes management in hospitalized patients with CKD, focusing on blood glucose monitoring, insulin dosing, and concerns about other diabetic agents.
FOCUS ON AVOIDING HYPOGLYCEMIA
CKD is common, estimated to affect more than 50 million people worldwide.2 Diabetes mellitus is the primary cause of kidney failure in 45% of dialysis patients with CKD.
Tight control comes with a cost
Hyperglycemia in hospitalized patients is associated with a higher risk of death, a higher risk of infections, and a longer hospital stay.3,4 In 2001, Van den Berghe et al5 found that intensive insulin therapy reduced the mortality rate in critically ill patients in the surgical intensive care unit. But subsequent studies6,7 found that intensive insulin therapy to achieve tight glycemic control increased rates of morbidity and mortality without adding clinical benefit.
Randomized clinical trials in outpatients have shown that tight control of blood glucose levels reduces microvascular and macrovascular complications in patients with type 1 diabetes.8–10 In the Diabetes Control and Complications Trial,9 compared with conventional therapy, intensive insulin therapy reduced the incidence of retinopathy progression (4.7 vs 1.2 cases per 100 patient-years, number needed to treat [NNT] = 3 for 10 years) and clinical neuropathy (9.8 vs 3.1 per 100 patient-years, NNT = 1.5 for 10 years). The long-term likelihood of a cardiovascular event was also significantly lower in the intensive treatment group (0.38 vs 0.80 events per 100 patient-years).9
Similarly, in the Epidemiology of Diabetes Interventions and Complications follow-up study, the intensive therapy group had fewer cardiovascular deaths.11 On the other hand, the risk of severe hypoglycemia and subsequent coma or seizure was significantly higher in the intensive therapy group than in the conventional therapy group (16.3 vs 5.4 per 100 patient-years).8
CKD increases hypoglycemia risk
Moen et al12 found that the incidence of hypoglycemia was significantly higher in patients with CKD (estimated glomerular filtration rate [GFR] < 60 mL/min) with or without diabetes, and that patients with both conditions were at greatest risk (Figure 1). Multiple factors contribute to the increased risk of hypoglycemia: patients with advanced CKD tend to have poor nutrition, resulting in reduced glycogen stores, and a smaller renal mass reduces renal gluconeogenesis and decreases the elimination of insulin and oral antidiabetic agents.
After the onset of diabetic nephropathy, progression of renal complications and overall life expectancy are influenced by earlier glycemic control.8 Development of diabetic nephropathy is commonly accompanied by changes in metabolic control, particularly an increased risk of hypoglycemia.13 In addition, episodes of severe hypoglycemia constitute an independent cardiovascular risk factor.14
Aggressive glycemic control in hospitalized patients, particularly those with advanced CKD, is associated with a risk of hypoglycemia without overall improvement in outcomes.15 Elderly patients with type 2 diabetes are similar to patients with CKD in that they have a reduced GFR and are thus more sensitive to insulin. In both groups, intensifying glycemic control, especially in the hospital, is associated with more frequent episodes of severe hypoglycemia.16 The focus should be not only on maintaining optimal blood glucose concentration, but also on preventing hypoglycemia.
‘Burnt-out’ diabetes
Paradoxically, patients with end-stage renal disease and type 2 diabetes often experience altered glucose homeostasis with markedly improved glycemic control. They may attain normoglycemia and normalization of hemoglobin A1c, a condition known as “burnt-out” diabetes. Its precise mechanism is not understood and its significance remains unclear (Table 1).17
HEMOGLOBIN A1c CAN BE FALSELY HIGH OR FALSELY LOW
Hemoglobin A1c measurement is used to diagnose diabetes and to assess long-term glycemic control. It is a measure of the fraction of hemoglobin that has been glycated by exposure to glucose. Because the average lifespan of a red cell is 120 days, the hemoglobin A1c value reflects the mean blood glucose concentration over the preceding 3 months.
But hemoglobin A1c measurement has limitations: any condition that alters the lifespan of erythrocytes leads to higher or lower hemoglobin A1c levels. Hemoglobin A1c levels are also affected by kidney dysfunction, hemolysis, and acidosis.18
Falsely high hemoglobin A1c levels are associated with conditions that prolong the lifespan of erythrocytes, such as asplenia. Iron deficiency also increases the average age of circulating red cells because of reduced red cell production. For patients in whom blood glucose measurements do not correlate with hemoglobin A1c measurements, iron deficiency anemia should be considered before altering a treatment regimen.
Falsely low hemoglobin A1c levels are associated with conditions of more rapid erythrocyte turnover, such as autoimmune hemolytic anemia, hereditary spherocytosis, and acute blood loss anemia. In patients with CKD, recombinant erythropoietin treatment lowers hemoglobin A1c levels by increasing the number of immature red cells, which are less likely to glycosylate.19
Morgan et al20 compared the association between hemoglobin A1c and blood glucose levels in diabetic patients with moderate to severe CKD not requiring dialysis and in diabetic patients with normal renal function and found no difference between these two groups, suggesting that hemoglobin A1c is reliable in this setting. But study results conflict for patients on dialysis, making the usefulness of hemoglobin A1c testing for those patients less clear. In one study, hemoglobin A1c testing underestimated glycemic control,20 but other studies found that glycemic control was overestimated.21,22
Alternatives to hemoglobin A1c
Other measures of long-term glycemic control such as fructosamine and glycated albumin levels are sometimes used in conditions in which hemoglobin A1c may not be reliable.
Albumin also undergoes glycation when exposed to glucose. Glycated albumin appears to be a better measure of glycemic control in patients with CKD and diabetes than serum fructosamine,23 which has failed to show a significant correlation with blood glucose levels in patients with CKD.24 However, because serum albumin has a short half-life, glycated albumin reflects glycemic control in only the approximately 1 to 2 weeks before sampling,25 so monthly monitoring is required.
Glycated albumin levels may be reduced due to increased albumin turnover in patients with nephrotic-range proteinuria and in diabetic patients on peritoneal dialysis. Several issues remain unclear, such as the appropriate target level of glycated albumin and at what stage of CKD it should replace hemoglobin A1c testing. If an improved assay that is unaffected by changes in serum albumin becomes available, it may be appropriate to use glycated albumin measurements to assess long-term glycemic control for patients with CKD.
In general, therapeutic decisions to achieve optimum glycemic control in patients with diabetes and CKD should be based on hemoglobin A1c testing, multiple glucose measurements, and patient symptoms of hypoglycemia or hyperglycemia. The best measure for assessing glycemic control in hospitalized patients with CKD remains multiple blood glucose testing daily.
INSULIN THERAPY PREFERRED
Although several studies have evaluated inpatient glycemic control,26–29 no guidelines have been published for hospitalized patients with diabetes and CKD. Insulin therapy is preferred for achieving glycemic control in acutely ill or hospitalized patients with diabetes. Oral hypoglycemic agents should be discontinued.
Regardless of the form of insulin chosen to treat diabetes, caution is needed for patients with kidney disease. During hospitalization, clinical changes are expected owing to illness and differences in caloric intake and physical activity, resulting in altered insulin sensitivity. Insulin-treated hospitalized patients require individualized care, including multiple daily blood glucose tests and insulin therapy modifications for ideal glycemic control.
For surgical or medical intensive care patients on insulin therapy, the target blood glucose level before meals should be 140 mg/dL, and the target random level should be less than 180 mg/dL.15,26–29
Basal-bolus insulin
Sliding-scale therapy should be avoided as the only method for glycemic control. Instead, scheduled subcutaneous basal insulin once or twice daily combined with rapid- or short-acting insulin with meals is recommended.
Basal-bolus insulin therapy, one of the most advanced and flexible insulin replacement therapies, mimics endogenous insulin release and offers great advantages in diabetes care. Using mealtime bolus insulin permits variation in the amount of food eaten; more insulin can be taken with a larger meal and less with smaller meals. A bolus approach offers the flexibility of administering rapid-acting insulin immediately after meals when oral intake is variable.
Individualize insulin therapy
Optimizing glycemic control requires an understanding of the altered pharmacokinetics and pharmacodynamics of insulin in patients with diabetic nephropathy. Table 2 shows the pharmacokinetic profiles of insulin preparations in healthy people. Analogue insulins, which are manufactured by recombinant DNA technology, have conformational changes in the insulin molecule that alter their pharmacokinetics and pharmacodynamics. The rapid-acting analogue insulins are absorbed quickly, making them suitable for postprandial glucose control.
Changes in GFR are associated with altered pharmacokinetics and pharmacodynamics of insulin,30,31 but unlike for oral antidiabetic agents, these properties are not well characterized for insulin preparations in patients with renal insufficiency.13,32–36
CKD may reduce insulin clearance. Rave et al32 reported that the clearance of regular human insulin was reduced by 30% to 40% in patients with type 1 diabetes and a mean estimated GFR of 54 mL/min. They found that the metabolic activity of insulin lispro was more robust than that of short-acting regular human insulin in patients with diabetic nephropathy. In another study, patients with diabetes treated with insulin aspart did not show any significant change in the required insulin dosage in relation to the renal filtration rate.34 Biesenbach et al33 found a 38% reduction in insulin requirements in patients with type 1 diabetes as estimated GFR decreased from 80 mL/min to 10 mL/min. Further studies are required to better understand the safety of insulin in treating hospitalized patients with diabetes and renal insufficiency.
Few studies have compared the pharmacodynamics of long-acting insulins in relation to declining renal function. The long-acting analogue insulins have less of a peak than human insulin and thus better mimic endogenous insulin secretion. For insulin detemir, Lindholm and Jacobsen found no significant differences in the pharmacokinetics related to the stages of CKD.35 When using the long-acting insulins glargine or detemir, one should consider giving much lower doses (half the initial starting dosage) and titrating the dosage until target fasting glucose concentrations are reached to prevent hypoglycemia.
Table 3 summarizes recommended insulin dosage adjustments in CKD based on the literature and our clinical experience.
Considerations for dialysis patients
Subcutaneously administered insulin is eliminated renally, unlike endogenous insulin, which undergoes first-pass metabolism in the liver.13,37 As renal function declines, insulin clearance decreases and the insulin dosage must be reduced to prevent hypoglycemia.
Patients on hemodialysis or peritoneal dialysis pose a challenge for insulin dosing. Hemodialysis improves insulin sensitivity but also increases insulin clearance, making it difficult to determine insulin requirements. Sobngwi et al38 conducted a study in diabetic patients with end-stage renal disease on hemodialysis, using a 24-hour euglycemic clamp. They found that exogenous basal insulin requirements were 25% lower on the day after hemodialysis compared with the day before, but premeal insulin requirements stayed the same.
Peritoneal dialysis exposes patients to a high glucose load via the peritoneum, which can worsen insulin resistance. Intraperitoneal administration of insulin during peritoneal dialysis provides a more physiologic effect than subcutaneous administration: it prevents fluctuations of blood glucose and the formation of insulin antibodies. But insulin requirements are higher owing to a dilutional effect and to insulin binding to the plastic surface of the dialysis fluid reservoir.39
GLYCEMIC CONTROL FOR PROCEDURES
No guidelines have been established regarding the optimal blood glucose range for diabetic patients with CKD undergoing diagnostic or surgical procedures. Given the risk of hypoglycemia in such settings, less-stringent targets are reasonable, ie, premeal blood glucose levels of 140 mg/dL and random blood glucose levels of less than 180 mg/dL.
Before surgery, consideration should be given to the type of diabetes, surgical procedure, and metabolic control. Patients on insulin detemir or glargine as part of a basal-bolus regimen with rapid-acting insulin may safely be given the full dose of their basal insulin the night before or the morning of their procedure. However, patients on neutral protamine Hagedorn (NPH) insulin as a part of their basal-bolus regimen should receive half of their usual dose due to a difference in pharmacokinetic profile compared with insulin glargine or detemir.
In insulin-treated patients undergoing prolonged procedures (eg, coronary artery bypass grafting, transplant):
- Discontinue subcutaneous insulin and start an intravenous insulin infusion, titrated to maintain a blood glucose range of 140 to 180 mg/dL
- Subcutaneous insulin management may be acceptable for patients undergoing shorter outpatient procedures
- Supplemental subcutaneous doses of short- or rapid-acting insulin preparations can be given for blood glucose elevation greater than 180 mg/dL.
AVOID ORAL AGENTS AND NONINSULIN INJECTABLES
Oral antidiabetic agents and noninsulin injectables (Table 4) should generally be avoided in hospitalized patients, especially for those with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, or for those given intravenous contrast. Most oral medications used to treat diabetes are affected by reduced kidney function, resulting in prolonged drug exposure and increased risk of hypoglycemia in patients with moderate to severe CKD (stages 3–5).
Metformin, a biguanide, is contraindicated in patients with high serum creatinine levels (> 1.5 mg/dL in men, > 1.4 mg/dL in women) because of the theoretical risk of lactic acidosis.40
Sulfonylurea clearance depends on kidney function.41 Severe prolonged episodes of hypoglycemia have been reported in dialysis patients taking these drugs, except with glipizide, which carries a lower risk.41,42
Repaglinide, a nonsulfonylurea insulin secretagogue, can be used in CKD stages 3 to 4 without any dosage adjustment.43
Thiazolidinediones have been reported to slow the progression of diabetic kidney disease independent of glycemic control.44 Adverse effects include fluid retention, edema, and congestive heart failure. Thiazolidinediones should not be used in patients with New York Heart Association class 3 or 4 heart failure,45 and so should not be prescribed in the hospital except for patients who are clinically stable or ready for discharge.
Quick-release bromocriptine, a dopamine receptor agonist, has been shown to be effective in lowering fasting plasma glucose levels and hemoglobin A1c, and improving glucose tolerance in obese patients with type 2 diabetes, although its usefulness in hospitalized patients with diabetes is not known.46,47
Dipeptidyl peptidase inhibitors. Sitagliptin and saxagliptin have been shown to be safe and effective in hospitalized patients with type 2 diabetes.48 However, except for linagliptin, dose reduction is recommended in patients with CKD stage 3 and higher.49–52
GLP-1 receptor agonists. Drugs of this class are potent agents for the reduction of glucose in the outpatient setting but are relatively contraindicated if the GFR is less than 30 mL/min, and they are currently not used in the hospital.
BLOOD GLUCOSE MONITORING IN HOSPITALIZED PATIENTS
Bedside blood glucose monitoring is recommended for all hospitalized patients with known diabetes with or without CKD, those with newly recognized hyperglycemia, and those who receive therapy associated with high risk for hyperglycemia, such as glucocorticoid therapy and enteral and parenteral nutrition. For patients on scheduled diets, fingerstick blood glucose monitoring is recommended before meals and at bedtime. In patients with no oral intake or on continuous enteral or parenteral nutrition, blood glucose monitoring every 4 to 6 hours is recommended. More frequent monitoring (eg, adding a 3:00 am check) may be prudent in patients with CKD.
Continuous glucose monitoring systems use a sensor inserted under the skin and transmit information via radio to a wireless monitor. Such systems are more expensive than conventional glucose monitoring but may enable better glucose control by providing real-time glucose measurements, with levels displayed at 5-minute or 1-minute intervals. Marshall et al53 confirmed this technology’s accuracy and precision in uremic patients on dialysis.
Considerations for peritoneal dialysis
For patients on peritoneal dialysis, glucose in the dialysate exacerbates hyperglycemia. Dialysis solutions with the glucose polymer icodextrin as the osmotic agent instead of glucose have been suggested to reduce glucose exposure.
Glucose monitoring systems measure interstitial fluid glucose by the glucose oxidase reaction and therefore are not affected by icodextrin. However, icodextrin is converted to maltose, a disaccharide composed of two glucose molecules, which can cause spuriously high readings in devices that use test strips containing the enzymes glucose dehydrogenase pyrroloquinoline quinone or glucose dye oxidoreductase. Spurious hyperglycemia may lead to giving too much insulin, in turn leading to symptomatic hypoglycemia.
Clinicians caring for patients receiving icodextrin should ensure that the glucose monitoring system uses only test strips that contain glucose oxidase, glucose dehydrogenase-nicotinamide adenine dinucleotide, or glucose dehydrogenase-flavin adenine dinucleotide, which are not affected by icodextrin.54
IMPROVING QUALITY
Hospitalized patients face many barriers to optimal glycemic control. Less experienced practitioners tend to have insufficient knowledge of insulin preparations and appropriate insulin dosing. Also, diabetes is often listed as a secondary diagnosis and so may be overlooked by the inpatient care team.
Educational programs should be instituted to overcome these barriers and improve knowledge related to inpatient diabetes care. When necessary, the appropriate use of consultants is important in hospitalized settings to improve quality and make hospital care more efficient and cost-effective.
No national benchmarks currently exist for inpatient diabetes care, and they need to be developed to ensure best practices. Physicians should take the initiative to remedy this by collaborating with other healthcare providers, such as dedicated diabetes educators, nursing staff, pharmacists, registered dietitians, and physicians with expertise in diabetes management, with the aim of achieving optimum glycemic control and minimizing hypoglycemia.
Managing glycemic control in hospitalized patients with chronic kidney disease (CKD) and diabetes mellitus is a challenge, with no published guidelines. In this setting, avoiding hypoglycemia takes precedence over meeting strict blood glucose targets. Optimal management is essential to reduce hypoglycemia and the risk of death from cardiovascular disease.1
This article reviews the evidence to guide diabetes management in hospitalized patients with CKD, focusing on blood glucose monitoring, insulin dosing, and concerns about other diabetic agents.
FOCUS ON AVOIDING HYPOGLYCEMIA
CKD is common, estimated to affect more than 50 million people worldwide.2 Diabetes mellitus is the primary cause of kidney failure in 45% of dialysis patients with CKD.
Tight control comes with a cost
Hyperglycemia in hospitalized patients is associated with a higher risk of death, a higher risk of infections, and a longer hospital stay.3,4 In 2001, Van den Berghe et al5 found that intensive insulin therapy reduced the mortality rate in critically ill patients in the surgical intensive care unit. But subsequent studies6,7 found that intensive insulin therapy to achieve tight glycemic control increased rates of morbidity and mortality without adding clinical benefit.
Randomized clinical trials in outpatients have shown that tight control of blood glucose levels reduces microvascular and macrovascular complications in patients with type 1 diabetes.8–10 In the Diabetes Control and Complications Trial,9 compared with conventional therapy, intensive insulin therapy reduced the incidence of retinopathy progression (4.7 vs 1.2 cases per 100 patient-years, number needed to treat [NNT] = 3 for 10 years) and clinical neuropathy (9.8 vs 3.1 per 100 patient-years, NNT = 1.5 for 10 years). The long-term likelihood of a cardiovascular event was also significantly lower in the intensive treatment group (0.38 vs 0.80 events per 100 patient-years).9
Similarly, in the Epidemiology of Diabetes Interventions and Complications follow-up study, the intensive therapy group had fewer cardiovascular deaths.11 On the other hand, the risk of severe hypoglycemia and subsequent coma or seizure was significantly higher in the intensive therapy group than in the conventional therapy group (16.3 vs 5.4 per 100 patient-years).8
CKD increases hypoglycemia risk
Moen et al12 found that the incidence of hypoglycemia was significantly higher in patients with CKD (estimated glomerular filtration rate [GFR] < 60 mL/min) with or without diabetes, and that patients with both conditions were at greatest risk (Figure 1). Multiple factors contribute to the increased risk of hypoglycemia: patients with advanced CKD tend to have poor nutrition, resulting in reduced glycogen stores, and a smaller renal mass reduces renal gluconeogenesis and decreases the elimination of insulin and oral antidiabetic agents.
After the onset of diabetic nephropathy, progression of renal complications and overall life expectancy are influenced by earlier glycemic control.8 Development of diabetic nephropathy is commonly accompanied by changes in metabolic control, particularly an increased risk of hypoglycemia.13 In addition, episodes of severe hypoglycemia constitute an independent cardiovascular risk factor.14
Aggressive glycemic control in hospitalized patients, particularly those with advanced CKD, is associated with a risk of hypoglycemia without overall improvement in outcomes.15 Elderly patients with type 2 diabetes are similar to patients with CKD in that they have a reduced GFR and are thus more sensitive to insulin. In both groups, intensifying glycemic control, especially in the hospital, is associated with more frequent episodes of severe hypoglycemia.16 The focus should be not only on maintaining optimal blood glucose concentration, but also on preventing hypoglycemia.
‘Burnt-out’ diabetes
Paradoxically, patients with end-stage renal disease and type 2 diabetes often experience altered glucose homeostasis with markedly improved glycemic control. They may attain normoglycemia and normalization of hemoglobin A1c, a condition known as “burnt-out” diabetes. Its precise mechanism is not understood and its significance remains unclear (Table 1).17
HEMOGLOBIN A1c CAN BE FALSELY HIGH OR FALSELY LOW
Hemoglobin A1c measurement is used to diagnose diabetes and to assess long-term glycemic control. It is a measure of the fraction of hemoglobin that has been glycated by exposure to glucose. Because the average lifespan of a red cell is 120 days, the hemoglobin A1c value reflects the mean blood glucose concentration over the preceding 3 months.
But hemoglobin A1c measurement has limitations: any condition that alters the lifespan of erythrocytes leads to higher or lower hemoglobin A1c levels. Hemoglobin A1c levels are also affected by kidney dysfunction, hemolysis, and acidosis.18
Falsely high hemoglobin A1c levels are associated with conditions that prolong the lifespan of erythrocytes, such as asplenia. Iron deficiency also increases the average age of circulating red cells because of reduced red cell production. For patients in whom blood glucose measurements do not correlate with hemoglobin A1c measurements, iron deficiency anemia should be considered before altering a treatment regimen.
Falsely low hemoglobin A1c levels are associated with conditions of more rapid erythrocyte turnover, such as autoimmune hemolytic anemia, hereditary spherocytosis, and acute blood loss anemia. In patients with CKD, recombinant erythropoietin treatment lowers hemoglobin A1c levels by increasing the number of immature red cells, which are less likely to glycosylate.19
Morgan et al20 compared the association between hemoglobin A1c and blood glucose levels in diabetic patients with moderate to severe CKD not requiring dialysis and in diabetic patients with normal renal function and found no difference between these two groups, suggesting that hemoglobin A1c is reliable in this setting. But study results conflict for patients on dialysis, making the usefulness of hemoglobin A1c testing for those patients less clear. In one study, hemoglobin A1c testing underestimated glycemic control,20 but other studies found that glycemic control was overestimated.21,22
Alternatives to hemoglobin A1c
Other measures of long-term glycemic control such as fructosamine and glycated albumin levels are sometimes used in conditions in which hemoglobin A1c may not be reliable.
Albumin also undergoes glycation when exposed to glucose. Glycated albumin appears to be a better measure of glycemic control in patients with CKD and diabetes than serum fructosamine,23 which has failed to show a significant correlation with blood glucose levels in patients with CKD.24 However, because serum albumin has a short half-life, glycated albumin reflects glycemic control in only the approximately 1 to 2 weeks before sampling,25 so monthly monitoring is required.
Glycated albumin levels may be reduced due to increased albumin turnover in patients with nephrotic-range proteinuria and in diabetic patients on peritoneal dialysis. Several issues remain unclear, such as the appropriate target level of glycated albumin and at what stage of CKD it should replace hemoglobin A1c testing. If an improved assay that is unaffected by changes in serum albumin becomes available, it may be appropriate to use glycated albumin measurements to assess long-term glycemic control for patients with CKD.
In general, therapeutic decisions to achieve optimum glycemic control in patients with diabetes and CKD should be based on hemoglobin A1c testing, multiple glucose measurements, and patient symptoms of hypoglycemia or hyperglycemia. The best measure for assessing glycemic control in hospitalized patients with CKD remains multiple blood glucose testing daily.
INSULIN THERAPY PREFERRED
Although several studies have evaluated inpatient glycemic control,26–29 no guidelines have been published for hospitalized patients with diabetes and CKD. Insulin therapy is preferred for achieving glycemic control in acutely ill or hospitalized patients with diabetes. Oral hypoglycemic agents should be discontinued.
Regardless of the form of insulin chosen to treat diabetes, caution is needed for patients with kidney disease. During hospitalization, clinical changes are expected owing to illness and differences in caloric intake and physical activity, resulting in altered insulin sensitivity. Insulin-treated hospitalized patients require individualized care, including multiple daily blood glucose tests and insulin therapy modifications for ideal glycemic control.
For surgical or medical intensive care patients on insulin therapy, the target blood glucose level before meals should be 140 mg/dL, and the target random level should be less than 180 mg/dL.15,26–29
Basal-bolus insulin
Sliding-scale therapy should be avoided as the only method for glycemic control. Instead, scheduled subcutaneous basal insulin once or twice daily combined with rapid- or short-acting insulin with meals is recommended.
Basal-bolus insulin therapy, one of the most advanced and flexible insulin replacement therapies, mimics endogenous insulin release and offers great advantages in diabetes care. Using mealtime bolus insulin permits variation in the amount of food eaten; more insulin can be taken with a larger meal and less with smaller meals. A bolus approach offers the flexibility of administering rapid-acting insulin immediately after meals when oral intake is variable.
Individualize insulin therapy
Optimizing glycemic control requires an understanding of the altered pharmacokinetics and pharmacodynamics of insulin in patients with diabetic nephropathy. Table 2 shows the pharmacokinetic profiles of insulin preparations in healthy people. Analogue insulins, which are manufactured by recombinant DNA technology, have conformational changes in the insulin molecule that alter their pharmacokinetics and pharmacodynamics. The rapid-acting analogue insulins are absorbed quickly, making them suitable for postprandial glucose control.
Changes in GFR are associated with altered pharmacokinetics and pharmacodynamics of insulin,30,31 but unlike for oral antidiabetic agents, these properties are not well characterized for insulin preparations in patients with renal insufficiency.13,32–36
CKD may reduce insulin clearance. Rave et al32 reported that the clearance of regular human insulin was reduced by 30% to 40% in patients with type 1 diabetes and a mean estimated GFR of 54 mL/min. They found that the metabolic activity of insulin lispro was more robust than that of short-acting regular human insulin in patients with diabetic nephropathy. In another study, patients with diabetes treated with insulin aspart did not show any significant change in the required insulin dosage in relation to the renal filtration rate.34 Biesenbach et al33 found a 38% reduction in insulin requirements in patients with type 1 diabetes as estimated GFR decreased from 80 mL/min to 10 mL/min. Further studies are required to better understand the safety of insulin in treating hospitalized patients with diabetes and renal insufficiency.
Few studies have compared the pharmacodynamics of long-acting insulins in relation to declining renal function. The long-acting analogue insulins have less of a peak than human insulin and thus better mimic endogenous insulin secretion. For insulin detemir, Lindholm and Jacobsen found no significant differences in the pharmacokinetics related to the stages of CKD.35 When using the long-acting insulins glargine or detemir, one should consider giving much lower doses (half the initial starting dosage) and titrating the dosage until target fasting glucose concentrations are reached to prevent hypoglycemia.
Table 3 summarizes recommended insulin dosage adjustments in CKD based on the literature and our clinical experience.
Considerations for dialysis patients
Subcutaneously administered insulin is eliminated renally, unlike endogenous insulin, which undergoes first-pass metabolism in the liver.13,37 As renal function declines, insulin clearance decreases and the insulin dosage must be reduced to prevent hypoglycemia.
Patients on hemodialysis or peritoneal dialysis pose a challenge for insulin dosing. Hemodialysis improves insulin sensitivity but also increases insulin clearance, making it difficult to determine insulin requirements. Sobngwi et al38 conducted a study in diabetic patients with end-stage renal disease on hemodialysis, using a 24-hour euglycemic clamp. They found that exogenous basal insulin requirements were 25% lower on the day after hemodialysis compared with the day before, but premeal insulin requirements stayed the same.
Peritoneal dialysis exposes patients to a high glucose load via the peritoneum, which can worsen insulin resistance. Intraperitoneal administration of insulin during peritoneal dialysis provides a more physiologic effect than subcutaneous administration: it prevents fluctuations of blood glucose and the formation of insulin antibodies. But insulin requirements are higher owing to a dilutional effect and to insulin binding to the plastic surface of the dialysis fluid reservoir.39
GLYCEMIC CONTROL FOR PROCEDURES
No guidelines have been established regarding the optimal blood glucose range for diabetic patients with CKD undergoing diagnostic or surgical procedures. Given the risk of hypoglycemia in such settings, less-stringent targets are reasonable, ie, premeal blood glucose levels of 140 mg/dL and random blood glucose levels of less than 180 mg/dL.
Before surgery, consideration should be given to the type of diabetes, surgical procedure, and metabolic control. Patients on insulin detemir or glargine as part of a basal-bolus regimen with rapid-acting insulin may safely be given the full dose of their basal insulin the night before or the morning of their procedure. However, patients on neutral protamine Hagedorn (NPH) insulin as a part of their basal-bolus regimen should receive half of their usual dose due to a difference in pharmacokinetic profile compared with insulin glargine or detemir.
In insulin-treated patients undergoing prolonged procedures (eg, coronary artery bypass grafting, transplant):
- Discontinue subcutaneous insulin and start an intravenous insulin infusion, titrated to maintain a blood glucose range of 140 to 180 mg/dL
- Subcutaneous insulin management may be acceptable for patients undergoing shorter outpatient procedures
- Supplemental subcutaneous doses of short- or rapid-acting insulin preparations can be given for blood glucose elevation greater than 180 mg/dL.
AVOID ORAL AGENTS AND NONINSULIN INJECTABLES
Oral antidiabetic agents and noninsulin injectables (Table 4) should generally be avoided in hospitalized patients, especially for those with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, or for those given intravenous contrast. Most oral medications used to treat diabetes are affected by reduced kidney function, resulting in prolonged drug exposure and increased risk of hypoglycemia in patients with moderate to severe CKD (stages 3–5).
Metformin, a biguanide, is contraindicated in patients with high serum creatinine levels (> 1.5 mg/dL in men, > 1.4 mg/dL in women) because of the theoretical risk of lactic acidosis.40
Sulfonylurea clearance depends on kidney function.41 Severe prolonged episodes of hypoglycemia have been reported in dialysis patients taking these drugs, except with glipizide, which carries a lower risk.41,42
Repaglinide, a nonsulfonylurea insulin secretagogue, can be used in CKD stages 3 to 4 without any dosage adjustment.43
Thiazolidinediones have been reported to slow the progression of diabetic kidney disease independent of glycemic control.44 Adverse effects include fluid retention, edema, and congestive heart failure. Thiazolidinediones should not be used in patients with New York Heart Association class 3 or 4 heart failure,45 and so should not be prescribed in the hospital except for patients who are clinically stable or ready for discharge.
Quick-release bromocriptine, a dopamine receptor agonist, has been shown to be effective in lowering fasting plasma glucose levels and hemoglobin A1c, and improving glucose tolerance in obese patients with type 2 diabetes, although its usefulness in hospitalized patients with diabetes is not known.46,47
Dipeptidyl peptidase inhibitors. Sitagliptin and saxagliptin have been shown to be safe and effective in hospitalized patients with type 2 diabetes.48 However, except for linagliptin, dose reduction is recommended in patients with CKD stage 3 and higher.49–52
GLP-1 receptor agonists. Drugs of this class are potent agents for the reduction of glucose in the outpatient setting but are relatively contraindicated if the GFR is less than 30 mL/min, and they are currently not used in the hospital.
BLOOD GLUCOSE MONITORING IN HOSPITALIZED PATIENTS
Bedside blood glucose monitoring is recommended for all hospitalized patients with known diabetes with or without CKD, those with newly recognized hyperglycemia, and those who receive therapy associated with high risk for hyperglycemia, such as glucocorticoid therapy and enteral and parenteral nutrition. For patients on scheduled diets, fingerstick blood glucose monitoring is recommended before meals and at bedtime. In patients with no oral intake or on continuous enteral or parenteral nutrition, blood glucose monitoring every 4 to 6 hours is recommended. More frequent monitoring (eg, adding a 3:00 am check) may be prudent in patients with CKD.
Continuous glucose monitoring systems use a sensor inserted under the skin and transmit information via radio to a wireless monitor. Such systems are more expensive than conventional glucose monitoring but may enable better glucose control by providing real-time glucose measurements, with levels displayed at 5-minute or 1-minute intervals. Marshall et al53 confirmed this technology’s accuracy and precision in uremic patients on dialysis.
Considerations for peritoneal dialysis
For patients on peritoneal dialysis, glucose in the dialysate exacerbates hyperglycemia. Dialysis solutions with the glucose polymer icodextrin as the osmotic agent instead of glucose have been suggested to reduce glucose exposure.
Glucose monitoring systems measure interstitial fluid glucose by the glucose oxidase reaction and therefore are not affected by icodextrin. However, icodextrin is converted to maltose, a disaccharide composed of two glucose molecules, which can cause spuriously high readings in devices that use test strips containing the enzymes glucose dehydrogenase pyrroloquinoline quinone or glucose dye oxidoreductase. Spurious hyperglycemia may lead to giving too much insulin, in turn leading to symptomatic hypoglycemia.
Clinicians caring for patients receiving icodextrin should ensure that the glucose monitoring system uses only test strips that contain glucose oxidase, glucose dehydrogenase-nicotinamide adenine dinucleotide, or glucose dehydrogenase-flavin adenine dinucleotide, which are not affected by icodextrin.54
IMPROVING QUALITY
Hospitalized patients face many barriers to optimal glycemic control. Less experienced practitioners tend to have insufficient knowledge of insulin preparations and appropriate insulin dosing. Also, diabetes is often listed as a secondary diagnosis and so may be overlooked by the inpatient care team.
Educational programs should be instituted to overcome these barriers and improve knowledge related to inpatient diabetes care. When necessary, the appropriate use of consultants is important in hospitalized settings to improve quality and make hospital care more efficient and cost-effective.
No national benchmarks currently exist for inpatient diabetes care, and they need to be developed to ensure best practices. Physicians should take the initiative to remedy this by collaborating with other healthcare providers, such as dedicated diabetes educators, nursing staff, pharmacists, registered dietitians, and physicians with expertise in diabetes management, with the aim of achieving optimum glycemic control and minimizing hypoglycemia.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305.
- Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess 2005; 9:iii–vi, xiii–163.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999; 22:1408–1414.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75:894–903.
- Nathan DM, Lachin J, Cleary P, et al; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348:2294–2303.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4:1121–1127.
- Iglesias P, Díez J. Insulin therapy in renal disease. Diabetes Obes Metab 2008; 10:811–823.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010; 23:148–156.
- De Marchi S, Cecchin E, Camurri C, et al. Origin of glycosylated hemoglobin A1 in chronic renal failure. Int J Artif Organs 1983; 6:77–82.
- Brown JN, Kemp DW, Brice KR. Class effect of erythropoietin therapy on hemoglobin A(1c) in a patient with diabetes mellitus and chronic kidney disease not undergoing hemodialysis. Pharmacotherapy 2009; 29:468–472.
- Morgan L, Marenah CB, Jeffcoate WJ, Morgan AG. Glycated proteins as indices of glycemic control in diabetic patients with chronic renal failure. Diabet Med 1996; 13:514–519.
- Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008; 73:1062–1068.
- Joy MS, Cefalu WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int 2010; 78(suppl 117):S41–S45.
- Alskar O, Korelli J, Duffull SB. A pharmacokinetic model for the glycation of albumin. J Pharmacokinet Pharmacodyn 2012; 39:273–282.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Bogun M, Inzucchi SE. Inpatient management of diabetes and hyperglycemia. Clin Ther 2013; 35:724–733.
- Miller DB. Glycemic targets in hospital and barriers to attaining them. Can J Diabetes 2014; 38:74–78.
- Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 1995; 38:565–572.
- Svensson M, Yu Z, Eriksson J. A small reduction in glomerular filtration is accompanied by insulin resistance in type I diabetes patients with diabetic nephropathy. Eur J Clin Invest 2002; 32:100–109.
- Rave K, Heise T, Pfutzner A, Heinemann L, Sawicki P. Impact of diabetic nephropathy on pharmacodynamics and pharmacokinetic properties of insulin in type I diabetic patients. Diabetes Care 2001; 24:886–890.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol 2005; 60:469–476.
- Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40:641–659.
- Bolli GB, Hahn AD, Schmidt R, et al. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care 2012; 35:2626–2630.
- Nielsen S. Time course and kinetics of proximal tubular processing of insulin. Am J Physiol 1992; 262:F813–F822.
- Sobngwi E, Enoru S, Ashuntantang G, et al. Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care 2010; 33:1409–1412.
- Quellhorst E. Insulin therapy during peritoneal dialysis: pros and cons of various forms of administration. J Am Soc Nephrol 2002; 13(suppl 1):S92–S96.
- Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med 1997; 102:99–110.
- Ahmed Z, Simon B, Choudhury D. Management of diabetes in patients with chronic kidney disease. Postgrad Med 2009; 121:52–60.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26(suppl 4):73–85.
- Hasslacher C; Multinational Repaglinide Renal Study Group. Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care 2003; 26:886–891.
- Iglesias P, Dies JJ. Peroxisome proliferator-activated receptor gamma agonists in renal disease. Eur J Endocrinol 2006; 154:613–621.
- Hollenberg NK. Considerations for management of fluid dynamic issues associated with thiazolidinediones. Am J Med 2003; 115(suppl. 8A) 111S–115S.
- Kamath V, Jones CN, Yip JC, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care 1997; 20:1697–1701.
- Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care 2000; 23:1154–1161.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10:545–555.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Onglyza package insert. www.azpicentral.com/onglyza/pi_onglyza.pdf. Accessed March 8, 2016.
- Gallwitz B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther Adv Endocrinol Metab 2013; 4:95–105.
- Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int 2003; 64:1480–1486.
- Schleis TG. Interference of maltose, icodextrin, galactose, or xylose with some blood glucose monitoring systems. Pharmacotherapy 2007; 27:1313–1321.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305.
- Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess 2005; 9:iii–vi, xiii–163.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999; 22:1408–1414.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75:894–903.
- Nathan DM, Lachin J, Cleary P, et al; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348:2294–2303.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4:1121–1127.
- Iglesias P, Díez J. Insulin therapy in renal disease. Diabetes Obes Metab 2008; 10:811–823.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010; 23:148–156.
- De Marchi S, Cecchin E, Camurri C, et al. Origin of glycosylated hemoglobin A1 in chronic renal failure. Int J Artif Organs 1983; 6:77–82.
- Brown JN, Kemp DW, Brice KR. Class effect of erythropoietin therapy on hemoglobin A(1c) in a patient with diabetes mellitus and chronic kidney disease not undergoing hemodialysis. Pharmacotherapy 2009; 29:468–472.
- Morgan L, Marenah CB, Jeffcoate WJ, Morgan AG. Glycated proteins as indices of glycemic control in diabetic patients with chronic renal failure. Diabet Med 1996; 13:514–519.
- Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008; 73:1062–1068.
- Joy MS, Cefalu WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int 2010; 78(suppl 117):S41–S45.
- Alskar O, Korelli J, Duffull SB. A pharmacokinetic model for the glycation of albumin. J Pharmacokinet Pharmacodyn 2012; 39:273–282.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Bogun M, Inzucchi SE. Inpatient management of diabetes and hyperglycemia. Clin Ther 2013; 35:724–733.
- Miller DB. Glycemic targets in hospital and barriers to attaining them. Can J Diabetes 2014; 38:74–78.
- Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 1995; 38:565–572.
- Svensson M, Yu Z, Eriksson J. A small reduction in glomerular filtration is accompanied by insulin resistance in type I diabetes patients with diabetic nephropathy. Eur J Clin Invest 2002; 32:100–109.
- Rave K, Heise T, Pfutzner A, Heinemann L, Sawicki P. Impact of diabetic nephropathy on pharmacodynamics and pharmacokinetic properties of insulin in type I diabetic patients. Diabetes Care 2001; 24:886–890.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol 2005; 60:469–476.
- Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40:641–659.
- Bolli GB, Hahn AD, Schmidt R, et al. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care 2012; 35:2626–2630.
- Nielsen S. Time course and kinetics of proximal tubular processing of insulin. Am J Physiol 1992; 262:F813–F822.
- Sobngwi E, Enoru S, Ashuntantang G, et al. Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care 2010; 33:1409–1412.
- Quellhorst E. Insulin therapy during peritoneal dialysis: pros and cons of various forms of administration. J Am Soc Nephrol 2002; 13(suppl 1):S92–S96.
- Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med 1997; 102:99–110.
- Ahmed Z, Simon B, Choudhury D. Management of diabetes in patients with chronic kidney disease. Postgrad Med 2009; 121:52–60.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26(suppl 4):73–85.
- Hasslacher C; Multinational Repaglinide Renal Study Group. Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care 2003; 26:886–891.
- Iglesias P, Dies JJ. Peroxisome proliferator-activated receptor gamma agonists in renal disease. Eur J Endocrinol 2006; 154:613–621.
- Hollenberg NK. Considerations for management of fluid dynamic issues associated with thiazolidinediones. Am J Med 2003; 115(suppl. 8A) 111S–115S.
- Kamath V, Jones CN, Yip JC, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care 1997; 20:1697–1701.
- Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care 2000; 23:1154–1161.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10:545–555.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Onglyza package insert. www.azpicentral.com/onglyza/pi_onglyza.pdf. Accessed March 8, 2016.
- Gallwitz B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther Adv Endocrinol Metab 2013; 4:95–105.
- Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int 2003; 64:1480–1486.
- Schleis TG. Interference of maltose, icodextrin, galactose, or xylose with some blood glucose monitoring systems. Pharmacotherapy 2007; 27:1313–1321.
KEY POINTS
- Hemoglobin A1c values are often unreliable in patients with end-stage renal disease; close monitoring by fingerstick testing or a continuous monitoring system is recommended during hospitalization.
- Insulin is the preferred treatment for hospitalized patients with diabetes; oral antidiabetic agents should be avoided.
- Blood glucose targets for hospitalized patients with diabetes or stress hyperglycemia should be less than 140 mg/dL before meals, and random values should be less than 180 mg/dL.
- A basal-bolus insulin approach is flexible and mimics endogenous insulin release.
- Many insulin-treated patients with type 2 diabetes and CKD stop needing insulin as kidney disease progresses.
IMWG issues renal impairment recommendations for myeloma patients
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: All patients diagnosed with multiple myeloma should be evaluated for renal impairment.
Major finding: Bortezomib-based regimens are the standard of care for patients with multiple myeloma.
Data source: Evidence-based clinical recommendations.
Disclosures: Many coauthors disclosed multiple relationships with companies that make antimyeloma therapies and other medications.
Test All Kidney Transplant Patients for Hepatitis E
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
FROM ANNALS OF INTERNAL MEDICINE
Test all kidney transplant patients for hepatitis E
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
On Twitter @richpizzi
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
On Twitter @richpizzi
All kidney transplant recipients with abnormal liver function test results should be tested for hepatitis E virus RNA, according to a recent finding by French investigators.
Hepatitis E virus (HEV) is most often transmitted through the fecal-oral route in contaminated drinking water or food, but it also can be transmitted by blood and blood products. A research letter from Dr. Vincent Mallett of the Université Paris Descartes Sorbonne Paris Cité and colleagues published in Annals of Internal Medicine reported a case of HEV transmission to a kidney transplant recipient through plasma exchange.
Nineteen months after a 48-year-old patient received a kidney transplant, Dr. Mallett and his colleagues detected HEV RNA genotype 3f in the patient’s blood based on sequencing, and the patient tested positive for anti-HEV IgG and negative for anti-HEV IgM. The physicians confirmed that the patient had been infected for more than 1 year by finding HEV RNA in a frozen plasma sample drawn 5 months after transplantation. The kidney donor had tested negative for HEV RNA, and the patient’s stored blood samples tested negative for HEV markers before transplantation.
The investigators said that the method of HEV transmission remained undetermined until the investigators tested for HEV RNA in stored samples of all 18 blood products used during the peritransplantation period. From a single sample of fresh frozen plasma from a donor who had tested negative on multiple occasions for hepatitis C virus, HIV-1 and -2, and hepatitis B virus before the plasma was used, the researchers recovered a strain of HEV identical to the one infecting the patient. This plasma had been used during a plasma exchange for treating acute humoral rejection.
Plasma exchange typically involves the removal of 2-5 L of plasma several times a week, Dr. Mallett and associates said, which often is replaced with donor plasma. If replacement involves 2.5 L (10 bags) of donor plasma, which is a typical amount, then the risk for HEV is 10 times greater than the risk involving a single bag.
“In some circumstances, replacement procedures use plasma that has been pooled from many donors and then treated with solvents and detergents to inactivate infectious agents,” they wrote. “However, HEV is not affected by this treatment, so pooling multiplies the risk for infection.”
On the basis of these findings, the coauthors said that “all kidney transplant recipients with abnormal liver function test results, especially those treated with plasma exchange, should be tested for HEV RNA.”
Read the letter in Annals of Internal Medicine (2016 Mar 1. doi: 10.7326/L15-0502).
On Twitter @richpizzi
FROM ANNALS OF INTERNAL MEDICINE