User login
Large survey reveals that few MS patients have long-term care insurance
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
REPORTING FROM ACTRIMS FORUM 2019
Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.
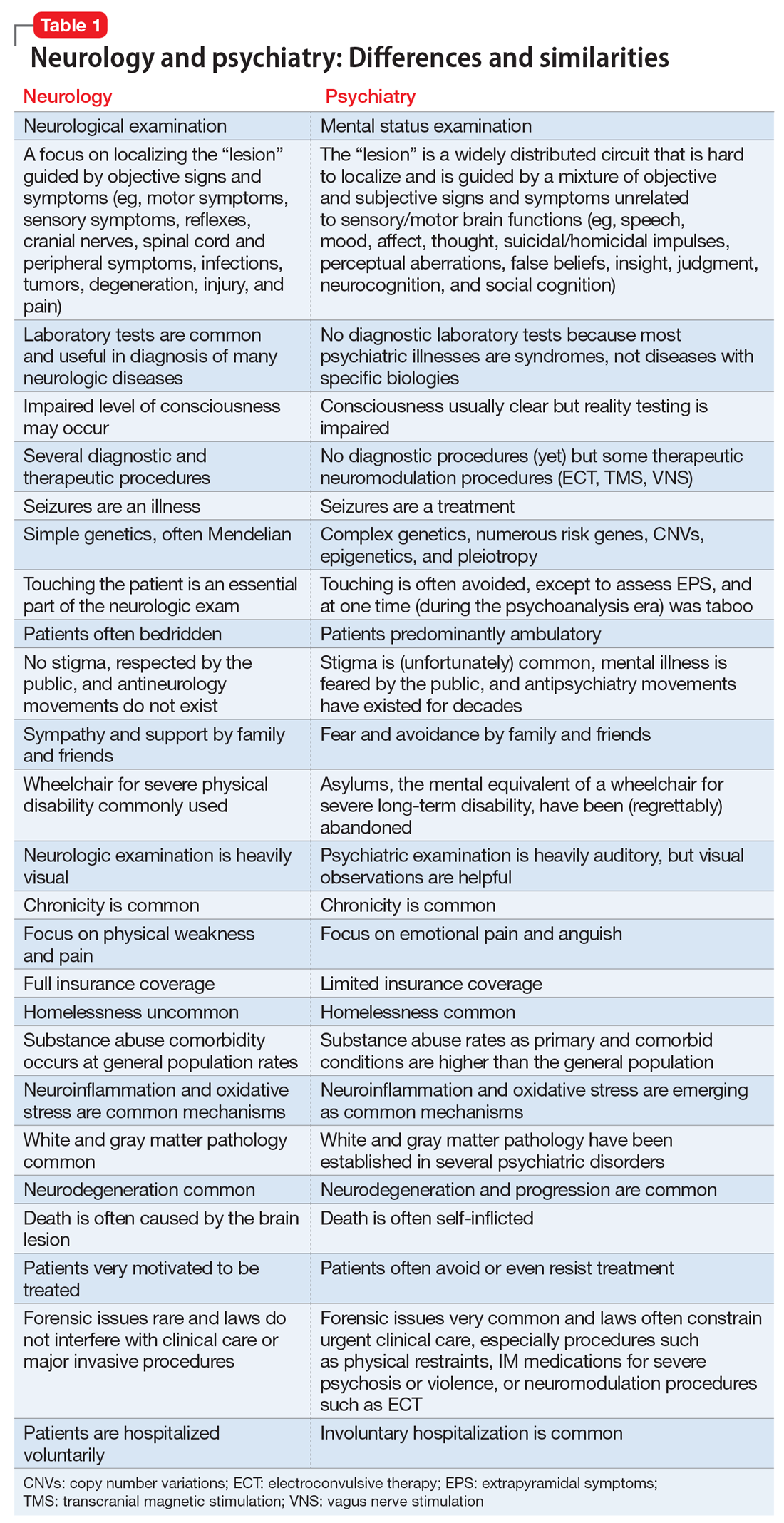
Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.

Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.

Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Myositis mimics: Clues for making the right diagnosis
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Spinal cord atrophy found to be accelerated in subset of RRMS patients
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: In patients with relapsing remitting MS (RRMS), upper cervical cord atrophy, as obtained from routine T1-weighted brain MRI, is a strong indicator of impending conversion to secondary progressive MS (SPMS).
Major finding: Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001).
Study details: A single-center, observational study of 54 RRMS patients who converted to SPMS during the 12-year observation and 54 RRMS patients who remained RRMS during the observation period.
Disclosures: Dr. Bischof reported having no financial disclosures.
Source: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
Stroke thrombolysis looks safe 31+ days after prior stroke
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
REPORTING FROM ISC 2019
Brain Biomarkers May Help Explain Severe PTSD Symptoms
A current theory holds that during a traumatic event, a person may learn to associate the people, locations, and objects in the situation with the trauma, and long after the event even the “safe” stimuli can trigger fearful and defensive responses. Experts believe it is an “overlearned response” to a threatening experience. But the way in which that learning happens is not well understood, say researchers from Yale University in New Haven, Connecticut, and the Icahn School of Medicine at Mount Sinai in New York City. Their study, though, may shed new light on how people with PTSD symptoms learn and unlearn fear.
In the study, funded in part by the National Institute of Mental Health, the researchers examined how the mental adjustments performed during learning and the way the brain tracks these adjustments relate to symptom severity.
They gave combat veterans with varying levels of PTSD symptom severity a reversal learning task. Participants were shown 2 mildly angry human faces and mildly shocked after viewing 1 face, but not the other. Then the task was reversed, with the aim of having the participants “unlearn” their original fear conditioning and testing their ability to relearn how to respond to negative surprises in the environment.
Although all participants were able to perform the reversal learning, the researchers found “pronounced differences in the ‘learning rates.’” Highly symptomatic veterans tended to overreact when what they expected to happen and what actually happened did not match up.
The researchers say they found biomarkers that could explain the different reactions. In the highly symptomatic veterans, 2 areas of the brain—the amygdala and striatum—were less able to track changes in threat level.
“One’s inability to adequately adjust expectations for potentially aversive outcomes has potential clinical relevance,” said Ilan Harpaz-Rotem, PhD, co-leader of the study, “as this deficit may lead to avoidance and depressive behavior.”
The researchers say their findings could give a “more fine-grained understanding of how learning processes may go awry in the aftermath of combat trauma.”
A current theory holds that during a traumatic event, a person may learn to associate the people, locations, and objects in the situation with the trauma, and long after the event even the “safe” stimuli can trigger fearful and defensive responses. Experts believe it is an “overlearned response” to a threatening experience. But the way in which that learning happens is not well understood, say researchers from Yale University in New Haven, Connecticut, and the Icahn School of Medicine at Mount Sinai in New York City. Their study, though, may shed new light on how people with PTSD symptoms learn and unlearn fear.
In the study, funded in part by the National Institute of Mental Health, the researchers examined how the mental adjustments performed during learning and the way the brain tracks these adjustments relate to symptom severity.
They gave combat veterans with varying levels of PTSD symptom severity a reversal learning task. Participants were shown 2 mildly angry human faces and mildly shocked after viewing 1 face, but not the other. Then the task was reversed, with the aim of having the participants “unlearn” their original fear conditioning and testing their ability to relearn how to respond to negative surprises in the environment.
Although all participants were able to perform the reversal learning, the researchers found “pronounced differences in the ‘learning rates.’” Highly symptomatic veterans tended to overreact when what they expected to happen and what actually happened did not match up.
The researchers say they found biomarkers that could explain the different reactions. In the highly symptomatic veterans, 2 areas of the brain—the amygdala and striatum—were less able to track changes in threat level.
“One’s inability to adequately adjust expectations for potentially aversive outcomes has potential clinical relevance,” said Ilan Harpaz-Rotem, PhD, co-leader of the study, “as this deficit may lead to avoidance and depressive behavior.”
The researchers say their findings could give a “more fine-grained understanding of how learning processes may go awry in the aftermath of combat trauma.”
A current theory holds that during a traumatic event, a person may learn to associate the people, locations, and objects in the situation with the trauma, and long after the event even the “safe” stimuli can trigger fearful and defensive responses. Experts believe it is an “overlearned response” to a threatening experience. But the way in which that learning happens is not well understood, say researchers from Yale University in New Haven, Connecticut, and the Icahn School of Medicine at Mount Sinai in New York City. Their study, though, may shed new light on how people with PTSD symptoms learn and unlearn fear.
In the study, funded in part by the National Institute of Mental Health, the researchers examined how the mental adjustments performed during learning and the way the brain tracks these adjustments relate to symptom severity.
They gave combat veterans with varying levels of PTSD symptom severity a reversal learning task. Participants were shown 2 mildly angry human faces and mildly shocked after viewing 1 face, but not the other. Then the task was reversed, with the aim of having the participants “unlearn” their original fear conditioning and testing their ability to relearn how to respond to negative surprises in the environment.
Although all participants were able to perform the reversal learning, the researchers found “pronounced differences in the ‘learning rates.’” Highly symptomatic veterans tended to overreact when what they expected to happen and what actually happened did not match up.
The researchers say they found biomarkers that could explain the different reactions. In the highly symptomatic veterans, 2 areas of the brain—the amygdala and striatum—were less able to track changes in threat level.
“One’s inability to adequately adjust expectations for potentially aversive outcomes has potential clinical relevance,” said Ilan Harpaz-Rotem, PhD, co-leader of the study, “as this deficit may lead to avoidance and depressive behavior.”
The researchers say their findings could give a “more fine-grained understanding of how learning processes may go awry in the aftermath of combat trauma.”
Noncardiac surgery has 7% covert stroke rate in elderly
HONOLULU – Covert strokes are relatively common in elderly patients who undergo noncardiac surgery, with a 7% incidence among a group of prospectively followed but generally unselected patients in a multicenter, international study.
By definition, these covert strokes were acutely asymptomatic, but showed evidence of clinical effects during the subsequent year. Twelve months after surgery, patients with acute, perioperative covert strokes found by systematic collection of postoperative MRI brain scans had a twofold increased rate of cognitive decline and a greater than twofold increased rate of delirium, compared with the patients who did not have evidence of a covert stroke, Marko Mrkobrada, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The message from these findings is that, when elderly patients exhibit confusion or delirium after noncardiac surgery, their physicians should have a high index of suspicion that a covert stroke may have occurred, Dr. Mrkobrada said in a video interview. It’s possible that typical stroke symptoms do not appear in many of the covert stroke patients because they are masked in the immediate postoperative period, he added.
Right now, the only way to screen for a covert stroke is with a brain MR, a test that generally costs several hundred dollars, which is too expensive for routine screening. Dr. Mrkobrada said that his team hopes further study will identify a biomarker that can flag patients with a covert stroke at a lower cost. For example, colleagues of Dr. Mrkobrada have successfully used high-sensitivity troponin T, a biomarker of myocardial injury, to identify patients who have myocardial injury after noncardiac surgery (MINS; JAMA. 2017 April 25;371[16]:1642-51). Study results also established that treating MINS patients with dabigatran improved their long-term clinical outcomes (Lancet. 2018 June 9;391[10137]:2325-34).
Covert stroke after noncardiac surgery “is the same concept” as MINS, said Dr. Mrkobrada, a researcher at the London Health Sciences Centre in Canada. “We find strokes that do not get picked up after noncardiac surgery just like MIs that are not picked up,” he said. It’s also possible that certain interventions may improve outcomes in patients with covert strokes, just as they have helped MINS patients, he suggested. Potentially helpful interventions could include aspirin, a statin, and improved blood pressure control. A major goal for his research group is finding a biomarker that makes diagnosing covert stroke as easy as using high sensitivity troponin T to diagnose MINS.
The NeuroVISION (Detection and Neurological Impact of Cerebrovascular Events In Noncardiac Surgery Patients: A Cohort EvaluatioN) study enrolled and tested 1,114 people aged 65 years or older scheduled for elective noncardiac surgery anticipated to keep them hospitalized for at least 2 days at any of 12 participating centers in nine countries. Patients underwent cognitive function testing before surgery and had a brain MR scan 2-9 days after surgery, and they were excluded if they developed an overt stroke prior to the scan. Patients underwent a second round of cognitive testing a year after surgery. Patients averaged 73 years old.
The screening MR scans identified covert strokes in 78 of the study subjects (7%). The 1-year cognitive tests showed measurable drops in cognitive function in 42% of those who had experience covert strokes and in 29% of everyone else. Those rates translated to a doubled odds ratio for cognitive decline after covert stroke, compared with people without covert stroke after adjustment for baseline between-group differences, a highly statistically significant between-group difference for the study’s primary endpoint. Delirium occurred 2.2-fold more often in the covert stroke patients after adjustment, and overt strokes during 1-year follow-up were 4.1-fold more common patients who’d experienced a covert stroke, compared with everyone else, after adjustment, Dr. Mrkobrada reported. NeuroVISION is the first large-scale study to assess the incidence and associations of covert strokes after noncardiac surgery, he noted.
SOURCE: Mrkobrada M. ISC 2019, Late-Breaking Abstract LB18.
HONOLULU – Covert strokes are relatively common in elderly patients who undergo noncardiac surgery, with a 7% incidence among a group of prospectively followed but generally unselected patients in a multicenter, international study.
By definition, these covert strokes were acutely asymptomatic, but showed evidence of clinical effects during the subsequent year. Twelve months after surgery, patients with acute, perioperative covert strokes found by systematic collection of postoperative MRI brain scans had a twofold increased rate of cognitive decline and a greater than twofold increased rate of delirium, compared with the patients who did not have evidence of a covert stroke, Marko Mrkobrada, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The message from these findings is that, when elderly patients exhibit confusion or delirium after noncardiac surgery, their physicians should have a high index of suspicion that a covert stroke may have occurred, Dr. Mrkobrada said in a video interview. It’s possible that typical stroke symptoms do not appear in many of the covert stroke patients because they are masked in the immediate postoperative period, he added.
Right now, the only way to screen for a covert stroke is with a brain MR, a test that generally costs several hundred dollars, which is too expensive for routine screening. Dr. Mrkobrada said that his team hopes further study will identify a biomarker that can flag patients with a covert stroke at a lower cost. For example, colleagues of Dr. Mrkobrada have successfully used high-sensitivity troponin T, a biomarker of myocardial injury, to identify patients who have myocardial injury after noncardiac surgery (MINS; JAMA. 2017 April 25;371[16]:1642-51). Study results also established that treating MINS patients with dabigatran improved their long-term clinical outcomes (Lancet. 2018 June 9;391[10137]:2325-34).
Covert stroke after noncardiac surgery “is the same concept” as MINS, said Dr. Mrkobrada, a researcher at the London Health Sciences Centre in Canada. “We find strokes that do not get picked up after noncardiac surgery just like MIs that are not picked up,” he said. It’s also possible that certain interventions may improve outcomes in patients with covert strokes, just as they have helped MINS patients, he suggested. Potentially helpful interventions could include aspirin, a statin, and improved blood pressure control. A major goal for his research group is finding a biomarker that makes diagnosing covert stroke as easy as using high sensitivity troponin T to diagnose MINS.
The NeuroVISION (Detection and Neurological Impact of Cerebrovascular Events In Noncardiac Surgery Patients: A Cohort EvaluatioN) study enrolled and tested 1,114 people aged 65 years or older scheduled for elective noncardiac surgery anticipated to keep them hospitalized for at least 2 days at any of 12 participating centers in nine countries. Patients underwent cognitive function testing before surgery and had a brain MR scan 2-9 days after surgery, and they were excluded if they developed an overt stroke prior to the scan. Patients underwent a second round of cognitive testing a year after surgery. Patients averaged 73 years old.
The screening MR scans identified covert strokes in 78 of the study subjects (7%). The 1-year cognitive tests showed measurable drops in cognitive function in 42% of those who had experience covert strokes and in 29% of everyone else. Those rates translated to a doubled odds ratio for cognitive decline after covert stroke, compared with people without covert stroke after adjustment for baseline between-group differences, a highly statistically significant between-group difference for the study’s primary endpoint. Delirium occurred 2.2-fold more often in the covert stroke patients after adjustment, and overt strokes during 1-year follow-up were 4.1-fold more common patients who’d experienced a covert stroke, compared with everyone else, after adjustment, Dr. Mrkobrada reported. NeuroVISION is the first large-scale study to assess the incidence and associations of covert strokes after noncardiac surgery, he noted.
SOURCE: Mrkobrada M. ISC 2019, Late-Breaking Abstract LB18.
HONOLULU – Covert strokes are relatively common in elderly patients who undergo noncardiac surgery, with a 7% incidence among a group of prospectively followed but generally unselected patients in a multicenter, international study.
By definition, these covert strokes were acutely asymptomatic, but showed evidence of clinical effects during the subsequent year. Twelve months after surgery, patients with acute, perioperative covert strokes found by systematic collection of postoperative MRI brain scans had a twofold increased rate of cognitive decline and a greater than twofold increased rate of delirium, compared with the patients who did not have evidence of a covert stroke, Marko Mrkobrada, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The message from these findings is that, when elderly patients exhibit confusion or delirium after noncardiac surgery, their physicians should have a high index of suspicion that a covert stroke may have occurred, Dr. Mrkobrada said in a video interview. It’s possible that typical stroke symptoms do not appear in many of the covert stroke patients because they are masked in the immediate postoperative period, he added.
Right now, the only way to screen for a covert stroke is with a brain MR, a test that generally costs several hundred dollars, which is too expensive for routine screening. Dr. Mrkobrada said that his team hopes further study will identify a biomarker that can flag patients with a covert stroke at a lower cost. For example, colleagues of Dr. Mrkobrada have successfully used high-sensitivity troponin T, a biomarker of myocardial injury, to identify patients who have myocardial injury after noncardiac surgery (MINS; JAMA. 2017 April 25;371[16]:1642-51). Study results also established that treating MINS patients with dabigatran improved their long-term clinical outcomes (Lancet. 2018 June 9;391[10137]:2325-34).
Covert stroke after noncardiac surgery “is the same concept” as MINS, said Dr. Mrkobrada, a researcher at the London Health Sciences Centre in Canada. “We find strokes that do not get picked up after noncardiac surgery just like MIs that are not picked up,” he said. It’s also possible that certain interventions may improve outcomes in patients with covert strokes, just as they have helped MINS patients, he suggested. Potentially helpful interventions could include aspirin, a statin, and improved blood pressure control. A major goal for his research group is finding a biomarker that makes diagnosing covert stroke as easy as using high sensitivity troponin T to diagnose MINS.
The NeuroVISION (Detection and Neurological Impact of Cerebrovascular Events In Noncardiac Surgery Patients: A Cohort EvaluatioN) study enrolled and tested 1,114 people aged 65 years or older scheduled for elective noncardiac surgery anticipated to keep them hospitalized for at least 2 days at any of 12 participating centers in nine countries. Patients underwent cognitive function testing before surgery and had a brain MR scan 2-9 days after surgery, and they were excluded if they developed an overt stroke prior to the scan. Patients underwent a second round of cognitive testing a year after surgery. Patients averaged 73 years old.
The screening MR scans identified covert strokes in 78 of the study subjects (7%). The 1-year cognitive tests showed measurable drops in cognitive function in 42% of those who had experience covert strokes and in 29% of everyone else. Those rates translated to a doubled odds ratio for cognitive decline after covert stroke, compared with people without covert stroke after adjustment for baseline between-group differences, a highly statistically significant between-group difference for the study’s primary endpoint. Delirium occurred 2.2-fold more often in the covert stroke patients after adjustment, and overt strokes during 1-year follow-up were 4.1-fold more common patients who’d experienced a covert stroke, compared with everyone else, after adjustment, Dr. Mrkobrada reported. NeuroVISION is the first large-scale study to assess the incidence and associations of covert strokes after noncardiac surgery, he noted.
SOURCE: Mrkobrada M. ISC 2019, Late-Breaking Abstract LB18.
REPORTING FROM ISC 2019
Key clinical point:
Major finding: Elderly patients who underwent noncardiac surgery had a 7% incidence of covert stroke.
Study details: NeuroVISION, a prospective, multicenter, observational study with 1,114 patients.
Disclosures: NeuroVISION did not receive commercial funding. Dr. Mrkobrada had no disclosures.
Source: Mrkobrada M. ISC 2019, Late-Breaking Abstract LB18.
Early intensive treatment of MS may benefit patients
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: After 5 years, the average increase in Expanded Disability Status Scale score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2).
Study details: A population-based cohort study of 592 patients with MS in southeast Wales.
Disclosures: Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
Source: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905.
When to suspect a severe skin reaction to an AED
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
EXPERT ANALYSIS FROM AES 2018
Minimally invasive ICH lysis safely helps when clot adequately shrinks
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
REPORTING FROM ISC 2019
Key clinical point: Minimally-invasive intracerebral clot lysis was safe and often effective when the residual clot shrank to 15 mL or less.
Major finding: One year after entry, 45% of MISTIE-treated patients and 41% of controls had a modified Rankin Scale score of 0-3.
Study details: MISTIE III, a multicenter, international, randomized trial of 499 patients.
Disclosures: MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
Source: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.















