User login
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER
Field Cancerization With Multiple Keratoacanthomas Successfully Treated With Topical and Intralesional 5-Fluorouracil
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
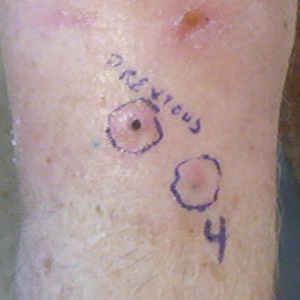
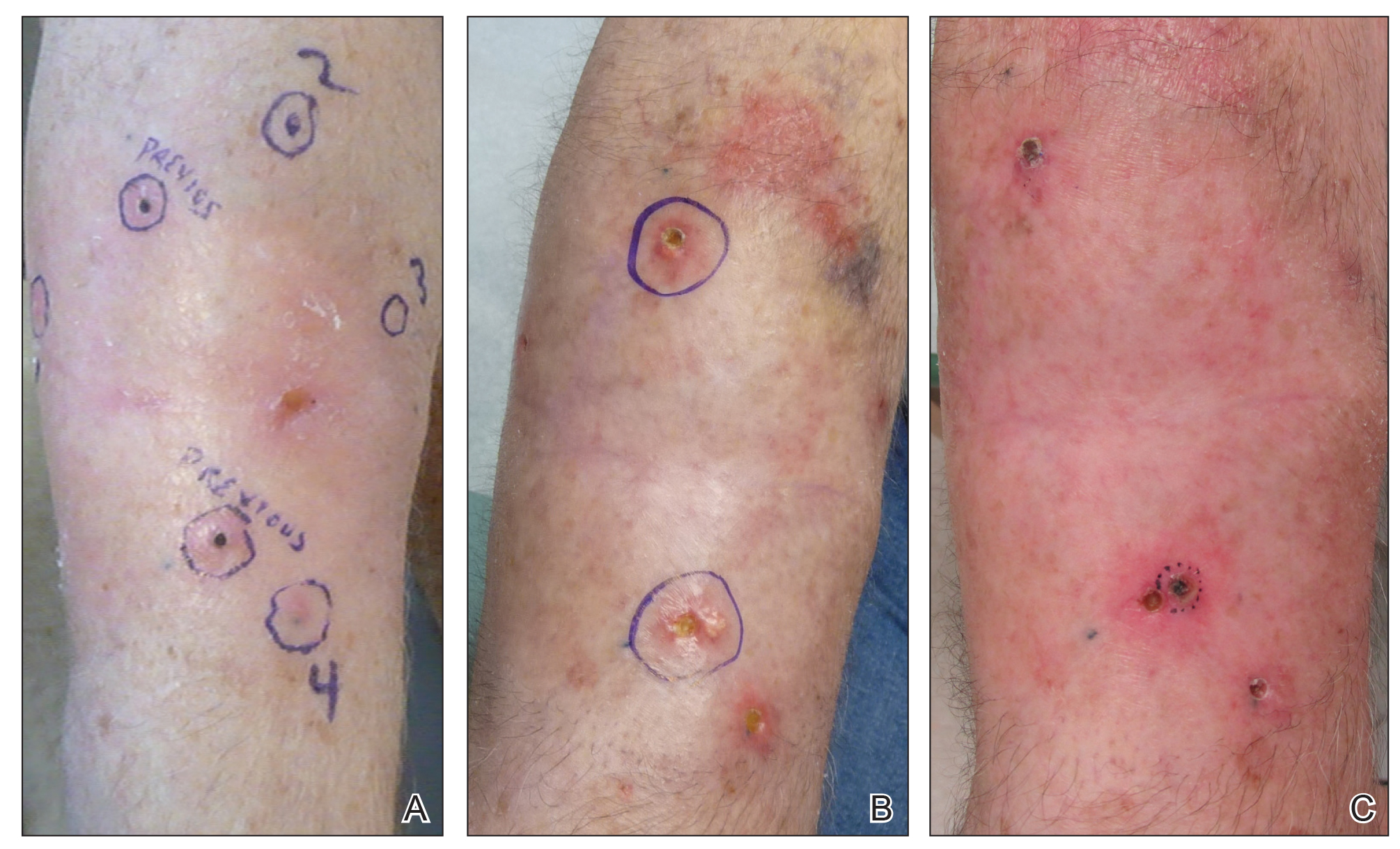
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.
Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Large study finds no link between TCI use, skin cancer in patients with AD
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
FROM JAMA DERMATOLOGY
FDA updates hydrochlorothiazide label to include nonmelanoma skin cancer risk
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

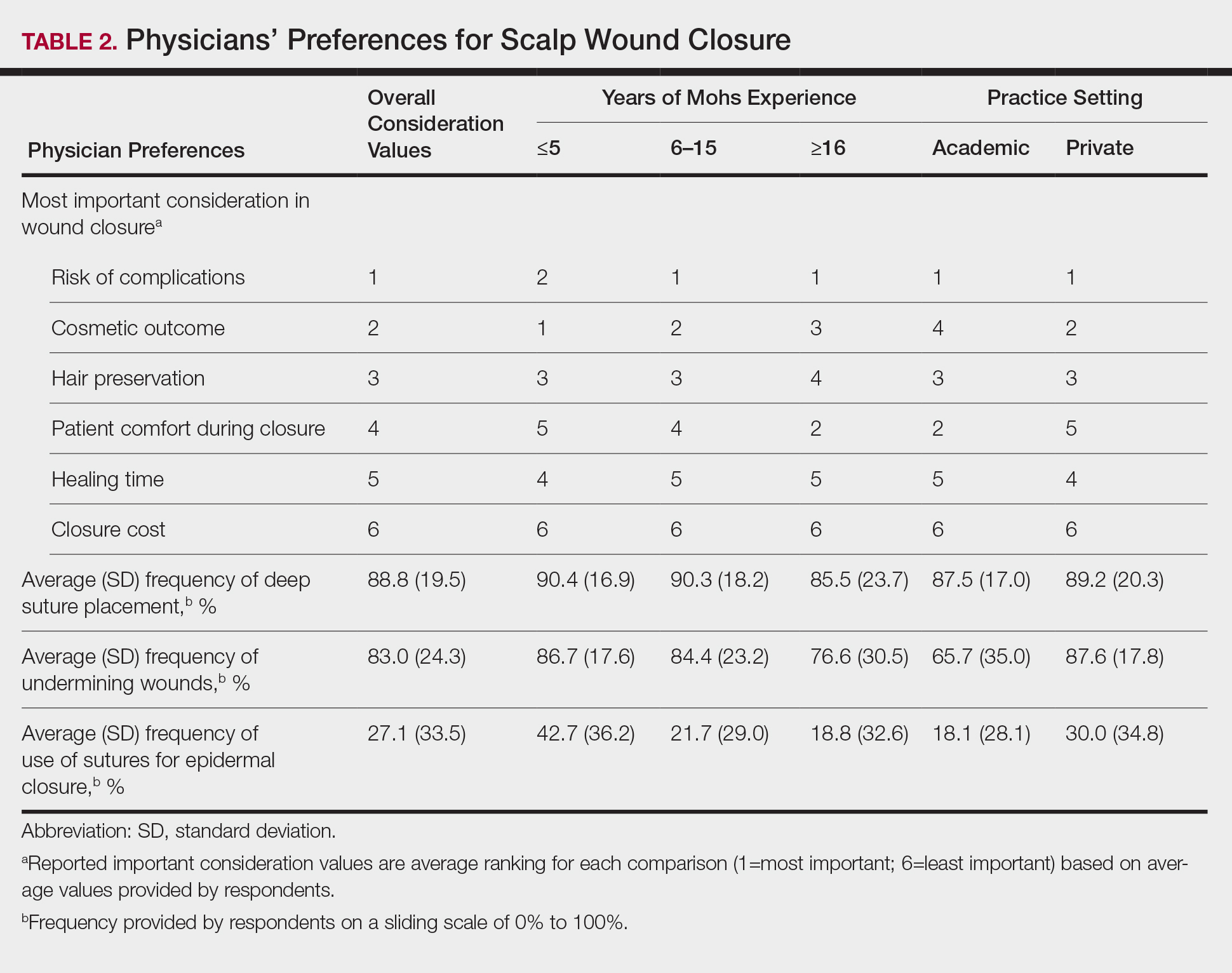
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
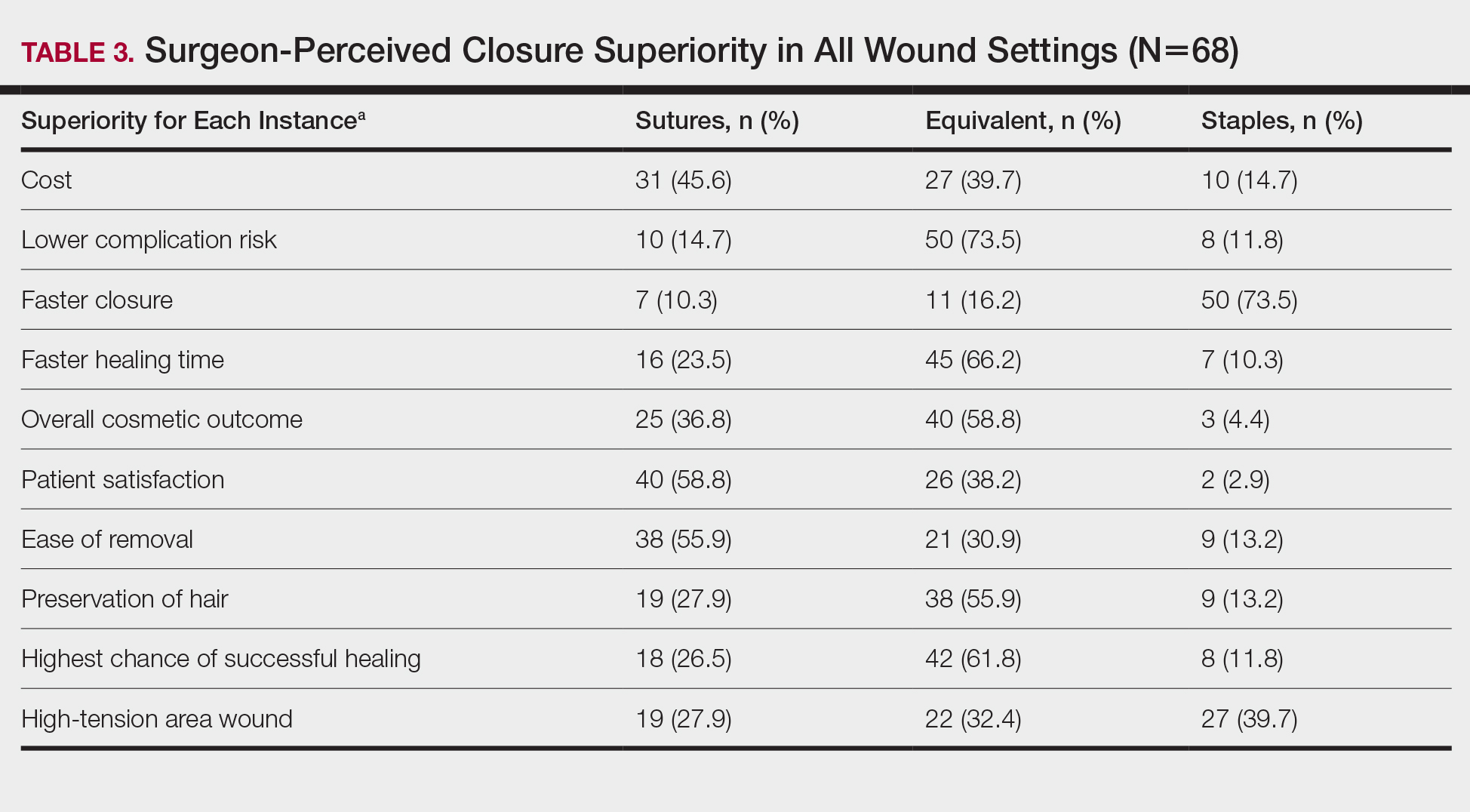
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
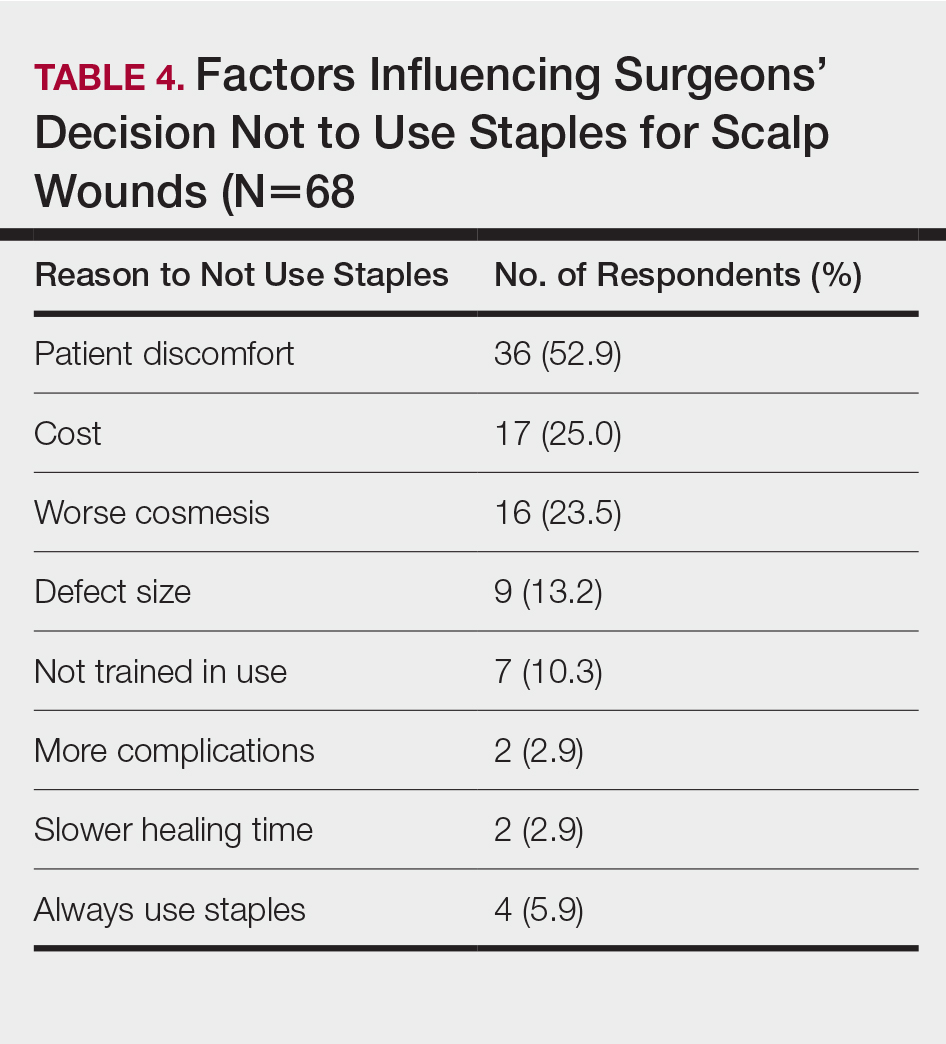
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.
Risk Factors and Management of Skin Cancer Among Active-Duty Servicemembers and Veterans
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.