User login
Shared medical appointments educate and encourage MS patients
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
REPORTING FROM ACTRIMS FORUM 2020
Depression, or something else?
CASE Suicidal behavior, severe headaches
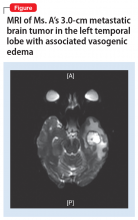
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
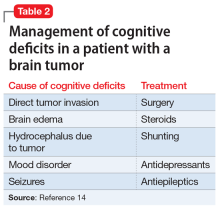
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
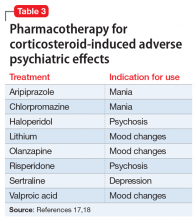
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
Incomplete MS relapse recovery predicted greater long-term disability
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
REPORTING FROM ACTRIMS FORUM 2020
Depression in MS predicted worsening of neurologic function
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
REPORTING FROM ACTRIMS Forum 2020
Incidence of cardiovascular events is doubled in patients with MS
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
REPORTING FROM ACTRIMS FORUM 2020
Cancer increase observed in modern era of MS drugs
WEST PALM BEACH, FLA. – Cancer incidence among patients with multiple sclerosis (MS) treated after the advent of immune therapies showed an increase, compared with prior generations, according to a large study of Norwegian MS patients.
“We detected a similar cancer risk among MS patients, compared to the general Norwegian population before 1996, [however] MS patients had increased risk of cancer compared to the general population after 1996,” first author Nina Grytten, PhD, of the department of neurology at the Norwegian Multiple Sclerosis Centre, Bergen, Norway, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“This finding suggests that clinicians should be aware of this increased risk of cancer when caring for MS patients.”
With the widespread use of disease-modifying therapies (DMTs) in patients with MS, such findings are always of interest to clinicians and patients alike, commented ACTRIMS president, Jeffrey A. Cohen, MD.
“Something that’s already on the mind of most people with MS is what are the long-term safety characteristics of these medicines because we’re talking about a life-long therapy for most people,” Dr. Cohen, who is the director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic, said in an interview.
“With such a large sample size and such a long study, this is on one hand reassuring and tells us the cancer risk is likely low, but it also suggests that it’s something we should pay attention to,” he said.
In previous research, Dr. Grytten and her team identified an increased risk of cancer among patients with MS in Norway, but conflicting results have been reported in other studies looking at cancer risk and MS.
The authors therefore sought to dig deeper into the risk in the Norwegian population, looking into the specifics of cancer incidence according to sex and the period of diagnosis.
For the study, they identified a total of 6,638 patients with MS from previous prevalence studies in Norway, as well as in the Norwegian MS Registry and Biobank.
The data from the cohort was matched with 36,957 Norwegian citizens without MS in a 5:1 ratio, with the participants matched according to age, gender, and county. The cohort was further linked to data from the Norwegian Cancer Registry for additional information on the year and type of cancer diagnosis, as well as cause and year of death data. The participants were born between 1930 and 1979.
Over the course of the full 65-year observation period, the cancer diagnosis rates were similar between participants with MS (774; 11.2%) and those without MS (4,017; 10.6%).
And in looking at cancer incidence rate ratios of those with MS, compared with controls between the years 1953 and 1995, the rate was similar (IRR, 1.05; 95% confidence interval, 0.97-1.14). However, after 1995, the rate increased, with a higher cancer incidence among MS patients, compared with those without MS (IRR, 1.40; 95% CI, 1.30-1.51).
Cancer rates were additionally higher among those with MS in cancers of various organs, including the brain (IRR, 1.75; 95% CI, 1.28-2.40), meninges (IRR, 2.28; 95% CI, 1.47-3.53), urinary organs (IRR, 2.06; 95% CI, 1.52-2.79), digestive system (IRR, 1.47; 95% CI, 1.20-1.80), endocrine glands (IRR, 1.64; 95% CI, 1.06-2.54), and respiratory organs (IRR, 2.05; 95% CI, 1.55-2.07).
Dr. Grytten noted, however, that the study cannot rule out various other possible causes for the differences. For instance, “cancer in urinary system and respiratory organs showed increased risk in MS both before and after introduction of disease-modifying therapies,” she noted. “Those are possibly caused by smoking, which is a habit more common among MS patients in Norway.”
Furthermore, “increased cancer in the central nervous system in MS could possibly be explained by frequent use of magnetic resonance imaging and the ability to detect CNS cancer at early stages.”
“There is increasing evidence that patients with MS are also more susceptible to other diseases, and increased cancer risk seems to be one of these comorbidities.”
However, the finding that increased cancers were observed after 1996 in other organs in MS patients as well does raise the issue of a possible role of DMTs.
Of note, mitoxantrone has been associated with an increased risk of leukemia and colorectal cancer.
And “other immunosuppressant drugs, including the MS drug fingolimod, are believed to possibly be linked to an increased cancer risk, although evidence has not yet been established,” Dr. Grytten said.
“The increased risk of cancer associated with MS was detected in the era of disease-modifying treatment of MS, and this association suggests that DMTs might possibly increase cancer risk.”
In general, “clinicians should be aware of comorbidity in MS,” Dr. Grytten said. “More data is needed on the long-time effects of immunomodulatory treatment.”
Dr. Cohen added that, in addition to mitoxantrone, azathioprine and cyclophosphamide have shown risk, but “clinical trials and follow-up studies of individual MS DMTs have not shown clear cut increased risk of cancer, which is reassuring.”
“Nevertheless, this study suggests that, in aggregate, there may be a mild increased risk. There are many other potential explanations, so the research needs to be followed up,” he said.
Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Torkildsen NG et al. ACTRIMS Forum 2020, Abstract P126.
WEST PALM BEACH, FLA. – Cancer incidence among patients with multiple sclerosis (MS) treated after the advent of immune therapies showed an increase, compared with prior generations, according to a large study of Norwegian MS patients.
“We detected a similar cancer risk among MS patients, compared to the general Norwegian population before 1996, [however] MS patients had increased risk of cancer compared to the general population after 1996,” first author Nina Grytten, PhD, of the department of neurology at the Norwegian Multiple Sclerosis Centre, Bergen, Norway, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“This finding suggests that clinicians should be aware of this increased risk of cancer when caring for MS patients.”
With the widespread use of disease-modifying therapies (DMTs) in patients with MS, such findings are always of interest to clinicians and patients alike, commented ACTRIMS president, Jeffrey A. Cohen, MD.
“Something that’s already on the mind of most people with MS is what are the long-term safety characteristics of these medicines because we’re talking about a life-long therapy for most people,” Dr. Cohen, who is the director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic, said in an interview.
“With such a large sample size and such a long study, this is on one hand reassuring and tells us the cancer risk is likely low, but it also suggests that it’s something we should pay attention to,” he said.
In previous research, Dr. Grytten and her team identified an increased risk of cancer among patients with MS in Norway, but conflicting results have been reported in other studies looking at cancer risk and MS.
The authors therefore sought to dig deeper into the risk in the Norwegian population, looking into the specifics of cancer incidence according to sex and the period of diagnosis.
For the study, they identified a total of 6,638 patients with MS from previous prevalence studies in Norway, as well as in the Norwegian MS Registry and Biobank.
The data from the cohort was matched with 36,957 Norwegian citizens without MS in a 5:1 ratio, with the participants matched according to age, gender, and county. The cohort was further linked to data from the Norwegian Cancer Registry for additional information on the year and type of cancer diagnosis, as well as cause and year of death data. The participants were born between 1930 and 1979.
Over the course of the full 65-year observation period, the cancer diagnosis rates were similar between participants with MS (774; 11.2%) and those without MS (4,017; 10.6%).
And in looking at cancer incidence rate ratios of those with MS, compared with controls between the years 1953 and 1995, the rate was similar (IRR, 1.05; 95% confidence interval, 0.97-1.14). However, after 1995, the rate increased, with a higher cancer incidence among MS patients, compared with those without MS (IRR, 1.40; 95% CI, 1.30-1.51).
Cancer rates were additionally higher among those with MS in cancers of various organs, including the brain (IRR, 1.75; 95% CI, 1.28-2.40), meninges (IRR, 2.28; 95% CI, 1.47-3.53), urinary organs (IRR, 2.06; 95% CI, 1.52-2.79), digestive system (IRR, 1.47; 95% CI, 1.20-1.80), endocrine glands (IRR, 1.64; 95% CI, 1.06-2.54), and respiratory organs (IRR, 2.05; 95% CI, 1.55-2.07).
Dr. Grytten noted, however, that the study cannot rule out various other possible causes for the differences. For instance, “cancer in urinary system and respiratory organs showed increased risk in MS both before and after introduction of disease-modifying therapies,” she noted. “Those are possibly caused by smoking, which is a habit more common among MS patients in Norway.”
Furthermore, “increased cancer in the central nervous system in MS could possibly be explained by frequent use of magnetic resonance imaging and the ability to detect CNS cancer at early stages.”
“There is increasing evidence that patients with MS are also more susceptible to other diseases, and increased cancer risk seems to be one of these comorbidities.”
However, the finding that increased cancers were observed after 1996 in other organs in MS patients as well does raise the issue of a possible role of DMTs.
Of note, mitoxantrone has been associated with an increased risk of leukemia and colorectal cancer.
And “other immunosuppressant drugs, including the MS drug fingolimod, are believed to possibly be linked to an increased cancer risk, although evidence has not yet been established,” Dr. Grytten said.
“The increased risk of cancer associated with MS was detected in the era of disease-modifying treatment of MS, and this association suggests that DMTs might possibly increase cancer risk.”
In general, “clinicians should be aware of comorbidity in MS,” Dr. Grytten said. “More data is needed on the long-time effects of immunomodulatory treatment.”
Dr. Cohen added that, in addition to mitoxantrone, azathioprine and cyclophosphamide have shown risk, but “clinical trials and follow-up studies of individual MS DMTs have not shown clear cut increased risk of cancer, which is reassuring.”
“Nevertheless, this study suggests that, in aggregate, there may be a mild increased risk. There are many other potential explanations, so the research needs to be followed up,” he said.
Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Torkildsen NG et al. ACTRIMS Forum 2020, Abstract P126.
WEST PALM BEACH, FLA. – Cancer incidence among patients with multiple sclerosis (MS) treated after the advent of immune therapies showed an increase, compared with prior generations, according to a large study of Norwegian MS patients.
“We detected a similar cancer risk among MS patients, compared to the general Norwegian population before 1996, [however] MS patients had increased risk of cancer compared to the general population after 1996,” first author Nina Grytten, PhD, of the department of neurology at the Norwegian Multiple Sclerosis Centre, Bergen, Norway, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“This finding suggests that clinicians should be aware of this increased risk of cancer when caring for MS patients.”
With the widespread use of disease-modifying therapies (DMTs) in patients with MS, such findings are always of interest to clinicians and patients alike, commented ACTRIMS president, Jeffrey A. Cohen, MD.
“Something that’s already on the mind of most people with MS is what are the long-term safety characteristics of these medicines because we’re talking about a life-long therapy for most people,” Dr. Cohen, who is the director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic, said in an interview.
“With such a large sample size and such a long study, this is on one hand reassuring and tells us the cancer risk is likely low, but it also suggests that it’s something we should pay attention to,” he said.
In previous research, Dr. Grytten and her team identified an increased risk of cancer among patients with MS in Norway, but conflicting results have been reported in other studies looking at cancer risk and MS.
The authors therefore sought to dig deeper into the risk in the Norwegian population, looking into the specifics of cancer incidence according to sex and the period of diagnosis.
For the study, they identified a total of 6,638 patients with MS from previous prevalence studies in Norway, as well as in the Norwegian MS Registry and Biobank.
The data from the cohort was matched with 36,957 Norwegian citizens without MS in a 5:1 ratio, with the participants matched according to age, gender, and county. The cohort was further linked to data from the Norwegian Cancer Registry for additional information on the year and type of cancer diagnosis, as well as cause and year of death data. The participants were born between 1930 and 1979.
Over the course of the full 65-year observation period, the cancer diagnosis rates were similar between participants with MS (774; 11.2%) and those without MS (4,017; 10.6%).
And in looking at cancer incidence rate ratios of those with MS, compared with controls between the years 1953 and 1995, the rate was similar (IRR, 1.05; 95% confidence interval, 0.97-1.14). However, after 1995, the rate increased, with a higher cancer incidence among MS patients, compared with those without MS (IRR, 1.40; 95% CI, 1.30-1.51).
Cancer rates were additionally higher among those with MS in cancers of various organs, including the brain (IRR, 1.75; 95% CI, 1.28-2.40), meninges (IRR, 2.28; 95% CI, 1.47-3.53), urinary organs (IRR, 2.06; 95% CI, 1.52-2.79), digestive system (IRR, 1.47; 95% CI, 1.20-1.80), endocrine glands (IRR, 1.64; 95% CI, 1.06-2.54), and respiratory organs (IRR, 2.05; 95% CI, 1.55-2.07).
Dr. Grytten noted, however, that the study cannot rule out various other possible causes for the differences. For instance, “cancer in urinary system and respiratory organs showed increased risk in MS both before and after introduction of disease-modifying therapies,” she noted. “Those are possibly caused by smoking, which is a habit more common among MS patients in Norway.”
Furthermore, “increased cancer in the central nervous system in MS could possibly be explained by frequent use of magnetic resonance imaging and the ability to detect CNS cancer at early stages.”
“There is increasing evidence that patients with MS are also more susceptible to other diseases, and increased cancer risk seems to be one of these comorbidities.”
However, the finding that increased cancers were observed after 1996 in other organs in MS patients as well does raise the issue of a possible role of DMTs.
Of note, mitoxantrone has been associated with an increased risk of leukemia and colorectal cancer.
And “other immunosuppressant drugs, including the MS drug fingolimod, are believed to possibly be linked to an increased cancer risk, although evidence has not yet been established,” Dr. Grytten said.
“The increased risk of cancer associated with MS was detected in the era of disease-modifying treatment of MS, and this association suggests that DMTs might possibly increase cancer risk.”
In general, “clinicians should be aware of comorbidity in MS,” Dr. Grytten said. “More data is needed on the long-time effects of immunomodulatory treatment.”
Dr. Cohen added that, in addition to mitoxantrone, azathioprine and cyclophosphamide have shown risk, but “clinical trials and follow-up studies of individual MS DMTs have not shown clear cut increased risk of cancer, which is reassuring.”
“Nevertheless, this study suggests that, in aggregate, there may be a mild increased risk. There are many other potential explanations, so the research needs to be followed up,” he said.
Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Torkildsen NG et al. ACTRIMS Forum 2020, Abstract P126.
REPORTING FROM ACTRIMS FORUM 2020
FDA OKs first orally disintegrating agent for rapid migraine relief
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.
Key differences found between pediatric- and adult-onset MS
WEST PALM BEACH, FLA. – , according to data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Among patients with POMS, researchers also have observed an association between fatigue and mood disorders on one hand and DMT choice on the other hand. “These findings confirm the unique features of POMS and suggest that DMT choice in POMS and AOMS may influence the frequency of fatigue and mood disorders,” said Mary Rensel, MD, staff neurologist in neuroimmunology at Cleveland Clinic’s Mellen Center for MS Treatment and Research, and colleagues.
POMS is defined as MS onset before age 18, and the disease characteristics of POMS and AOMS are distinct. The former diagnosis is rare, which has limited the amount of data collected on POMS to date.
MS Partners Advancing Technology and Health Solutions (MS-PATHS), sponsored by Biogen, is a multicenter initiative in which researchers collect MS performance measures longitudinally at each patient visit. MS-PATHS data include sociodemographic information, patient-reported outcomes (PROs), functional outcomes (FOs), MS phenotype, and DMT. Using MS-PATHS data, Dr. Rensel and colleagues sought to determine differences in sociodemographics, MS phenotype, PRO, FO, and DMT among patients with POMS and between patients with POMS and those with AOMS.
The investigators analyzed data cut 9 of the MS-PATHS database for their study. They included 637 participants with POMS and matched them with patients with AOMS, based on disease duration, in a 1:5 ratio. Dr. Rensel and colleagues categorized DMTs as high, mid, or low efficacy. They calculated descriptive statistics to characterize the study population. In addition, they compared MS FOs and PROs in the matched cohort using the Wilcoxon rank-sum test. Finally, linear regression analysis allowed the investigators to identify differences in the data set, while adjusting for important covariates.
The matched cohort included 5,857 patients with AOMS and 600 patients with POMS. The patients with AOMS had an average age of 49.8 years. About 87.5% of these patients were white, and 73.5% were female. The POMS patients had an average age of 31.49 years. Overall, 76.7% of these patients were white, and 73.2% were female. Dr. Rensel and colleagues found significant differences between the two groups in age at encounter, disease duration, race, insurance, Patient Determined Disease Steps (PDDS), education, employment, FOs, PROs, and DMT.
Patients with POMS used high-efficacy DMT more frequently than those with AOMS. The rate of depression was similar between patients with AOMS and those with POMS. Depression, anxiety, and fatigue were associated with DMT potency in AOMS, and anxiety and fatigue were associated with DMT groups in POMS.
Racial differences between POMS and AOMS have been reported previously, said Dr. Rensel. First-generation immigrant children have an increased risk of POMS, compared with other children. “In our data set, we had more Asians, more blacks, and fewer Caucasians in the POMS group,” said Dr. Rensel. People from a socioeconomically challenged environment have an increased risk of POMS, and this observation may explain the difference in insurance coverage between the POMS and AOMS groups, she added. Socioeconomic challenges also may explain the difference in the rate of higher education between the two groups.
“Why were the POMS cases associated with higher-efficacy DMT when only one oral MS DMT is [Food and Drug Administration]-approved for POMS?” Dr. Rensel asked. “This is likely due to the fact that POMS cases tend to have higher disease activity with more relapses and more brain lesions, leading to the choice of higher efficacy DMTs that are currently not FDA-approved for POMS.”
These data “may help [clinicians] caring for kids and teens, especially non-Caucasian [patients], to consider MS on the differential diagnosis,” Dr. Rensel added. “Mood disorders in POMS were as common as mood disorders in AOMS, so these should be screened for in this POMS population.”
Dr. Rensel has received funding for consulting, research, or patient education from Biogen, Genentech, Genzyme, Medimmune, MSAA, NMSS, Novartis, TSerono, and Teva.
SOURCE: Rensel M et al. ACTRIMS Forum 2020, Abstract P042.
WEST PALM BEACH, FLA. – , according to data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Among patients with POMS, researchers also have observed an association between fatigue and mood disorders on one hand and DMT choice on the other hand. “These findings confirm the unique features of POMS and suggest that DMT choice in POMS and AOMS may influence the frequency of fatigue and mood disorders,” said Mary Rensel, MD, staff neurologist in neuroimmunology at Cleveland Clinic’s Mellen Center for MS Treatment and Research, and colleagues.
POMS is defined as MS onset before age 18, and the disease characteristics of POMS and AOMS are distinct. The former diagnosis is rare, which has limited the amount of data collected on POMS to date.
MS Partners Advancing Technology and Health Solutions (MS-PATHS), sponsored by Biogen, is a multicenter initiative in which researchers collect MS performance measures longitudinally at each patient visit. MS-PATHS data include sociodemographic information, patient-reported outcomes (PROs), functional outcomes (FOs), MS phenotype, and DMT. Using MS-PATHS data, Dr. Rensel and colleagues sought to determine differences in sociodemographics, MS phenotype, PRO, FO, and DMT among patients with POMS and between patients with POMS and those with AOMS.
The investigators analyzed data cut 9 of the MS-PATHS database for their study. They included 637 participants with POMS and matched them with patients with AOMS, based on disease duration, in a 1:5 ratio. Dr. Rensel and colleagues categorized DMTs as high, mid, or low efficacy. They calculated descriptive statistics to characterize the study population. In addition, they compared MS FOs and PROs in the matched cohort using the Wilcoxon rank-sum test. Finally, linear regression analysis allowed the investigators to identify differences in the data set, while adjusting for important covariates.
The matched cohort included 5,857 patients with AOMS and 600 patients with POMS. The patients with AOMS had an average age of 49.8 years. About 87.5% of these patients were white, and 73.5% were female. The POMS patients had an average age of 31.49 years. Overall, 76.7% of these patients were white, and 73.2% were female. Dr. Rensel and colleagues found significant differences between the two groups in age at encounter, disease duration, race, insurance, Patient Determined Disease Steps (PDDS), education, employment, FOs, PROs, and DMT.
Patients with POMS used high-efficacy DMT more frequently than those with AOMS. The rate of depression was similar between patients with AOMS and those with POMS. Depression, anxiety, and fatigue were associated with DMT potency in AOMS, and anxiety and fatigue were associated with DMT groups in POMS.
Racial differences between POMS and AOMS have been reported previously, said Dr. Rensel. First-generation immigrant children have an increased risk of POMS, compared with other children. “In our data set, we had more Asians, more blacks, and fewer Caucasians in the POMS group,” said Dr. Rensel. People from a socioeconomically challenged environment have an increased risk of POMS, and this observation may explain the difference in insurance coverage between the POMS and AOMS groups, she added. Socioeconomic challenges also may explain the difference in the rate of higher education between the two groups.
“Why were the POMS cases associated with higher-efficacy DMT when only one oral MS DMT is [Food and Drug Administration]-approved for POMS?” Dr. Rensel asked. “This is likely due to the fact that POMS cases tend to have higher disease activity with more relapses and more brain lesions, leading to the choice of higher efficacy DMTs that are currently not FDA-approved for POMS.”
These data “may help [clinicians] caring for kids and teens, especially non-Caucasian [patients], to consider MS on the differential diagnosis,” Dr. Rensel added. “Mood disorders in POMS were as common as mood disorders in AOMS, so these should be screened for in this POMS population.”
Dr. Rensel has received funding for consulting, research, or patient education from Biogen, Genentech, Genzyme, Medimmune, MSAA, NMSS, Novartis, TSerono, and Teva.
SOURCE: Rensel M et al. ACTRIMS Forum 2020, Abstract P042.
WEST PALM BEACH, FLA. – , according to data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Among patients with POMS, researchers also have observed an association between fatigue and mood disorders on one hand and DMT choice on the other hand. “These findings confirm the unique features of POMS and suggest that DMT choice in POMS and AOMS may influence the frequency of fatigue and mood disorders,” said Mary Rensel, MD, staff neurologist in neuroimmunology at Cleveland Clinic’s Mellen Center for MS Treatment and Research, and colleagues.
POMS is defined as MS onset before age 18, and the disease characteristics of POMS and AOMS are distinct. The former diagnosis is rare, which has limited the amount of data collected on POMS to date.
MS Partners Advancing Technology and Health Solutions (MS-PATHS), sponsored by Biogen, is a multicenter initiative in which researchers collect MS performance measures longitudinally at each patient visit. MS-PATHS data include sociodemographic information, patient-reported outcomes (PROs), functional outcomes (FOs), MS phenotype, and DMT. Using MS-PATHS data, Dr. Rensel and colleagues sought to determine differences in sociodemographics, MS phenotype, PRO, FO, and DMT among patients with POMS and between patients with POMS and those with AOMS.
The investigators analyzed data cut 9 of the MS-PATHS database for their study. They included 637 participants with POMS and matched them with patients with AOMS, based on disease duration, in a 1:5 ratio. Dr. Rensel and colleagues categorized DMTs as high, mid, or low efficacy. They calculated descriptive statistics to characterize the study population. In addition, they compared MS FOs and PROs in the matched cohort using the Wilcoxon rank-sum test. Finally, linear regression analysis allowed the investigators to identify differences in the data set, while adjusting for important covariates.
The matched cohort included 5,857 patients with AOMS and 600 patients with POMS. The patients with AOMS had an average age of 49.8 years. About 87.5% of these patients were white, and 73.5% were female. The POMS patients had an average age of 31.49 years. Overall, 76.7% of these patients were white, and 73.2% were female. Dr. Rensel and colleagues found significant differences between the two groups in age at encounter, disease duration, race, insurance, Patient Determined Disease Steps (PDDS), education, employment, FOs, PROs, and DMT.
Patients with POMS used high-efficacy DMT more frequently than those with AOMS. The rate of depression was similar between patients with AOMS and those with POMS. Depression, anxiety, and fatigue were associated with DMT potency in AOMS, and anxiety and fatigue were associated with DMT groups in POMS.
Racial differences between POMS and AOMS have been reported previously, said Dr. Rensel. First-generation immigrant children have an increased risk of POMS, compared with other children. “In our data set, we had more Asians, more blacks, and fewer Caucasians in the POMS group,” said Dr. Rensel. People from a socioeconomically challenged environment have an increased risk of POMS, and this observation may explain the difference in insurance coverage between the POMS and AOMS groups, she added. Socioeconomic challenges also may explain the difference in the rate of higher education between the two groups.
“Why were the POMS cases associated with higher-efficacy DMT when only one oral MS DMT is [Food and Drug Administration]-approved for POMS?” Dr. Rensel asked. “This is likely due to the fact that POMS cases tend to have higher disease activity with more relapses and more brain lesions, leading to the choice of higher efficacy DMTs that are currently not FDA-approved for POMS.”
These data “may help [clinicians] caring for kids and teens, especially non-Caucasian [patients], to consider MS on the differential diagnosis,” Dr. Rensel added. “Mood disorders in POMS were as common as mood disorders in AOMS, so these should be screened for in this POMS population.”
Dr. Rensel has received funding for consulting, research, or patient education from Biogen, Genentech, Genzyme, Medimmune, MSAA, NMSS, Novartis, TSerono, and Teva.
SOURCE: Rensel M et al. ACTRIMS Forum 2020, Abstract P042.
REPORTING FROM ACTRIMS FORUM 2020
Increased risk of infection seen in patients with MS
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
REPORTING FROM ACTRIMS FORUM 2020
Pregnancy linked to slowed MS progression
The effect increases with multiple children
WEST PALM BEACH, FLA. – Women who have no history of a full-term pregnancy show an earlier onset of progressive multiple sclerosis (MS) compared to those who do have pregnancies, and the apparent onset-delaying effect appears to increase with the number of pregnancies, according to new research adding to speculation of the effects of pregnancy in MS.
“Our results suggest that a higher number of full-term pregnancies than average is associated with later onset of progressive MS, while having no full-term pregnancies is associated with significantly younger age at progressive MS onset,” first author Burcu Zeydan, MD, an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minn., said in an interview.
The study was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The findings, which also link early menopause with faster disease progression, offer important insights into the broader effects of pregnancy on MS, said ACTRIMS president Jeffrey A. Cohen, MD, director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
“We know pregnancy affects the short term disease activity – relapses tend to quiet down during pregnancy – but what has been somewhat conflicting is whether it affects the long-term prognosis or is just a temporary effect,” he said in an interview.
“So that is the main interest in this study, and it does indicate that pregnancy affects the long-term prognosis and provides some insight into the mechanism by which it might do that.”
While being female is in fact considered the most important risk factor for MS susceptibility, pregnancy has been suggested to have a protective role in disease progression, but more research is needed on the nature of the effect – and its mechanisms.
For the study, Dr. Zeydan and colleagues evaluated data on 202 patients with MS who were part of a Mayo Clinic survey, including 134 women and 68 men.
They found that women who had no full-term pregnancies (n = 32), had an earlier onset of progressive MS (mean age 41.4 ± 12.6 years) compared to women giving birth to 1 or more children (n = 95; 47.1 ± 9.7 years; P = .012).
In addition, the mean age of progressive MS onset also increased with a dose-effect trend according to number of full pregnancies (no children, 41.4 ± 12.6 years; 1-3 children: 46.4 ± 9.2 years; 4 or more children: 52.6 ± 12.9 years; P = .002).
A look at a subgroup of patients with secondary progressive MS also showed an earlier mean age of onset among women who had no full pregnancies (n = 19; 41.5 ± 9.2 years) compared to women with 1 or more full pregnancies (n = 57; 47.3 ± 10.6 years; P = .049).
The later disease onset associated with pregnancy was also seen in relapsing-remitting MS: Mean age of onset was earlier women with no pregnancies (27.5 ± 7.0 years) compared to those with one or more children (33.0 ± 9.4 years; P = .021).
The trends of later onset with more pregnancies was also observed with the mean age of onset of secondary progressive MS (no full pregnancies: onset at 41.5 ± 9.2 years; 1-3 pregnancies: 46.2 ± 9.9 years; 4 or more pregnancies, onset 52.6 ± 12.9 years; P = .010).
And likewise, the later mean age of onset of relapsing-remitting MS was seen with additional pregnancies (no full pregnancies: 27.5 ± 7.0 years; 1-3 pregnancies: 32.4 ± 9.3 years; 4 or more pregnancies: 35.8 ± 9.8 years; P = .012).
“The dose effect was clearly a surprise (having no full-term pregnancies vs. 1-3 vs. 4 or more),” Dr. Zeydan said.
“In addition to the significant difference between having no versus one or more full-term pregnancies, the clear dose-effect consolidates our results related to the association between the number of pregnancies and age at progressive MS onset.”
Early menopause also linked to shorter progression to secondary progressive MS
The study also showed that women with premature or early menopause had a shorter duration of progressing from relapsing-remitting MS to secondary progressive MS (n = 26; 12.9 ± 9.0 years) compared to women with normal age at menopause (n = 39; 17.8 ± 10.3 years).
The pattern was similar for women experiencing the onset of secondary progressive MS after menopause, with a shorter progression among those with early menopause (P = .012).
The patterns in early menopause are consistent with previous observations regarding menopause and MS progression, Dr. Cohen said.
“When women go through menopause, estradiol and pregnancy-related factors further decline and we know this coincides temporally with the development of progressive MS in women,” he noted.
Compared to men, women with premature or early menopause furthermore had a longer duration from relapsing-remitting MS to secondary progressive MS (P = .008), and women with secondary progressive MS also had also had an earlier age of relapsing-remitting MS onset than men (P = .018).
Possible mechanisms and applications of the findings
The mechanisms of pregnancy that could include a complex interaction between estrogen and factors such as astrocyte and microglia function, Dr. Zeydan explained.
“Estrogen, through various mechanisms of eliminating toxicity of highly activated neurons – including preventing proinflammatory molecule release, supporting mitochondria function thereby eliminating energy failure, and promoting remyelination – helps neuronal plasticity and delays neurodegeneration, which is closely related to the progressive phase of MS,” she said.
“One could easily make the probable association, while yet to be proven, that our findings may relate to these mechanisms,” Dr. Zeydan said.
The logical question of whether hormone replacement or some type of therapy that could mimic the effects of pregnancy could also benefit in delaying MS onset remained to be seen, Dr. Zeydan said.
“While we believe that is possible, particularly for delaying the onset of progressive phase, definitive evidence is lacking at this time,” Dr. Zeydan said.
“However, our study ultimately may lead to such a trial.”
In the meantime, the findings provide additional insights that may be beneficial in sharing with patients regarding pregnancy,” she said.
“As the contemporary problem in MS care is to delay or prevent progressive MS onset, our findings may suggest that how we counsel women with MS who are planning to get pregnant, or contemplating surgically induced menopause, or how we consider hormone therapies during perimenopause may impact the course of their disease.”
Dr. Zeydan cautioned, however, that “our findings do not confirm causality beyond an association.”
“More studies are needed in this important issue in a disease that affects women three times more than men.”
Dr. Zeydan had no disclosures to report. Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Zeydan B et al. ACTRIMS Forum 2020, Abstract P135.
The effect increases with multiple children
The effect increases with multiple children
WEST PALM BEACH, FLA. – Women who have no history of a full-term pregnancy show an earlier onset of progressive multiple sclerosis (MS) compared to those who do have pregnancies, and the apparent onset-delaying effect appears to increase with the number of pregnancies, according to new research adding to speculation of the effects of pregnancy in MS.
“Our results suggest that a higher number of full-term pregnancies than average is associated with later onset of progressive MS, while having no full-term pregnancies is associated with significantly younger age at progressive MS onset,” first author Burcu Zeydan, MD, an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minn., said in an interview.
The study was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The findings, which also link early menopause with faster disease progression, offer important insights into the broader effects of pregnancy on MS, said ACTRIMS president Jeffrey A. Cohen, MD, director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
“We know pregnancy affects the short term disease activity – relapses tend to quiet down during pregnancy – but what has been somewhat conflicting is whether it affects the long-term prognosis or is just a temporary effect,” he said in an interview.
“So that is the main interest in this study, and it does indicate that pregnancy affects the long-term prognosis and provides some insight into the mechanism by which it might do that.”
While being female is in fact considered the most important risk factor for MS susceptibility, pregnancy has been suggested to have a protective role in disease progression, but more research is needed on the nature of the effect – and its mechanisms.
For the study, Dr. Zeydan and colleagues evaluated data on 202 patients with MS who were part of a Mayo Clinic survey, including 134 women and 68 men.
They found that women who had no full-term pregnancies (n = 32), had an earlier onset of progressive MS (mean age 41.4 ± 12.6 years) compared to women giving birth to 1 or more children (n = 95; 47.1 ± 9.7 years; P = .012).
In addition, the mean age of progressive MS onset also increased with a dose-effect trend according to number of full pregnancies (no children, 41.4 ± 12.6 years; 1-3 children: 46.4 ± 9.2 years; 4 or more children: 52.6 ± 12.9 years; P = .002).
A look at a subgroup of patients with secondary progressive MS also showed an earlier mean age of onset among women who had no full pregnancies (n = 19; 41.5 ± 9.2 years) compared to women with 1 or more full pregnancies (n = 57; 47.3 ± 10.6 years; P = .049).
The later disease onset associated with pregnancy was also seen in relapsing-remitting MS: Mean age of onset was earlier women with no pregnancies (27.5 ± 7.0 years) compared to those with one or more children (33.0 ± 9.4 years; P = .021).
The trends of later onset with more pregnancies was also observed with the mean age of onset of secondary progressive MS (no full pregnancies: onset at 41.5 ± 9.2 years; 1-3 pregnancies: 46.2 ± 9.9 years; 4 or more pregnancies, onset 52.6 ± 12.9 years; P = .010).
And likewise, the later mean age of onset of relapsing-remitting MS was seen with additional pregnancies (no full pregnancies: 27.5 ± 7.0 years; 1-3 pregnancies: 32.4 ± 9.3 years; 4 or more pregnancies: 35.8 ± 9.8 years; P = .012).
“The dose effect was clearly a surprise (having no full-term pregnancies vs. 1-3 vs. 4 or more),” Dr. Zeydan said.
“In addition to the significant difference between having no versus one or more full-term pregnancies, the clear dose-effect consolidates our results related to the association between the number of pregnancies and age at progressive MS onset.”
Early menopause also linked to shorter progression to secondary progressive MS
The study also showed that women with premature or early menopause had a shorter duration of progressing from relapsing-remitting MS to secondary progressive MS (n = 26; 12.9 ± 9.0 years) compared to women with normal age at menopause (n = 39; 17.8 ± 10.3 years).
The pattern was similar for women experiencing the onset of secondary progressive MS after menopause, with a shorter progression among those with early menopause (P = .012).
The patterns in early menopause are consistent with previous observations regarding menopause and MS progression, Dr. Cohen said.
“When women go through menopause, estradiol and pregnancy-related factors further decline and we know this coincides temporally with the development of progressive MS in women,” he noted.
Compared to men, women with premature or early menopause furthermore had a longer duration from relapsing-remitting MS to secondary progressive MS (P = .008), and women with secondary progressive MS also had also had an earlier age of relapsing-remitting MS onset than men (P = .018).
Possible mechanisms and applications of the findings
The mechanisms of pregnancy that could include a complex interaction between estrogen and factors such as astrocyte and microglia function, Dr. Zeydan explained.
“Estrogen, through various mechanisms of eliminating toxicity of highly activated neurons – including preventing proinflammatory molecule release, supporting mitochondria function thereby eliminating energy failure, and promoting remyelination – helps neuronal plasticity and delays neurodegeneration, which is closely related to the progressive phase of MS,” she said.
“One could easily make the probable association, while yet to be proven, that our findings may relate to these mechanisms,” Dr. Zeydan said.
The logical question of whether hormone replacement or some type of therapy that could mimic the effects of pregnancy could also benefit in delaying MS onset remained to be seen, Dr. Zeydan said.
“While we believe that is possible, particularly for delaying the onset of progressive phase, definitive evidence is lacking at this time,” Dr. Zeydan said.
“However, our study ultimately may lead to such a trial.”
In the meantime, the findings provide additional insights that may be beneficial in sharing with patients regarding pregnancy,” she said.
“As the contemporary problem in MS care is to delay or prevent progressive MS onset, our findings may suggest that how we counsel women with MS who are planning to get pregnant, or contemplating surgically induced menopause, or how we consider hormone therapies during perimenopause may impact the course of their disease.”
Dr. Zeydan cautioned, however, that “our findings do not confirm causality beyond an association.”
“More studies are needed in this important issue in a disease that affects women three times more than men.”
Dr. Zeydan had no disclosures to report. Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Zeydan B et al. ACTRIMS Forum 2020, Abstract P135.
WEST PALM BEACH, FLA. – Women who have no history of a full-term pregnancy show an earlier onset of progressive multiple sclerosis (MS) compared to those who do have pregnancies, and the apparent onset-delaying effect appears to increase with the number of pregnancies, according to new research adding to speculation of the effects of pregnancy in MS.
“Our results suggest that a higher number of full-term pregnancies than average is associated with later onset of progressive MS, while having no full-term pregnancies is associated with significantly younger age at progressive MS onset,” first author Burcu Zeydan, MD, an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minn., said in an interview.
The study was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The findings, which also link early menopause with faster disease progression, offer important insights into the broader effects of pregnancy on MS, said ACTRIMS president Jeffrey A. Cohen, MD, director of Experimental Therapeutics at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
“We know pregnancy affects the short term disease activity – relapses tend to quiet down during pregnancy – but what has been somewhat conflicting is whether it affects the long-term prognosis or is just a temporary effect,” he said in an interview.
“So that is the main interest in this study, and it does indicate that pregnancy affects the long-term prognosis and provides some insight into the mechanism by which it might do that.”
While being female is in fact considered the most important risk factor for MS susceptibility, pregnancy has been suggested to have a protective role in disease progression, but more research is needed on the nature of the effect – and its mechanisms.
For the study, Dr. Zeydan and colleagues evaluated data on 202 patients with MS who were part of a Mayo Clinic survey, including 134 women and 68 men.
They found that women who had no full-term pregnancies (n = 32), had an earlier onset of progressive MS (mean age 41.4 ± 12.6 years) compared to women giving birth to 1 or more children (n = 95; 47.1 ± 9.7 years; P = .012).
In addition, the mean age of progressive MS onset also increased with a dose-effect trend according to number of full pregnancies (no children, 41.4 ± 12.6 years; 1-3 children: 46.4 ± 9.2 years; 4 or more children: 52.6 ± 12.9 years; P = .002).
A look at a subgroup of patients with secondary progressive MS also showed an earlier mean age of onset among women who had no full pregnancies (n = 19; 41.5 ± 9.2 years) compared to women with 1 or more full pregnancies (n = 57; 47.3 ± 10.6 years; P = .049).
The later disease onset associated with pregnancy was also seen in relapsing-remitting MS: Mean age of onset was earlier women with no pregnancies (27.5 ± 7.0 years) compared to those with one or more children (33.0 ± 9.4 years; P = .021).
The trends of later onset with more pregnancies was also observed with the mean age of onset of secondary progressive MS (no full pregnancies: onset at 41.5 ± 9.2 years; 1-3 pregnancies: 46.2 ± 9.9 years; 4 or more pregnancies, onset 52.6 ± 12.9 years; P = .010).
And likewise, the later mean age of onset of relapsing-remitting MS was seen with additional pregnancies (no full pregnancies: 27.5 ± 7.0 years; 1-3 pregnancies: 32.4 ± 9.3 years; 4 or more pregnancies: 35.8 ± 9.8 years; P = .012).
“The dose effect was clearly a surprise (having no full-term pregnancies vs. 1-3 vs. 4 or more),” Dr. Zeydan said.
“In addition to the significant difference between having no versus one or more full-term pregnancies, the clear dose-effect consolidates our results related to the association between the number of pregnancies and age at progressive MS onset.”
Early menopause also linked to shorter progression to secondary progressive MS
The study also showed that women with premature or early menopause had a shorter duration of progressing from relapsing-remitting MS to secondary progressive MS (n = 26; 12.9 ± 9.0 years) compared to women with normal age at menopause (n = 39; 17.8 ± 10.3 years).
The pattern was similar for women experiencing the onset of secondary progressive MS after menopause, with a shorter progression among those with early menopause (P = .012).
The patterns in early menopause are consistent with previous observations regarding menopause and MS progression, Dr. Cohen said.
“When women go through menopause, estradiol and pregnancy-related factors further decline and we know this coincides temporally with the development of progressive MS in women,” he noted.
Compared to men, women with premature or early menopause furthermore had a longer duration from relapsing-remitting MS to secondary progressive MS (P = .008), and women with secondary progressive MS also had also had an earlier age of relapsing-remitting MS onset than men (P = .018).
Possible mechanisms and applications of the findings
The mechanisms of pregnancy that could include a complex interaction between estrogen and factors such as astrocyte and microglia function, Dr. Zeydan explained.
“Estrogen, through various mechanisms of eliminating toxicity of highly activated neurons – including preventing proinflammatory molecule release, supporting mitochondria function thereby eliminating energy failure, and promoting remyelination – helps neuronal plasticity and delays neurodegeneration, which is closely related to the progressive phase of MS,” she said.
“One could easily make the probable association, while yet to be proven, that our findings may relate to these mechanisms,” Dr. Zeydan said.
The logical question of whether hormone replacement or some type of therapy that could mimic the effects of pregnancy could also benefit in delaying MS onset remained to be seen, Dr. Zeydan said.
“While we believe that is possible, particularly for delaying the onset of progressive phase, definitive evidence is lacking at this time,” Dr. Zeydan said.
“However, our study ultimately may lead to such a trial.”
In the meantime, the findings provide additional insights that may be beneficial in sharing with patients regarding pregnancy,” she said.
“As the contemporary problem in MS care is to delay or prevent progressive MS onset, our findings may suggest that how we counsel women with MS who are planning to get pregnant, or contemplating surgically induced menopause, or how we consider hormone therapies during perimenopause may impact the course of their disease.”
Dr. Zeydan cautioned, however, that “our findings do not confirm causality beyond an association.”
“More studies are needed in this important issue in a disease that affects women three times more than men.”
Dr. Zeydan had no disclosures to report. Dr. Cohen reported receiving personal compensation for consulting for Adamas, Convelo, MedDay, Mylan, and Population Council; and serving as an Editor of Multiple Sclerosis Journal.
SOURCE: Zeydan B et al. ACTRIMS Forum 2020, Abstract P135.
REPORTING FROM ACTRIMS FORUM 2020