User login
Fentanyl vaccine a potential ‘game changer’ for opioid crisis
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
FROM PHARMACEUTICS
Screen time may help concussion recovery
research shows.
Now a study suggests that getting back on TikTok and Snapchat may help, too.
After surveying 700 patients ages 8-16 following an injury, researchers for the Pediatric Emergency Research Canada A-CAP study team found that
A “moderate” amount was between 2 and 7 hours per day on various screens. “That includes their phones, computers, and televisions,” says lead author Molly Cairncross, PhD, of Simon Fraser University, Vancouver.
People in the study who reported either less or more screen time than that in the 7-10 days after injury also reported more symptoms, such as headaches and fatigue, during the first month. After that month, all the participants reported similar symptoms, regardless of their early screen use – suggesting that screen time makes little difference long term in pediatric concussion recovery.
The findings differ from a 2021 study by researchers at the University of Massachusetts, Boston, that found screen time slowed recovery. Why the clashing results? “I think what it comes down to are differences in study design,” says Dr. Cairncross. While the earlier study measured screen use in the first 48 hours, and recovery over 10 days, “we focused on screen time use over the first 7-10 days, and tracked recovery over 6 months,” she says.
“Taken together, the studies suggest a need to find balance – not too little and not too much time on screens for kids and teens following a concussion,” Dr. Cairncross says.
Ultimately, the findings support moderation rather than blanket restrictions on screen time as the best way to manage pediatric concussion, especially after the first 48 hours.
“It’s actually unsurprising,” says Sarah Brittain, MS, a speech-language pathologist and founder of Colorado Brain Recovery in Wheat Ridge, who was not involved in the study. “An early return to both cognitive and physical activity in a controlled fashion is really important. Sitting in a dark room and resting is not the answer and has been disproven in the literature.”
Old advice involved lying in a quiet, dark room for days, but recent evidence reveals that such “cocoon therapy” may actually prolong symptoms.
“With time, we have found this can negatively impact quality of life and depression scores, especially in teenagers,” says Katherine Labiner, MD, a child neurologist at Pediatrix Child Neurology Consultants of Austin, Tex., who was not involved in the study.
So, how might screens help? Dr. Labiner, Ms. Brittain, and Dr. Cairncross all point to the importance of connection – not the Internet kind, but the social kind. Children and teens use smartphones and computers to stay connected with peers, so banning screen time could have a negative impact on mental health by leading to loneliness, separation, and lack of social support.
“Depression can prolong the course of recovery,” says Ms. Brittain.
It’s worth noting that screen time could trigger visual symptoms in some patients, she says. “If someone feels worse within 2 minutes of being on a screen, that’s a good indicator that screens aren’t working for them,” Ms. Brittain says. “If being on a screen makes them dizzy or wiped out, or the words on the screen look like they’re moving when they’re not, that means it’s time to back off.”
She advises parents to watch for behavior changes like increased crankiness, impatience, and/or fatigue, which could mean that the child has returned to screen time – or any activity – too soon and should scale back until symptoms subside.
“The most important thing to stress with concussion is full recovery before complete return to activity,” Dr. Labiner says.
A version of this article first appeared on Medscape.com.
research shows.
Now a study suggests that getting back on TikTok and Snapchat may help, too.
After surveying 700 patients ages 8-16 following an injury, researchers for the Pediatric Emergency Research Canada A-CAP study team found that
A “moderate” amount was between 2 and 7 hours per day on various screens. “That includes their phones, computers, and televisions,” says lead author Molly Cairncross, PhD, of Simon Fraser University, Vancouver.
People in the study who reported either less or more screen time than that in the 7-10 days after injury also reported more symptoms, such as headaches and fatigue, during the first month. After that month, all the participants reported similar symptoms, regardless of their early screen use – suggesting that screen time makes little difference long term in pediatric concussion recovery.
The findings differ from a 2021 study by researchers at the University of Massachusetts, Boston, that found screen time slowed recovery. Why the clashing results? “I think what it comes down to are differences in study design,” says Dr. Cairncross. While the earlier study measured screen use in the first 48 hours, and recovery over 10 days, “we focused on screen time use over the first 7-10 days, and tracked recovery over 6 months,” she says.
“Taken together, the studies suggest a need to find balance – not too little and not too much time on screens for kids and teens following a concussion,” Dr. Cairncross says.
Ultimately, the findings support moderation rather than blanket restrictions on screen time as the best way to manage pediatric concussion, especially after the first 48 hours.
“It’s actually unsurprising,” says Sarah Brittain, MS, a speech-language pathologist and founder of Colorado Brain Recovery in Wheat Ridge, who was not involved in the study. “An early return to both cognitive and physical activity in a controlled fashion is really important. Sitting in a dark room and resting is not the answer and has been disproven in the literature.”
Old advice involved lying in a quiet, dark room for days, but recent evidence reveals that such “cocoon therapy” may actually prolong symptoms.
“With time, we have found this can negatively impact quality of life and depression scores, especially in teenagers,” says Katherine Labiner, MD, a child neurologist at Pediatrix Child Neurology Consultants of Austin, Tex., who was not involved in the study.
So, how might screens help? Dr. Labiner, Ms. Brittain, and Dr. Cairncross all point to the importance of connection – not the Internet kind, but the social kind. Children and teens use smartphones and computers to stay connected with peers, so banning screen time could have a negative impact on mental health by leading to loneliness, separation, and lack of social support.
“Depression can prolong the course of recovery,” says Ms. Brittain.
It’s worth noting that screen time could trigger visual symptoms in some patients, she says. “If someone feels worse within 2 minutes of being on a screen, that’s a good indicator that screens aren’t working for them,” Ms. Brittain says. “If being on a screen makes them dizzy or wiped out, or the words on the screen look like they’re moving when they’re not, that means it’s time to back off.”
She advises parents to watch for behavior changes like increased crankiness, impatience, and/or fatigue, which could mean that the child has returned to screen time – or any activity – too soon and should scale back until symptoms subside.
“The most important thing to stress with concussion is full recovery before complete return to activity,” Dr. Labiner says.
A version of this article first appeared on Medscape.com.
research shows.
Now a study suggests that getting back on TikTok and Snapchat may help, too.
After surveying 700 patients ages 8-16 following an injury, researchers for the Pediatric Emergency Research Canada A-CAP study team found that
A “moderate” amount was between 2 and 7 hours per day on various screens. “That includes their phones, computers, and televisions,” says lead author Molly Cairncross, PhD, of Simon Fraser University, Vancouver.
People in the study who reported either less or more screen time than that in the 7-10 days after injury also reported more symptoms, such as headaches and fatigue, during the first month. After that month, all the participants reported similar symptoms, regardless of their early screen use – suggesting that screen time makes little difference long term in pediatric concussion recovery.
The findings differ from a 2021 study by researchers at the University of Massachusetts, Boston, that found screen time slowed recovery. Why the clashing results? “I think what it comes down to are differences in study design,” says Dr. Cairncross. While the earlier study measured screen use in the first 48 hours, and recovery over 10 days, “we focused on screen time use over the first 7-10 days, and tracked recovery over 6 months,” she says.
“Taken together, the studies suggest a need to find balance – not too little and not too much time on screens for kids and teens following a concussion,” Dr. Cairncross says.
Ultimately, the findings support moderation rather than blanket restrictions on screen time as the best way to manage pediatric concussion, especially after the first 48 hours.
“It’s actually unsurprising,” says Sarah Brittain, MS, a speech-language pathologist and founder of Colorado Brain Recovery in Wheat Ridge, who was not involved in the study. “An early return to both cognitive and physical activity in a controlled fashion is really important. Sitting in a dark room and resting is not the answer and has been disproven in the literature.”
Old advice involved lying in a quiet, dark room for days, but recent evidence reveals that such “cocoon therapy” may actually prolong symptoms.
“With time, we have found this can negatively impact quality of life and depression scores, especially in teenagers,” says Katherine Labiner, MD, a child neurologist at Pediatrix Child Neurology Consultants of Austin, Tex., who was not involved in the study.
So, how might screens help? Dr. Labiner, Ms. Brittain, and Dr. Cairncross all point to the importance of connection – not the Internet kind, but the social kind. Children and teens use smartphones and computers to stay connected with peers, so banning screen time could have a negative impact on mental health by leading to loneliness, separation, and lack of social support.
“Depression can prolong the course of recovery,” says Ms. Brittain.
It’s worth noting that screen time could trigger visual symptoms in some patients, she says. “If someone feels worse within 2 minutes of being on a screen, that’s a good indicator that screens aren’t working for them,” Ms. Brittain says. “If being on a screen makes them dizzy or wiped out, or the words on the screen look like they’re moving when they’re not, that means it’s time to back off.”
She advises parents to watch for behavior changes like increased crankiness, impatience, and/or fatigue, which could mean that the child has returned to screen time – or any activity – too soon and should scale back until symptoms subside.
“The most important thing to stress with concussion is full recovery before complete return to activity,” Dr. Labiner says.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Neurosurgery Operating Room Efficiency During the COVID-19 Era
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 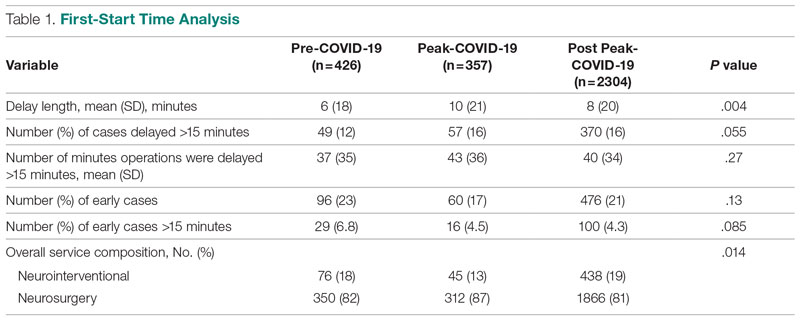
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.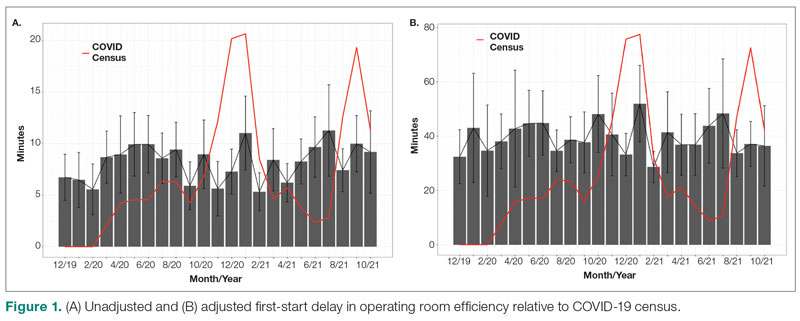
Turnover Time
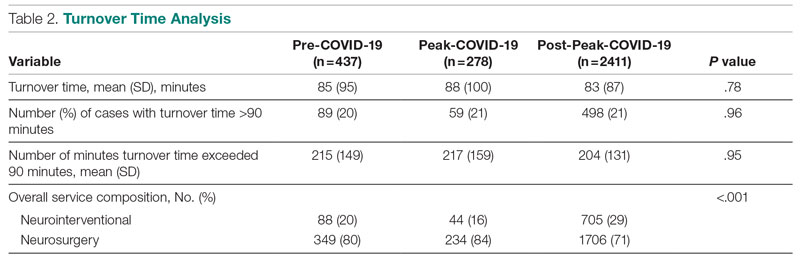
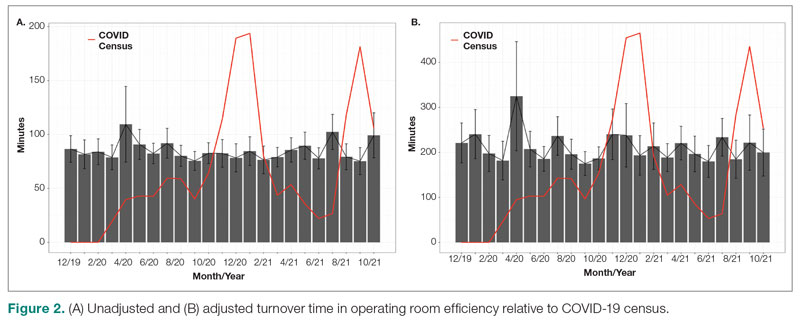
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.
Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
Anesthetic Choices and Postoperative Delirium Incidence: Propofol vs Sevoflurane
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
U.S. dementia rate drops as education, women’s employment rises
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From PNAS
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Imaging IDs brain activity related to dissociative symptoms
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Nutrition for cognition: A missed opportunity in U.S. seniors?
, new research shows. Researchers assessed the memory function of more than 3,500 persons who used SNAP or did not use SNAP over a period of 20 years. They found that those who didn’t use the food benefits program experienced 2 more years of cognitive aging compared with program users.
Of the 3,555 individuals included in the study, all were eligible to use the benefits, but only 559 did, leaving 2,996 participants who did not take advantage of the program.
Low program participation levels translate into a missed opportunity to prevent dementia, said study investigator Adina Zeki Al Hazzouri, PhD, assistant professor of epidemiology at the Columbia Aging Center at Columbia University Mailman School of Public Health in New York.
She said that prior research has shown that stigma may prevent older Americans from using SNAP. “Educational programs are needed to reduce the stigma that the public holds towards SNAP use,” she said.
Policy change could increase usage among older individuals, Dr. Zeki Al Hazzouri noted. Such changes could include simplifying enrollment and reporting procedures, shortening recertification periods, and increasing benefit levels.
The study was published online in Neurology.
Memory preservation
Dr. Zeki Al Hazzouri and her team assessed respondents from the Health and Retirement Study (HRS), a representative sample of Americans aged 50 and older. All respondents who were eligible to participate in SNAP in 1996 were followed every 2 years until 2016.
At each assessment, HRS respondents completed memory tests, including immediate and delayed word recall. For those who were too impaired to complete the interview, proxy informants – typically, their spouses or family members – assessed the memory and cognition of their family members using validated instruments, such as the 16-item Informant Questionnaire for Cognitive Decline.
Investigators used a validated memory function composite score, which is benchmarked against the memory assessments and evaluations of the Aging, Demographics, and Memory Study (ADAMS) cohort.
The team found that compared with nonusers, SNAP users were more likely to be women, Black, and born in the southern United States. They were less likely to be married and had more chronic conditions, such as high blood pressure, diabetes, cancer, heart problems, psychiatric problems, and arthritis.
One important study limitation was that SNAP use was measured only once during the study, the investigators noted. Ideally, Dr. Zeki Al Hazzouri said, future research would examine cumulative SNAP use history and explore the pathways that might account for the association between SNAP use and memory decline.
While findings suggest that there were no significant differences in baseline memory function between SNAP users and nonusers, users experienced approximately 2 fewer years of cognitive aging over a 10-year period than those who didn’t use the program.
Dr. Zeki Al Hazzouri speculated that SNAP benefits may slow cognitive aging by contributing to overall brain health and that, in comparison with nonusers, SNAP users absorb more nutrients, which promote neuronal integrity.
The investigators theorized that SNAP benefits may reduce stress from financial hardship, which has been linked to premature cognitive aging in other research.
“SNAP may also increase the purchasing power and investment in other health preserving behaviors, but also resulting in better access to care, which may in turn result in better disease management and management of risk factors for cognitive function,” the investigators wrote.
An underutilized program
In an accompanying editorial, Steven Albert, PhD, Philip B. Hallen Endowed Chair in Community Health and Social Justice at the University of Pittsburgh, noted that in 2020, among households with people aged 50 and older in the United States, more than 9 million Americans experienced food insecurity.
Furthermore, he pointed out, research from 2018 showed that 71% of people aged 60 and older who met income eligibility for SNAP did not participate in the program. “SNAP is an underutilized food security program involving substantial income supplements for older people with low incomes.
“Against the backdrop of so many failures of pharmacotherapy for dementia and the so far inexorable increase in the prevalence of dementia due to population aging, are we missing an opportunity to support cognitive health by failing to enroll the 14 million Americans who are over age 60 and eligible for SNAP but who do not participate?” Dr. Albert asked. He suggested that it would be helpful to determine this through a randomized promotion trial.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Researchers assessed the memory function of more than 3,500 persons who used SNAP or did not use SNAP over a period of 20 years. They found that those who didn’t use the food benefits program experienced 2 more years of cognitive aging compared with program users.
Of the 3,555 individuals included in the study, all were eligible to use the benefits, but only 559 did, leaving 2,996 participants who did not take advantage of the program.
Low program participation levels translate into a missed opportunity to prevent dementia, said study investigator Adina Zeki Al Hazzouri, PhD, assistant professor of epidemiology at the Columbia Aging Center at Columbia University Mailman School of Public Health in New York.
She said that prior research has shown that stigma may prevent older Americans from using SNAP. “Educational programs are needed to reduce the stigma that the public holds towards SNAP use,” she said.
Policy change could increase usage among older individuals, Dr. Zeki Al Hazzouri noted. Such changes could include simplifying enrollment and reporting procedures, shortening recertification periods, and increasing benefit levels.
The study was published online in Neurology.
Memory preservation
Dr. Zeki Al Hazzouri and her team assessed respondents from the Health and Retirement Study (HRS), a representative sample of Americans aged 50 and older. All respondents who were eligible to participate in SNAP in 1996 were followed every 2 years until 2016.
At each assessment, HRS respondents completed memory tests, including immediate and delayed word recall. For those who were too impaired to complete the interview, proxy informants – typically, their spouses or family members – assessed the memory and cognition of their family members using validated instruments, such as the 16-item Informant Questionnaire for Cognitive Decline.
Investigators used a validated memory function composite score, which is benchmarked against the memory assessments and evaluations of the Aging, Demographics, and Memory Study (ADAMS) cohort.
The team found that compared with nonusers, SNAP users were more likely to be women, Black, and born in the southern United States. They were less likely to be married and had more chronic conditions, such as high blood pressure, diabetes, cancer, heart problems, psychiatric problems, and arthritis.
One important study limitation was that SNAP use was measured only once during the study, the investigators noted. Ideally, Dr. Zeki Al Hazzouri said, future research would examine cumulative SNAP use history and explore the pathways that might account for the association between SNAP use and memory decline.
While findings suggest that there were no significant differences in baseline memory function between SNAP users and nonusers, users experienced approximately 2 fewer years of cognitive aging over a 10-year period than those who didn’t use the program.
Dr. Zeki Al Hazzouri speculated that SNAP benefits may slow cognitive aging by contributing to overall brain health and that, in comparison with nonusers, SNAP users absorb more nutrients, which promote neuronal integrity.
The investigators theorized that SNAP benefits may reduce stress from financial hardship, which has been linked to premature cognitive aging in other research.
“SNAP may also increase the purchasing power and investment in other health preserving behaviors, but also resulting in better access to care, which may in turn result in better disease management and management of risk factors for cognitive function,” the investigators wrote.
An underutilized program
In an accompanying editorial, Steven Albert, PhD, Philip B. Hallen Endowed Chair in Community Health and Social Justice at the University of Pittsburgh, noted that in 2020, among households with people aged 50 and older in the United States, more than 9 million Americans experienced food insecurity.
Furthermore, he pointed out, research from 2018 showed that 71% of people aged 60 and older who met income eligibility for SNAP did not participate in the program. “SNAP is an underutilized food security program involving substantial income supplements for older people with low incomes.
“Against the backdrop of so many failures of pharmacotherapy for dementia and the so far inexorable increase in the prevalence of dementia due to population aging, are we missing an opportunity to support cognitive health by failing to enroll the 14 million Americans who are over age 60 and eligible for SNAP but who do not participate?” Dr. Albert asked. He suggested that it would be helpful to determine this through a randomized promotion trial.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Researchers assessed the memory function of more than 3,500 persons who used SNAP or did not use SNAP over a period of 20 years. They found that those who didn’t use the food benefits program experienced 2 more years of cognitive aging compared with program users.
Of the 3,555 individuals included in the study, all were eligible to use the benefits, but only 559 did, leaving 2,996 participants who did not take advantage of the program.
Low program participation levels translate into a missed opportunity to prevent dementia, said study investigator Adina Zeki Al Hazzouri, PhD, assistant professor of epidemiology at the Columbia Aging Center at Columbia University Mailman School of Public Health in New York.
She said that prior research has shown that stigma may prevent older Americans from using SNAP. “Educational programs are needed to reduce the stigma that the public holds towards SNAP use,” she said.
Policy change could increase usage among older individuals, Dr. Zeki Al Hazzouri noted. Such changes could include simplifying enrollment and reporting procedures, shortening recertification periods, and increasing benefit levels.
The study was published online in Neurology.
Memory preservation
Dr. Zeki Al Hazzouri and her team assessed respondents from the Health and Retirement Study (HRS), a representative sample of Americans aged 50 and older. All respondents who were eligible to participate in SNAP in 1996 were followed every 2 years until 2016.
At each assessment, HRS respondents completed memory tests, including immediate and delayed word recall. For those who were too impaired to complete the interview, proxy informants – typically, their spouses or family members – assessed the memory and cognition of their family members using validated instruments, such as the 16-item Informant Questionnaire for Cognitive Decline.
Investigators used a validated memory function composite score, which is benchmarked against the memory assessments and evaluations of the Aging, Demographics, and Memory Study (ADAMS) cohort.
The team found that compared with nonusers, SNAP users were more likely to be women, Black, and born in the southern United States. They were less likely to be married and had more chronic conditions, such as high blood pressure, diabetes, cancer, heart problems, psychiatric problems, and arthritis.
One important study limitation was that SNAP use was measured only once during the study, the investigators noted. Ideally, Dr. Zeki Al Hazzouri said, future research would examine cumulative SNAP use history and explore the pathways that might account for the association between SNAP use and memory decline.
While findings suggest that there were no significant differences in baseline memory function between SNAP users and nonusers, users experienced approximately 2 fewer years of cognitive aging over a 10-year period than those who didn’t use the program.
Dr. Zeki Al Hazzouri speculated that SNAP benefits may slow cognitive aging by contributing to overall brain health and that, in comparison with nonusers, SNAP users absorb more nutrients, which promote neuronal integrity.
The investigators theorized that SNAP benefits may reduce stress from financial hardship, which has been linked to premature cognitive aging in other research.
“SNAP may also increase the purchasing power and investment in other health preserving behaviors, but also resulting in better access to care, which may in turn result in better disease management and management of risk factors for cognitive function,” the investigators wrote.
An underutilized program
In an accompanying editorial, Steven Albert, PhD, Philip B. Hallen Endowed Chair in Community Health and Social Justice at the University of Pittsburgh, noted that in 2020, among households with people aged 50 and older in the United States, more than 9 million Americans experienced food insecurity.
Furthermore, he pointed out, research from 2018 showed that 71% of people aged 60 and older who met income eligibility for SNAP did not participate in the program. “SNAP is an underutilized food security program involving substantial income supplements for older people with low incomes.
“Against the backdrop of so many failures of pharmacotherapy for dementia and the so far inexorable increase in the prevalence of dementia due to population aging, are we missing an opportunity to support cognitive health by failing to enroll the 14 million Americans who are over age 60 and eligible for SNAP but who do not participate?” Dr. Albert asked. He suggested that it would be helpful to determine this through a randomized promotion trial.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Neurology