User login
Field Cancerization in Dermatology: Updates on Treatment Considerations and Emerging Therapies
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
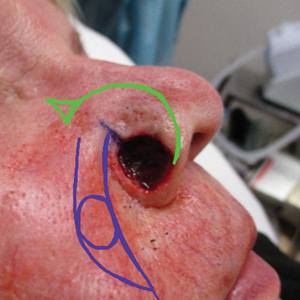
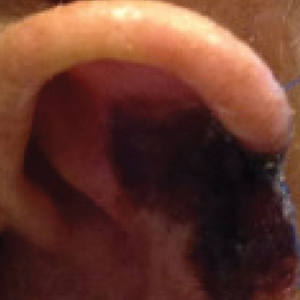
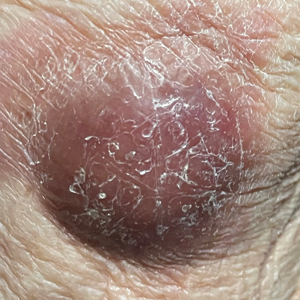
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
Pick your sunscreen carefully: 75% don’t pass muster
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
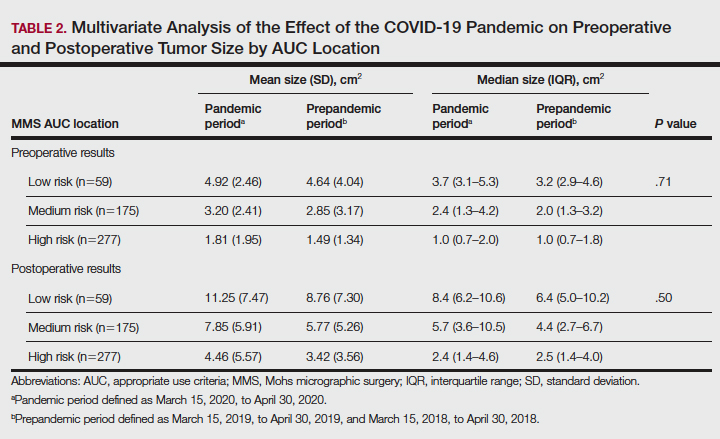
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
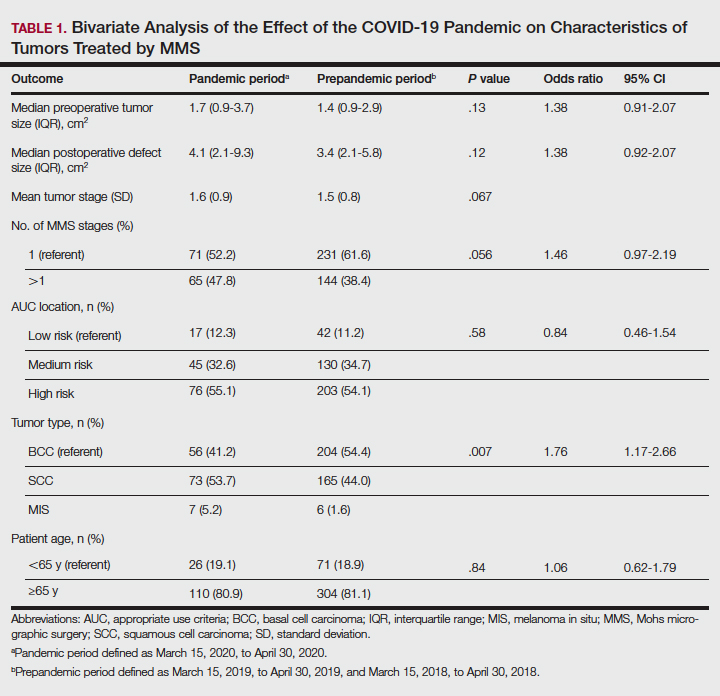
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Surgical Planning for Mohs Defect Reconstruction in the Digital Age
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Q&A with Hubert (Hugh) Greenway, MD
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
Which solid organ transplant recipients face the highest risk of skin cancer?
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
AT AAD 22
Necrosis of the Ear Following Skin Cancer Resection
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.
Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.
Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).
The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Practice Points
- The auricular vasculature supply is complex and forms several anastomoses and arcades, making it susceptible to vascular compromise.
- Damage to the auricular helical arcades or perforating branches can result in postoperative tissue necrosis.
- Clinicians should pay special attention during operative planning for Mohs micrographic surgery to account for the external ear vascular arcades and, when possible, should choose the least invasive treatment and closure options available.
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
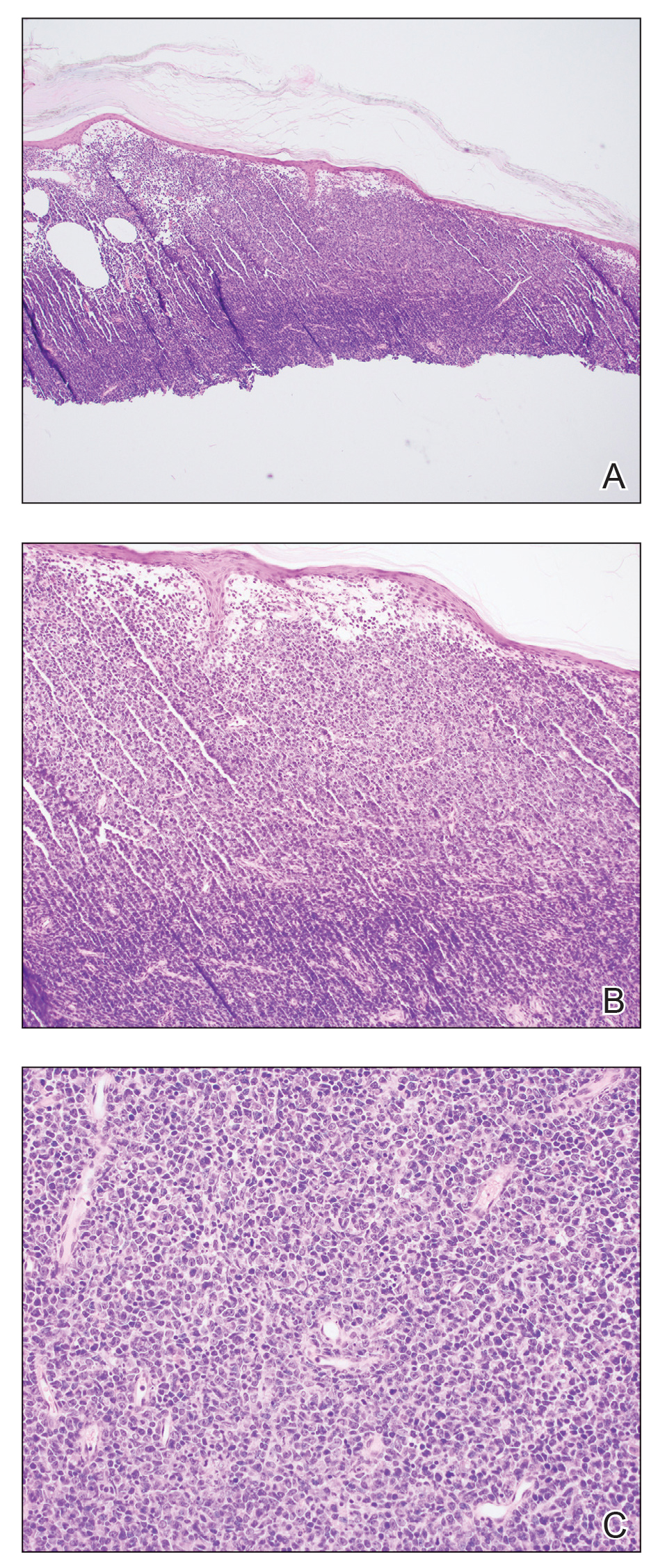
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.
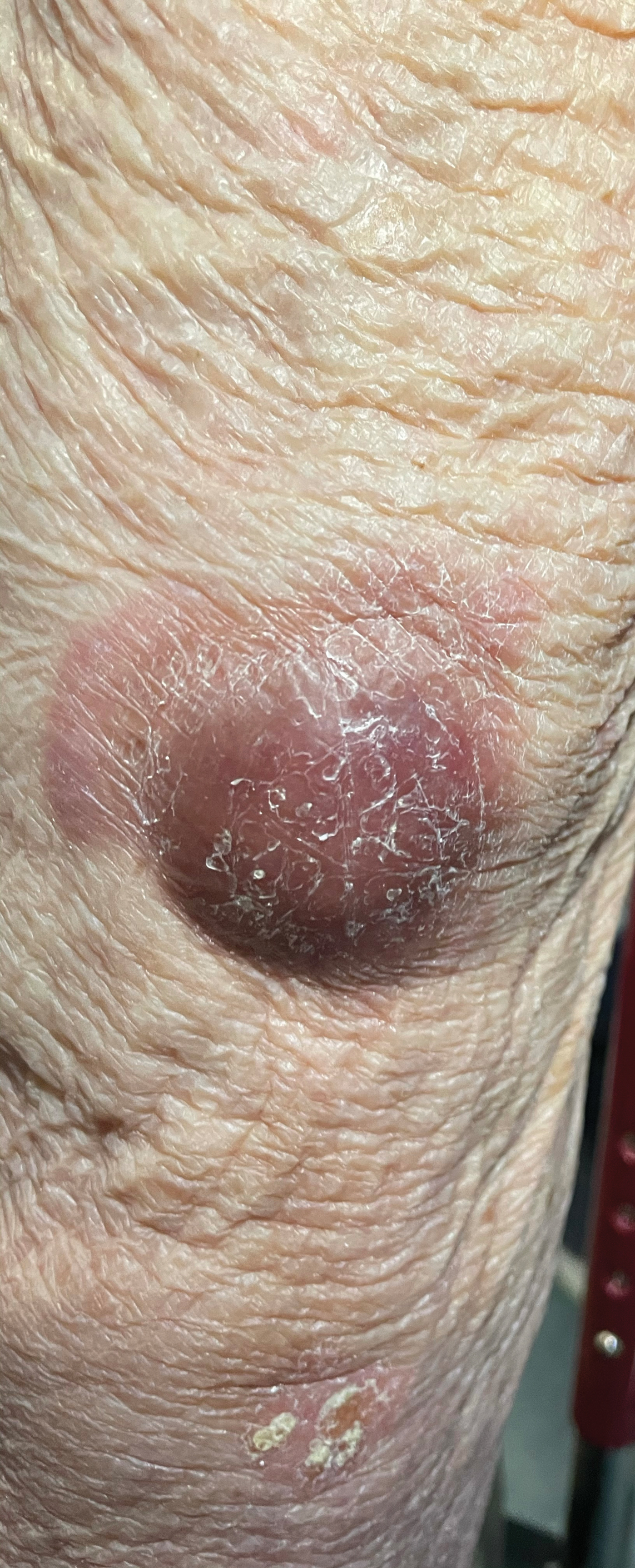
PLA testing brings nuance to the diagnosis of early-stage melanoma
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
AT AAD 22
Study finds discrepancies in biopsy decisions, diagnoses based on skin type
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.