User login
OCT may help predict disease activity in CIS
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
REPORTING FROM ACTRIMS FORUM 2020
More evidence backs LDL below 70 to reduce recurrent stroke
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
REPORTING FROM ISC 2020
Pediatrics Board Review: Neonatal Seizures
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
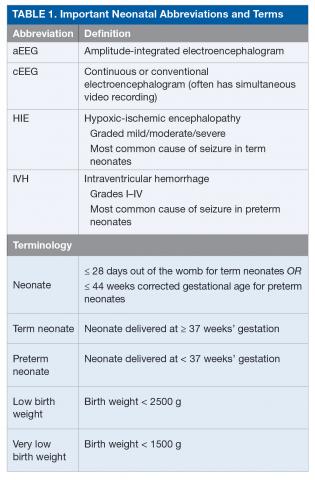
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
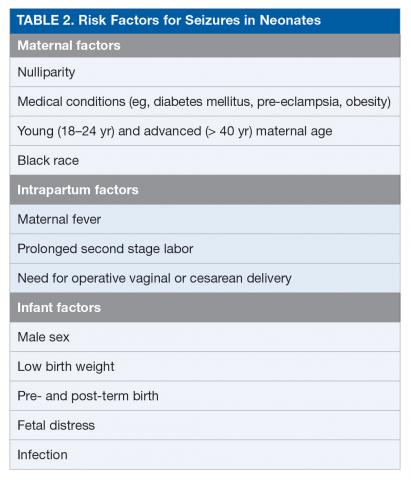
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
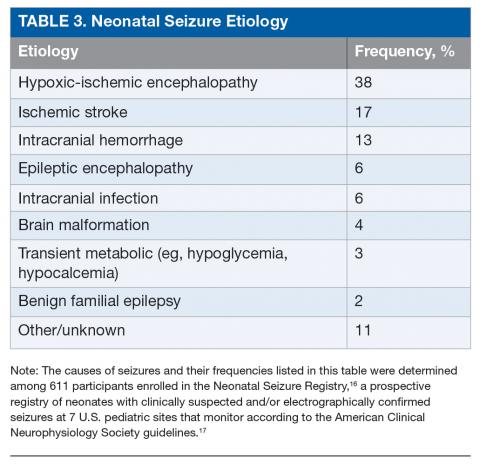
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34 Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
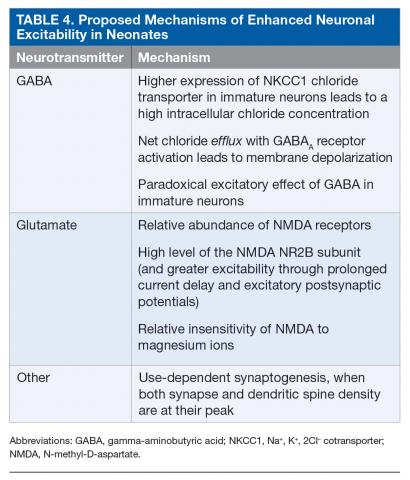
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44 In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47 Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
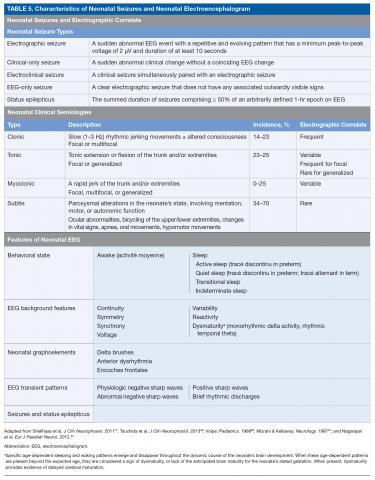
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
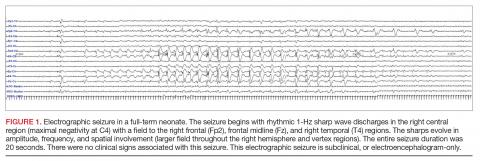
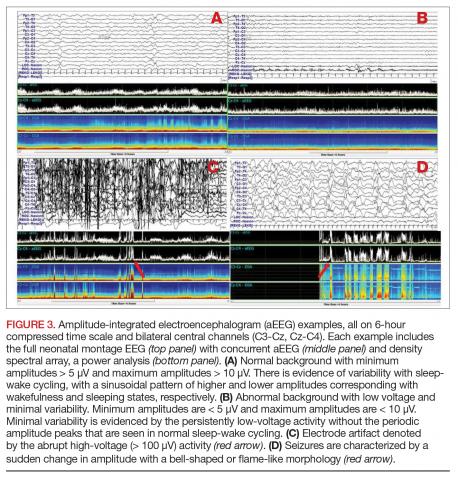
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50 The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51 Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52 Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17 The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23 A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59 While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9 Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60 Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62 A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
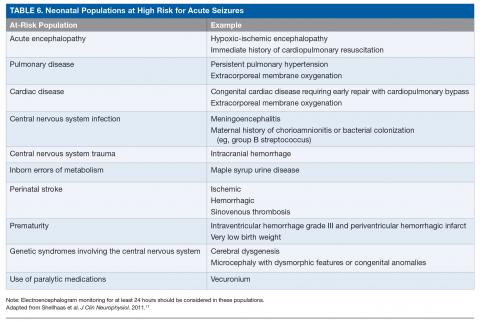
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
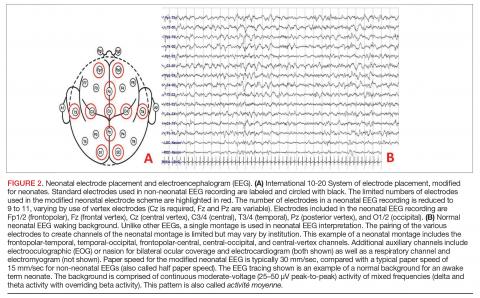
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17 However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74 Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83 For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
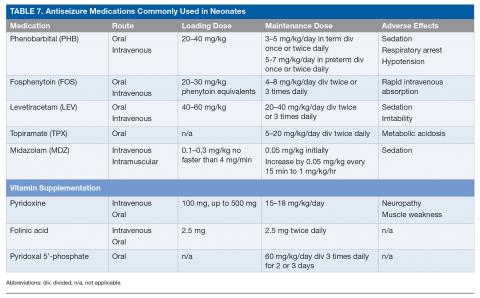
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85 Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86 Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63 The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21 In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7 In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47 Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6 Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34 Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44 In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47 Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50 The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51 Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52 Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17 The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23 A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59 While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9 Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60 Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62 A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17 However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74 Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83 For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85 Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86 Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63 The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21 In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7 In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47 Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6 Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34 Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44 In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47 Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50 The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51 Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52 Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17 The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23 A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59 While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9 Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60 Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62 A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17 However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74 Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83 For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85 Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86 Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63 The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21 In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7 In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47 Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6 Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
FDA approves first IV migraine prevention drug
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
FROM MEDSCAPE.COM
First clinical evidence of neuroprotection in acute stroke?
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
TNK dose in large-vessel stroke: 0.25 mg/kg is sufficient
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY
ARCADIA: Predicting risk of atrial cardiopathy poststroke
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.
Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
REPORTING FROM ISC 2020