User login
Interferon-free regimen improves response in HCV
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Major finding: For patients with genotype 1a infection, SVR occurred in 97% with ribavirin and 90.2% without ribavirin. For patients with genotype 1b infection, rates were 99.5% and 99%, respectively.
Data source: Two randomized, phase III trials of previously untreated patients with hepatitis C and no cirrhosis who were treated with 12 weeks of ABT-450/r, ombitasvir, and dasabuvir with or without ribavirin.
Disclosures: AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
VIDEO: Is wine good for the kidneys?
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
AT SCM 2014
Antimicrobial prophylaxis halves UTI risk in pediatric vesicoureteral reflux
VANCOUVER – Two years of low-dose trimethoprim-sulfamethoxazole prophylaxis halved the risk of recurrent urinary tract infections, but did not prevent renal scarring in a trial of 607 children with vesicoureteral reflux that was published online May 4 in the New England Journal of Medicine, and presented concurrently at the annual meeting of the Pediatric Academic Societies.
Within 112 days of their first or second febrile or symptomatic urinary tract infection (UTI), 302 young children diagnosed with vesicoureteral reflux (VUR) by voiding cystourethrogram were randomized to 3 mg of trimethoprim plus 15 mg of sulfamethoxazole per kilogram; and 305 other VUR children were randomized to placebo.
Thirty-nine (13%) children who received antimicrobial prophylaxis developed a recurrent febrile or symptomatic UTI, compared with 72 (24%) who received a placebo (hazard ratio for risk of recurrence, 0.50; 95% confidence interval, 0.34-0.74). Prophylaxis was particularly effective in children whose index UTI was febrile (HR, 0.41; 95% CI, 0.26-0.64) and in those with baseline bladder and bowel dysfunction (HR, 0.21; 95% CI, 0.08-0.58).
Nuclear imaging showed no significant between-group differences in the incidence of renal scarring (11.9% in the treated group vs. 10.2% in the placebo group; P = 0.55), severe renal scars (4.0% vs. 2.6%; P = 0.37), or new renal scars since baseline (8.2% vs. 8.4%; P = 0.94) at trial completion (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMoa1401811]).
"This study showed unequivocal evidence that antimicrobial prophylaxis reduced at least in half the likelihood of children having recurrent UTIs. Rates of renal scarring ... were low and not reduced by prophylaxis, perhaps because most children were enrolled after their first infection and because parents, instructed to be vigilant, sought early medical attention," said Dr. Alejandro Hoberman, a professor of pediatrics at the University of Pittsburgh and lead investigator in the multicenter study, dubbed the RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial.
"As long as evidence supporting the benefit of prophylaxis was dubious, the recommendation of a watchful-waiting approach, without performance of a voiding cystourethrographic study, seemed reasonable, because the imaging findings would not affect the nature of treatment. However, our finding that antimicrobial prophylaxis was associated with a reduced risk of recurrence may warrant reconsideration of that recommendation," the investigators said.
Eight children would have had to be treated for 2 years to prevent one case of febrile or symptomatic UTI. Several audience members, after hearing the results, wondered if the benefits of prophylaxis outweighed the costs, given that there was no effect on the incidence of renal scarring in the short term, and the difficulty and expense of performing voiding cystourethrographic studies, among other concerns.
Dr. Hoberman plans to investigate the cost-effectiveness implications of the findings, and, in the meantime, he noted that the study offers proof that prophylaxis helps prevent recurrent UTIs, something that was uncertain in the past. Also, he noted, the study was not powered to detect a difference in renal scarring as a primary outcome.
The children were aged 2-71 months (median age, 12 months), and 92% were girls. Eighty percent had grade II or III vesicoureteral reflux, and 48% had bilateral reflux.
Among 87 children with a first recurrence caused by Escherichia coli, the proportion of isolates that were resistant to trimethoprim-sulfamethoxazole was 63% in the prophylaxis group and 19% in the placebo group. "Not unexpectedly, recurrences that did occur in children who received prophylaxis were more likely to have been caused by a resistant pathogen," the investigators said.
Parents of 77% of the children reported that they had given the study medication at least 75% of the time, and parents of 85% reported administering it at least 50% of the time. There was no significant difference in reported adherence between the study groups.
Dr. Hoberman had no disclosures. The work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
"As in most studies of complex conditions, unresolved questions remain. Only one form of antibiotic prophylaxis was used [in the study]; therefore, the effectiveness of other prophylactic antibiotic strategies remains untested. The evaluation of scarring was determined after only 2 years, leaving the long-term degree of renal injury unknown.
Sadly, the decision to use antibiotic prophylaxis in children with reflux remains a clinical dilemma, despite this well-done study. In the face of the emergence of antibiotic resistance, the lack of a significant between-group difference in renal parenchymal scarring, and questions about generalizability, the RIVUR study results would imply that the general recommendation of prophylactic antibiotics for vesicoureteral reflux in young children awaits more evidence before universal adoption."
Dr. Julie Ingelfinger is the senior consultant in pediatric nephrology at the Massachusetts General Hospital in Boston. Dr. F. Bruder Stapleton is a professor and chair of pediatrics at the University of Washington in Seattle. They made their comments in an editorial that accompanied the study (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMe1404774]), and they had no relevant disclosures.
"As in most studies of complex conditions, unresolved questions remain. Only one form of antibiotic prophylaxis was used [in the study]; therefore, the effectiveness of other prophylactic antibiotic strategies remains untested. The evaluation of scarring was determined after only 2 years, leaving the long-term degree of renal injury unknown.
Sadly, the decision to use antibiotic prophylaxis in children with reflux remains a clinical dilemma, despite this well-done study. In the face of the emergence of antibiotic resistance, the lack of a significant between-group difference in renal parenchymal scarring, and questions about generalizability, the RIVUR study results would imply that the general recommendation of prophylactic antibiotics for vesicoureteral reflux in young children awaits more evidence before universal adoption."
Dr. Julie Ingelfinger is the senior consultant in pediatric nephrology at the Massachusetts General Hospital in Boston. Dr. F. Bruder Stapleton is a professor and chair of pediatrics at the University of Washington in Seattle. They made their comments in an editorial that accompanied the study (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMe1404774]), and they had no relevant disclosures.
"As in most studies of complex conditions, unresolved questions remain. Only one form of antibiotic prophylaxis was used [in the study]; therefore, the effectiveness of other prophylactic antibiotic strategies remains untested. The evaluation of scarring was determined after only 2 years, leaving the long-term degree of renal injury unknown.
Sadly, the decision to use antibiotic prophylaxis in children with reflux remains a clinical dilemma, despite this well-done study. In the face of the emergence of antibiotic resistance, the lack of a significant between-group difference in renal parenchymal scarring, and questions about generalizability, the RIVUR study results would imply that the general recommendation of prophylactic antibiotics for vesicoureteral reflux in young children awaits more evidence before universal adoption."
Dr. Julie Ingelfinger is the senior consultant in pediatric nephrology at the Massachusetts General Hospital in Boston. Dr. F. Bruder Stapleton is a professor and chair of pediatrics at the University of Washington in Seattle. They made their comments in an editorial that accompanied the study (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMe1404774]), and they had no relevant disclosures.
VANCOUVER – Two years of low-dose trimethoprim-sulfamethoxazole prophylaxis halved the risk of recurrent urinary tract infections, but did not prevent renal scarring in a trial of 607 children with vesicoureteral reflux that was published online May 4 in the New England Journal of Medicine, and presented concurrently at the annual meeting of the Pediatric Academic Societies.
Within 112 days of their first or second febrile or symptomatic urinary tract infection (UTI), 302 young children diagnosed with vesicoureteral reflux (VUR) by voiding cystourethrogram were randomized to 3 mg of trimethoprim plus 15 mg of sulfamethoxazole per kilogram; and 305 other VUR children were randomized to placebo.
Thirty-nine (13%) children who received antimicrobial prophylaxis developed a recurrent febrile or symptomatic UTI, compared with 72 (24%) who received a placebo (hazard ratio for risk of recurrence, 0.50; 95% confidence interval, 0.34-0.74). Prophylaxis was particularly effective in children whose index UTI was febrile (HR, 0.41; 95% CI, 0.26-0.64) and in those with baseline bladder and bowel dysfunction (HR, 0.21; 95% CI, 0.08-0.58).
Nuclear imaging showed no significant between-group differences in the incidence of renal scarring (11.9% in the treated group vs. 10.2% in the placebo group; P = 0.55), severe renal scars (4.0% vs. 2.6%; P = 0.37), or new renal scars since baseline (8.2% vs. 8.4%; P = 0.94) at trial completion (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMoa1401811]).
"This study showed unequivocal evidence that antimicrobial prophylaxis reduced at least in half the likelihood of children having recurrent UTIs. Rates of renal scarring ... were low and not reduced by prophylaxis, perhaps because most children were enrolled after their first infection and because parents, instructed to be vigilant, sought early medical attention," said Dr. Alejandro Hoberman, a professor of pediatrics at the University of Pittsburgh and lead investigator in the multicenter study, dubbed the RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial.
"As long as evidence supporting the benefit of prophylaxis was dubious, the recommendation of a watchful-waiting approach, without performance of a voiding cystourethrographic study, seemed reasonable, because the imaging findings would not affect the nature of treatment. However, our finding that antimicrobial prophylaxis was associated with a reduced risk of recurrence may warrant reconsideration of that recommendation," the investigators said.
Eight children would have had to be treated for 2 years to prevent one case of febrile or symptomatic UTI. Several audience members, after hearing the results, wondered if the benefits of prophylaxis outweighed the costs, given that there was no effect on the incidence of renal scarring in the short term, and the difficulty and expense of performing voiding cystourethrographic studies, among other concerns.
Dr. Hoberman plans to investigate the cost-effectiveness implications of the findings, and, in the meantime, he noted that the study offers proof that prophylaxis helps prevent recurrent UTIs, something that was uncertain in the past. Also, he noted, the study was not powered to detect a difference in renal scarring as a primary outcome.
The children were aged 2-71 months (median age, 12 months), and 92% were girls. Eighty percent had grade II or III vesicoureteral reflux, and 48% had bilateral reflux.
Among 87 children with a first recurrence caused by Escherichia coli, the proportion of isolates that were resistant to trimethoprim-sulfamethoxazole was 63% in the prophylaxis group and 19% in the placebo group. "Not unexpectedly, recurrences that did occur in children who received prophylaxis were more likely to have been caused by a resistant pathogen," the investigators said.
Parents of 77% of the children reported that they had given the study medication at least 75% of the time, and parents of 85% reported administering it at least 50% of the time. There was no significant difference in reported adherence between the study groups.
Dr. Hoberman had no disclosures. The work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
VANCOUVER – Two years of low-dose trimethoprim-sulfamethoxazole prophylaxis halved the risk of recurrent urinary tract infections, but did not prevent renal scarring in a trial of 607 children with vesicoureteral reflux that was published online May 4 in the New England Journal of Medicine, and presented concurrently at the annual meeting of the Pediatric Academic Societies.
Within 112 days of their first or second febrile or symptomatic urinary tract infection (UTI), 302 young children diagnosed with vesicoureteral reflux (VUR) by voiding cystourethrogram were randomized to 3 mg of trimethoprim plus 15 mg of sulfamethoxazole per kilogram; and 305 other VUR children were randomized to placebo.
Thirty-nine (13%) children who received antimicrobial prophylaxis developed a recurrent febrile or symptomatic UTI, compared with 72 (24%) who received a placebo (hazard ratio for risk of recurrence, 0.50; 95% confidence interval, 0.34-0.74). Prophylaxis was particularly effective in children whose index UTI was febrile (HR, 0.41; 95% CI, 0.26-0.64) and in those with baseline bladder and bowel dysfunction (HR, 0.21; 95% CI, 0.08-0.58).
Nuclear imaging showed no significant between-group differences in the incidence of renal scarring (11.9% in the treated group vs. 10.2% in the placebo group; P = 0.55), severe renal scars (4.0% vs. 2.6%; P = 0.37), or new renal scars since baseline (8.2% vs. 8.4%; P = 0.94) at trial completion (N. Eng. J. Med. 2014 May 4 [doi: 10.1056/NEJMoa1401811]).
"This study showed unequivocal evidence that antimicrobial prophylaxis reduced at least in half the likelihood of children having recurrent UTIs. Rates of renal scarring ... were low and not reduced by prophylaxis, perhaps because most children were enrolled after their first infection and because parents, instructed to be vigilant, sought early medical attention," said Dr. Alejandro Hoberman, a professor of pediatrics at the University of Pittsburgh and lead investigator in the multicenter study, dubbed the RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial.
"As long as evidence supporting the benefit of prophylaxis was dubious, the recommendation of a watchful-waiting approach, without performance of a voiding cystourethrographic study, seemed reasonable, because the imaging findings would not affect the nature of treatment. However, our finding that antimicrobial prophylaxis was associated with a reduced risk of recurrence may warrant reconsideration of that recommendation," the investigators said.
Eight children would have had to be treated for 2 years to prevent one case of febrile or symptomatic UTI. Several audience members, after hearing the results, wondered if the benefits of prophylaxis outweighed the costs, given that there was no effect on the incidence of renal scarring in the short term, and the difficulty and expense of performing voiding cystourethrographic studies, among other concerns.
Dr. Hoberman plans to investigate the cost-effectiveness implications of the findings, and, in the meantime, he noted that the study offers proof that prophylaxis helps prevent recurrent UTIs, something that was uncertain in the past. Also, he noted, the study was not powered to detect a difference in renal scarring as a primary outcome.
The children were aged 2-71 months (median age, 12 months), and 92% were girls. Eighty percent had grade II or III vesicoureteral reflux, and 48% had bilateral reflux.
Among 87 children with a first recurrence caused by Escherichia coli, the proportion of isolates that were resistant to trimethoprim-sulfamethoxazole was 63% in the prophylaxis group and 19% in the placebo group. "Not unexpectedly, recurrences that did occur in children who received prophylaxis were more likely to have been caused by a resistant pathogen," the investigators said.
Parents of 77% of the children reported that they had given the study medication at least 75% of the time, and parents of 85% reported administering it at least 50% of the time. There was no significant difference in reported adherence between the study groups.
Dr. Hoberman had no disclosures. The work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
AT THE PAS ANNUAL MEETING
Major finding: Over 2 years, 13% of children with vesicoureteral reflux who received antimicrobial prophylaxis developed a recurrent febrile or symptomatic UTI, compared with 24% who received a placebo (HR for risk of recurrence, 0.50; 95% CI, 0.34-0.74).
Data Source: Randomized, placebo-controlled trial in 607 children aged 2-71 months.
Disclosures: The lead investigator had no disclosures. The work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
The generalist, the specialist, and the patient with chronic kidney disease
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
Managing advanced chronic kidney disease: A primary care guide
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
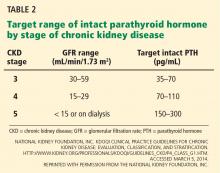
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
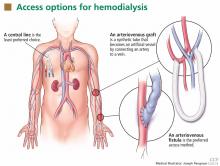
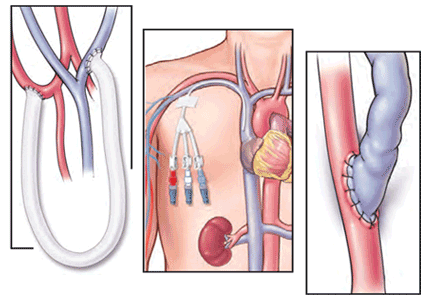
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
KEY POINTS
- Steps to stabilize renal function include blood pressure and diabetes control.
- Patients have a very high risk of cardiovascular disease, and one should try to reduce modifiable risk factors such as hypertension (which is also a risk factor for the progression of CKD) and hyperlipidemia.
- In addition to controlling blood pressure, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce proteinuria, a risk factor for progression of CKD.
- Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and, in advanced CKD, hypocalcemia, all leading to disorders of bone mineral metabolism. Low vitamin D levels should be raised with supplements, and high phosphorus levels should be lowered with dietary restriction and phosphate binders.
Novel complex shows unique benefits in chronic kidney disease
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.
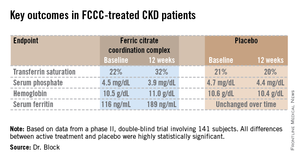
"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
AT SCM 14
Major finding: FCCC safely repleted low iron stores, reduced elevated serum phosphate, raised hemoglobin, and slashed FGF23 levels, compared with placebo.
Data source: A prospective, double-blind, placebo-controlled, 12-week trial in which 141 patients with non–dialysis-dependent CKD, iron-deficiency anemia, and elevated serum phosphate were assigned to FCCC or placebo.
Disclosures: The study was sponsored by Keryx Biopharmaceuticals. Dr. Block is a consultant to the company and was principal investigator in the trial.
Anemia, A1C, and Rhabdomyolysis
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Chronic kidney disease may raise cancer risk
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
AT SCM 14
Major finding: Patients with chronic kidney disease had an adjusted 55% increased risk of cancer-related mortality during a mean 8.9 years of prospective follow-up, compared with those with normal kidney function.
Data source: This was a post hoc analysis of 31,896 participants in the prospective ALLHAT study.
Disclosures: ALLHAT was funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Herbal medicines and supplements carry risk of hepatoxicity
LONDON – Herbal medicines and other home remedies or supplements are a significant cause of hepatotoxicity experts warned recently during a symposium at the International Liver Congress, which unintentionally coincided with World Homeopathy Awareness Week.
Although they are not at the very top of the list when it comes to drug-induced liver injury (DILI) – that accolade being reserved for antimicrobial agents used to treat tuberculosis – the use of homeopathy-based approaches are potentially on the increase in the western world and the use of such substances are often not reported to physicians.
"Herbal medicines represent a significant cause of liver injury," Dr. Dominique Larrey (Central University Hospital, Montpellier, France), said at the meeting that was sponsored by the European Association for the Study of the Liver. "Herbs can cause almost the whole spectrum of hepatic and biliary lesions, acute hepatitis being the most frequent one," he added.
The rise of herbal medicine
Dr. Larrey, who works in the liver and transplantation department of Saint Eloi Hospital, also in Montpellier, noted that the use of herbs in traditional medicine was very important in many parts of the world, notably in Asia, Africa, and Central and South America. They are used for both traditional and cultural reasons, he added, are often easy to access and are low cost in comparison to regulated medicines.
Their use is probably on the increase in western countries for a variety of reasons, Dr. Larrey suggested, such as the migration of people from cultures in which the use of traditional medicines is high, to the thinking that "what is natural can only be good" and "herbal medicines are considered completely innocuous in contrast to classical drugs." Furthermore, the lack of satisfactory treatments for some severe diseases – cancer, multiple sclerosis, AIDS, and hepatitis C virus infection to name a few – mean that people often are willing to try out complementary or alternative medicines (CAM).
In the United States, the total sale of herbal remedies in 2010 was an estimated $5.2 billion per year, having increased around 3% a year over the past decade, Dr. Larrey pointed out.
The problem is that patients do not often tell their doctors about their use of CAM. A staggering 90% of patients taking the anticoagulant drug warfarin – which is renowned for having a very narrow therapeutic window and careful monitoring is required – were taking herbal medicines in one study, he said.
Prospective studies on the use of herbal medicines in western countries are scarce but those that have been conducted specifically in patients with liver disease suggest that as many as one-fifth (Hepatology 2008;47:605-12) to one-third (Gastroenterology 2001;120[Suppl 1]:A228) might be taking herbal remedies unbeknownst to their doctor.
The problem of assessment
There are limited data on how frequently herbal medicines cause liver damage, but estimates range from 2% to 16%, Dr. Larrey observed, adding that reported cases could be just the tip of the iceberg.
"Herbal medicine hepatoxicity is clearly underestimated for many reasons," he suggested. First, their intake is hard to analyze. Second, the mechanism of liver damage is often uncertain, and third, it is hard to confirm causality. Indeed, herbal medicines do not have to undergo the rigorous testing or regulation in the same way that prescribed medicines do, and sales via the Internet make them easily available to all.
There is then the uncertainty of what is really in the preparations, if they contain the right plant at all or the wrong part of it, and then whether or not they have been stored correctly, or if they have been contaminated with other liver-damaging agents or microorganisms.
Advice for physicians
DILI from prescription and nonprescription medicines is an important but rare event in the westernized world, Dr. Robert Fontana of the University of Michigan in Ann Arbor said in an interview. However, because it can bring about very bad and unpredictable liver injury, it is of great importance for hepatologists and general family physicians alike.
"In the United States and I think worldwide, the frequency in use of [herbal treatments] is increasing and as we start to see registry data I think we will start to see more and more cases [of hepatoxicity]," Dr. Fontana said.
Dr. Fontana is part of the National Institutes of Health–funded Drug-Induced Liver Injury Network (DILIN), a multicenter, prospective registry looking at the etiologies, risk factors, and outcomes of DILI in the United States (Drug Saf. 2009;32:55-68). Data from the registry show the prevalence of herbal and dietary supplements is around 9% (n = 300) in confirmed DILI cases.
"Patients need to tell their doctors what they are taking," he advised, adding that, as physicians, "we all need to be aware and maybe ask more questions of our patients."
The LiverTox website – produced by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Library of Medicine – is a valuable online and freely available resource for determining if a medication, herbal, or other supplement is known to cause liver problems. This is going to have a new chapter on herbal medicines, Dr. Fontana said, and is worth using in daily practice to help advise patients on the prescription or CAM they might be taking.
Dr. Larrey and Dr. Fontana had no disclosures relevant to their comments.
New Chinese herbal medicine inhibits HCV activity
A compound named SBEL1 after the laboratory in which it was discovered has multiple effects on the hepatitis C virus (HCV) life cycle, according to data from a late-breaking poster presented at the meeting.
Researchers from the Systems Biology of Epithelia Laboratory at the National Taiwan University, Taipei, screened six herbal medicines and found that one of these – SBEL1 – inhibited HCV activity by about 90% in infected cells.
Cheng-Wei Lin and Ming-Jiun Yu pretreated liver cells with the herbal extract and then infected these cells with HCV. Compared with control cells, SBEL1-treated cells contained 23% less viral protein. This suggested that SBEL1 prevented HCV from entering the pretreated cells.
Their findings also suggested that SBEL1 reduced internal-ribosome entry site–mediated translation, a process vital for viral protein production, and might also have interfered with the RNA replication process.
"SBEL1 has demonstrated significant inhibition of HCV at multiple stages of the viral life cycle," Dr. Markus Peck-Radosavljevic, the secretary-general of the European Association for the Study of the Liver, said in a press release issued by the Society.
Dr. Peck-Radosavljevic (University of Vienna, Austria), who was not involved in the research, added that this "is an exciting discovery because it allows us to gain a deeper understanding of the virus and its interactions with other compounds. Ultimately, this adds to our library of knowledge that may bring us closer to improving future treatment options."
LONDON – Herbal medicines and other home remedies or supplements are a significant cause of hepatotoxicity experts warned recently during a symposium at the International Liver Congress, which unintentionally coincided with World Homeopathy Awareness Week.
Although they are not at the very top of the list when it comes to drug-induced liver injury (DILI) – that accolade being reserved for antimicrobial agents used to treat tuberculosis – the use of homeopathy-based approaches are potentially on the increase in the western world and the use of such substances are often not reported to physicians.
"Herbal medicines represent a significant cause of liver injury," Dr. Dominique Larrey (Central University Hospital, Montpellier, France), said at the meeting that was sponsored by the European Association for the Study of the Liver. "Herbs can cause almost the whole spectrum of hepatic and biliary lesions, acute hepatitis being the most frequent one," he added.
The rise of herbal medicine
Dr. Larrey, who works in the liver and transplantation department of Saint Eloi Hospital, also in Montpellier, noted that the use of herbs in traditional medicine was very important in many parts of the world, notably in Asia, Africa, and Central and South America. They are used for both traditional and cultural reasons, he added, are often easy to access and are low cost in comparison to regulated medicines.
Their use is probably on the increase in western countries for a variety of reasons, Dr. Larrey suggested, such as the migration of people from cultures in which the use of traditional medicines is high, to the thinking that "what is natural can only be good" and "herbal medicines are considered completely innocuous in contrast to classical drugs." Furthermore, the lack of satisfactory treatments for some severe diseases – cancer, multiple sclerosis, AIDS, and hepatitis C virus infection to name a few – mean that people often are willing to try out complementary or alternative medicines (CAM).
In the United States, the total sale of herbal remedies in 2010 was an estimated $5.2 billion per year, having increased around 3% a year over the past decade, Dr. Larrey pointed out.
The problem is that patients do not often tell their doctors about their use of CAM. A staggering 90% of patients taking the anticoagulant drug warfarin – which is renowned for having a very narrow therapeutic window and careful monitoring is required – were taking herbal medicines in one study, he said.
Prospective studies on the use of herbal medicines in western countries are scarce but those that have been conducted specifically in patients with liver disease suggest that as many as one-fifth (Hepatology 2008;47:605-12) to one-third (Gastroenterology 2001;120[Suppl 1]:A228) might be taking herbal remedies unbeknownst to their doctor.
The problem of assessment
There are limited data on how frequently herbal medicines cause liver damage, but estimates range from 2% to 16%, Dr. Larrey observed, adding that reported cases could be just the tip of the iceberg.
"Herbal medicine hepatoxicity is clearly underestimated for many reasons," he suggested. First, their intake is hard to analyze. Second, the mechanism of liver damage is often uncertain, and third, it is hard to confirm causality. Indeed, herbal medicines do not have to undergo the rigorous testing or regulation in the same way that prescribed medicines do, and sales via the Internet make them easily available to all.
There is then the uncertainty of what is really in the preparations, if they contain the right plant at all or the wrong part of it, and then whether or not they have been stored correctly, or if they have been contaminated with other liver-damaging agents or microorganisms.
Advice for physicians
DILI from prescription and nonprescription medicines is an important but rare event in the westernized world, Dr. Robert Fontana of the University of Michigan in Ann Arbor said in an interview. However, because it can bring about very bad and unpredictable liver injury, it is of great importance for hepatologists and general family physicians alike.
"In the United States and I think worldwide, the frequency in use of [herbal treatments] is increasing and as we start to see registry data I think we will start to see more and more cases [of hepatoxicity]," Dr. Fontana said.
Dr. Fontana is part of the National Institutes of Health–funded Drug-Induced Liver Injury Network (DILIN), a multicenter, prospective registry looking at the etiologies, risk factors, and outcomes of DILI in the United States (Drug Saf. 2009;32:55-68). Data from the registry show the prevalence of herbal and dietary supplements is around 9% (n = 300) in confirmed DILI cases.
"Patients need to tell their doctors what they are taking," he advised, adding that, as physicians, "we all need to be aware and maybe ask more questions of our patients."
The LiverTox website – produced by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Library of Medicine – is a valuable online and freely available resource for determining if a medication, herbal, or other supplement is known to cause liver problems. This is going to have a new chapter on herbal medicines, Dr. Fontana said, and is worth using in daily practice to help advise patients on the prescription or CAM they might be taking.
Dr. Larrey and Dr. Fontana had no disclosures relevant to their comments.
New Chinese herbal medicine inhibits HCV activity
A compound named SBEL1 after the laboratory in which it was discovered has multiple effects on the hepatitis C virus (HCV) life cycle, according to data from a late-breaking poster presented at the meeting.
Researchers from the Systems Biology of Epithelia Laboratory at the National Taiwan University, Taipei, screened six herbal medicines and found that one of these – SBEL1 – inhibited HCV activity by about 90% in infected cells.
Cheng-Wei Lin and Ming-Jiun Yu pretreated liver cells with the herbal extract and then infected these cells with HCV. Compared with control cells, SBEL1-treated cells contained 23% less viral protein. This suggested that SBEL1 prevented HCV from entering the pretreated cells.
Their findings also suggested that SBEL1 reduced internal-ribosome entry site–mediated translation, a process vital for viral protein production, and might also have interfered with the RNA replication process.
"SBEL1 has demonstrated significant inhibition of HCV at multiple stages of the viral life cycle," Dr. Markus Peck-Radosavljevic, the secretary-general of the European Association for the Study of the Liver, said in a press release issued by the Society.
Dr. Peck-Radosavljevic (University of Vienna, Austria), who was not involved in the research, added that this "is an exciting discovery because it allows us to gain a deeper understanding of the virus and its interactions with other compounds. Ultimately, this adds to our library of knowledge that may bring us closer to improving future treatment options."
LONDON – Herbal medicines and other home remedies or supplements are a significant cause of hepatotoxicity experts warned recently during a symposium at the International Liver Congress, which unintentionally coincided with World Homeopathy Awareness Week.
Although they are not at the very top of the list when it comes to drug-induced liver injury (DILI) – that accolade being reserved for antimicrobial agents used to treat tuberculosis – the use of homeopathy-based approaches are potentially on the increase in the western world and the use of such substances are often not reported to physicians.
"Herbal medicines represent a significant cause of liver injury," Dr. Dominique Larrey (Central University Hospital, Montpellier, France), said at the meeting that was sponsored by the European Association for the Study of the Liver. "Herbs can cause almost the whole spectrum of hepatic and biliary lesions, acute hepatitis being the most frequent one," he added.
The rise of herbal medicine
Dr. Larrey, who works in the liver and transplantation department of Saint Eloi Hospital, also in Montpellier, noted that the use of herbs in traditional medicine was very important in many parts of the world, notably in Asia, Africa, and Central and South America. They are used for both traditional and cultural reasons, he added, are often easy to access and are low cost in comparison to regulated medicines.
Their use is probably on the increase in western countries for a variety of reasons, Dr. Larrey suggested, such as the migration of people from cultures in which the use of traditional medicines is high, to the thinking that "what is natural can only be good" and "herbal medicines are considered completely innocuous in contrast to classical drugs." Furthermore, the lack of satisfactory treatments for some severe diseases – cancer, multiple sclerosis, AIDS, and hepatitis C virus infection to name a few – mean that people often are willing to try out complementary or alternative medicines (CAM).
In the United States, the total sale of herbal remedies in 2010 was an estimated $5.2 billion per year, having increased around 3% a year over the past decade, Dr. Larrey pointed out.
The problem is that patients do not often tell their doctors about their use of CAM. A staggering 90% of patients taking the anticoagulant drug warfarin – which is renowned for having a very narrow therapeutic window and careful monitoring is required – were taking herbal medicines in one study, he said.
Prospective studies on the use of herbal medicines in western countries are scarce but those that have been conducted specifically in patients with liver disease suggest that as many as one-fifth (Hepatology 2008;47:605-12) to one-third (Gastroenterology 2001;120[Suppl 1]:A228) might be taking herbal remedies unbeknownst to their doctor.
The problem of assessment
There are limited data on how frequently herbal medicines cause liver damage, but estimates range from 2% to 16%, Dr. Larrey observed, adding that reported cases could be just the tip of the iceberg.
"Herbal medicine hepatoxicity is clearly underestimated for many reasons," he suggested. First, their intake is hard to analyze. Second, the mechanism of liver damage is often uncertain, and third, it is hard to confirm causality. Indeed, herbal medicines do not have to undergo the rigorous testing or regulation in the same way that prescribed medicines do, and sales via the Internet make them easily available to all.
There is then the uncertainty of what is really in the preparations, if they contain the right plant at all or the wrong part of it, and then whether or not they have been stored correctly, or if they have been contaminated with other liver-damaging agents or microorganisms.
Advice for physicians
DILI from prescription and nonprescription medicines is an important but rare event in the westernized world, Dr. Robert Fontana of the University of Michigan in Ann Arbor said in an interview. However, because it can bring about very bad and unpredictable liver injury, it is of great importance for hepatologists and general family physicians alike.
"In the United States and I think worldwide, the frequency in use of [herbal treatments] is increasing and as we start to see registry data I think we will start to see more and more cases [of hepatoxicity]," Dr. Fontana said.
Dr. Fontana is part of the National Institutes of Health–funded Drug-Induced Liver Injury Network (DILIN), a multicenter, prospective registry looking at the etiologies, risk factors, and outcomes of DILI in the United States (Drug Saf. 2009;32:55-68). Data from the registry show the prevalence of herbal and dietary supplements is around 9% (n = 300) in confirmed DILI cases.
"Patients need to tell their doctors what they are taking," he advised, adding that, as physicians, "we all need to be aware and maybe ask more questions of our patients."
The LiverTox website – produced by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Library of Medicine – is a valuable online and freely available resource for determining if a medication, herbal, or other supplement is known to cause liver problems. This is going to have a new chapter on herbal medicines, Dr. Fontana said, and is worth using in daily practice to help advise patients on the prescription or CAM they might be taking.
Dr. Larrey and Dr. Fontana had no disclosures relevant to their comments.
New Chinese herbal medicine inhibits HCV activity
A compound named SBEL1 after the laboratory in which it was discovered has multiple effects on the hepatitis C virus (HCV) life cycle, according to data from a late-breaking poster presented at the meeting.
Researchers from the Systems Biology of Epithelia Laboratory at the National Taiwan University, Taipei, screened six herbal medicines and found that one of these – SBEL1 – inhibited HCV activity by about 90% in infected cells.
Cheng-Wei Lin and Ming-Jiun Yu pretreated liver cells with the herbal extract and then infected these cells with HCV. Compared with control cells, SBEL1-treated cells contained 23% less viral protein. This suggested that SBEL1 prevented HCV from entering the pretreated cells.
Their findings also suggested that SBEL1 reduced internal-ribosome entry site–mediated translation, a process vital for viral protein production, and might also have interfered with the RNA replication process.
"SBEL1 has demonstrated significant inhibition of HCV at multiple stages of the viral life cycle," Dr. Markus Peck-Radosavljevic, the secretary-general of the European Association for the Study of the Liver, said in a press release issued by the Society.
Dr. Peck-Radosavljevic (University of Vienna, Austria), who was not involved in the research, added that this "is an exciting discovery because it allows us to gain a deeper understanding of the virus and its interactions with other compounds. Ultimately, this adds to our library of knowledge that may bring us closer to improving future treatment options."
AT THE INTERNATIONAL LIVER CONGRESS 2014
Lorazepam not superior to diazepam in pediatric status epilepticus
The efficacy and safety of lorazepam were not superior to diazepam in a clinical trial of pediatric status epilepticus, investigators reported online April 22 in JAMA.
Both drugs effectively halted status epilepticus in more than 70% of children and adolescents and caused severe respiratory depression in less than 20%, said Dr. James Chamberlain of the Children’s National Medical Center, Washington, and his associates in the Pediatric Emergency Care Applied Research Network (PECARN).
"Taken together with the results of the RAMPART trial, it would appear that either diazepam, lorazepam, or midazolam could be chosen as a reasonable first-line therapy" for pediatric status epilepticus, the researchers said. Many prehospital systems prefer diazepam because it does not require refrigeration, they noted (JAMA 2014;311:1652-60 [doi:10.1001/jama.2014.2625]).
The multicenter, randomized, double-blind trial included 272 patients aged 3 months to less than 18 years who presented to one of 11 academic pediatric emergency departments with generalized tonic-clonic convulsive status epilepticus. Patients received intravenous diazepam (0.2 mg/kg) or lorazepam (0.1 mg/kg), with half the dose repeated at 5 minutes if needed. If status epilepticus continued at 12 minutes, patients received fosphenytoin or phenytoin.
Status epilepticus ceased by 10 minutes and did not recur for at least 30 minutes in 97 (72.9%) patients given lorazepam and in 101 (72.1%) patients treated with diazepam. The absolute efficacy difference was 0.8% (95% confidence interval, –11.4% to 9.8%). Severe respiratory depression – the primary safety outcome – occurred in 17.6% of lorazepam patients and 16.0% of diazepam patients, for an absolute risk difference of just 1.6% (95% CI, –9.9% to 6.8%), Dr. James Chamberlain and his associates reported.
Rates of secondary outcomes also were similar, except that sedation was more common in the lorazepam group (66.9% vs. 50% for diazepam; absolute risk difference, 16.9%; 95% CI, 6.1% to 27.7%).
The study was a superiority trial, not a noninferiority trial, so the two drugs could not be assumed to be statistically equivalent, the researchers said. "Although the point estimates for both efficacy and safety outcomes are similar in the two groups, the confidence intervals suggest that one medication could be superior in efficacy by as much as approximately 10% to 11% and in safety by approximately 7% to 10%," they wrote. They concluded that new treatment options for pediatric status epilepticus are needed, given the extent of medication failure and respiratory depression observed in both study groups.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Dr. Pamela Okada reported receiving a grant from NICHD during the study. Dr. Prashant Mahajan, Dr. Richard Lichenstein, and Dr. Joseph Grubenhoff reported having received grants from the National Institutes of Health during the study. The remaining authors reported no relevant financial disclosures.
The efficacy and safety of lorazepam were not superior to diazepam in a clinical trial of pediatric status epilepticus, investigators reported online April 22 in JAMA.
Both drugs effectively halted status epilepticus in more than 70% of children and adolescents and caused severe respiratory depression in less than 20%, said Dr. James Chamberlain of the Children’s National Medical Center, Washington, and his associates in the Pediatric Emergency Care Applied Research Network (PECARN).
"Taken together with the results of the RAMPART trial, it would appear that either diazepam, lorazepam, or midazolam could be chosen as a reasonable first-line therapy" for pediatric status epilepticus, the researchers said. Many prehospital systems prefer diazepam because it does not require refrigeration, they noted (JAMA 2014;311:1652-60 [doi:10.1001/jama.2014.2625]).
The multicenter, randomized, double-blind trial included 272 patients aged 3 months to less than 18 years who presented to one of 11 academic pediatric emergency departments with generalized tonic-clonic convulsive status epilepticus. Patients received intravenous diazepam (0.2 mg/kg) or lorazepam (0.1 mg/kg), with half the dose repeated at 5 minutes if needed. If status epilepticus continued at 12 minutes, patients received fosphenytoin or phenytoin.
Status epilepticus ceased by 10 minutes and did not recur for at least 30 minutes in 97 (72.9%) patients given lorazepam and in 101 (72.1%) patients treated with diazepam. The absolute efficacy difference was 0.8% (95% confidence interval, –11.4% to 9.8%). Severe respiratory depression – the primary safety outcome – occurred in 17.6% of lorazepam patients and 16.0% of diazepam patients, for an absolute risk difference of just 1.6% (95% CI, –9.9% to 6.8%), Dr. James Chamberlain and his associates reported.
Rates of secondary outcomes also were similar, except that sedation was more common in the lorazepam group (66.9% vs. 50% for diazepam; absolute risk difference, 16.9%; 95% CI, 6.1% to 27.7%).
The study was a superiority trial, not a noninferiority trial, so the two drugs could not be assumed to be statistically equivalent, the researchers said. "Although the point estimates for both efficacy and safety outcomes are similar in the two groups, the confidence intervals suggest that one medication could be superior in efficacy by as much as approximately 10% to 11% and in safety by approximately 7% to 10%," they wrote. They concluded that new treatment options for pediatric status epilepticus are needed, given the extent of medication failure and respiratory depression observed in both study groups.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Dr. Pamela Okada reported receiving a grant from NICHD during the study. Dr. Prashant Mahajan, Dr. Richard Lichenstein, and Dr. Joseph Grubenhoff reported having received grants from the National Institutes of Health during the study. The remaining authors reported no relevant financial disclosures.
The efficacy and safety of lorazepam were not superior to diazepam in a clinical trial of pediatric status epilepticus, investigators reported online April 22 in JAMA.
Both drugs effectively halted status epilepticus in more than 70% of children and adolescents and caused severe respiratory depression in less than 20%, said Dr. James Chamberlain of the Children’s National Medical Center, Washington, and his associates in the Pediatric Emergency Care Applied Research Network (PECARN).
"Taken together with the results of the RAMPART trial, it would appear that either diazepam, lorazepam, or midazolam could be chosen as a reasonable first-line therapy" for pediatric status epilepticus, the researchers said. Many prehospital systems prefer diazepam because it does not require refrigeration, they noted (JAMA 2014;311:1652-60 [doi:10.1001/jama.2014.2625]).
The multicenter, randomized, double-blind trial included 272 patients aged 3 months to less than 18 years who presented to one of 11 academic pediatric emergency departments with generalized tonic-clonic convulsive status epilepticus. Patients received intravenous diazepam (0.2 mg/kg) or lorazepam (0.1 mg/kg), with half the dose repeated at 5 minutes if needed. If status epilepticus continued at 12 minutes, patients received fosphenytoin or phenytoin.
Status epilepticus ceased by 10 minutes and did not recur for at least 30 minutes in 97 (72.9%) patients given lorazepam and in 101 (72.1%) patients treated with diazepam. The absolute efficacy difference was 0.8% (95% confidence interval, –11.4% to 9.8%). Severe respiratory depression – the primary safety outcome – occurred in 17.6% of lorazepam patients and 16.0% of diazepam patients, for an absolute risk difference of just 1.6% (95% CI, –9.9% to 6.8%), Dr. James Chamberlain and his associates reported.
Rates of secondary outcomes also were similar, except that sedation was more common in the lorazepam group (66.9% vs. 50% for diazepam; absolute risk difference, 16.9%; 95% CI, 6.1% to 27.7%).
The study was a superiority trial, not a noninferiority trial, so the two drugs could not be assumed to be statistically equivalent, the researchers said. "Although the point estimates for both efficacy and safety outcomes are similar in the two groups, the confidence intervals suggest that one medication could be superior in efficacy by as much as approximately 10% to 11% and in safety by approximately 7% to 10%," they wrote. They concluded that new treatment options for pediatric status epilepticus are needed, given the extent of medication failure and respiratory depression observed in both study groups.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Dr. Pamela Okada reported receiving a grant from NICHD during the study. Dr. Prashant Mahajan, Dr. Richard Lichenstein, and Dr. Joseph Grubenhoff reported having received grants from the National Institutes of Health during the study. The remaining authors reported no relevant financial disclosures.
FROM JAMA
Major finding: Status epilepticus ceased by 10 minutes and did not recur for at least 30 minutes in 101 (72.1%) patients treated with diazepam and 97 (72.9%) patients given lorazepam, for an absolute efficacy difference of 0.8% (95% confidence interval, –11.4% to 9.8%).
Data source: A randomized, double-blind, multicenter superiority trial of 273 pediatric patients with convulsive status epilepticus.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Dr. Pamela Okada reported receiving a grant from NICHD during the study. Dr. Prashant Mahajan, Dr. Richard Lichenstein, and Dr. Joseph Grubenhoff reported having received grants from the National Institutes of Health during the study. The remaining authors reported no relevant financial disclosures.