User login
Intrauterine vacuum device treatment of postpartum hemorrhage
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
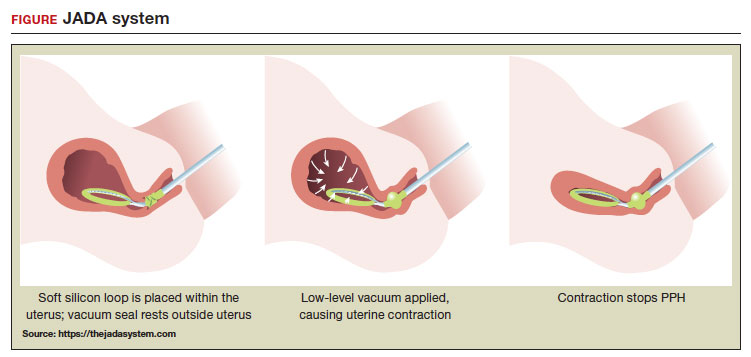 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
Zuranolone: A novel postpartum depression treatment, with lingering questions
Postpartum depression (PPD) remains the most common complication in modern obstetrics, and a leading cause of postpartum mortality in the first year of life. The last 15 years have brought considerable progress with respect to adoption of systematic screening for PPD across America. Screening for PPD, most often using the Edinburgh Postnatal Depression Scale (EPDS), has become part of routine obstetrical care, and is also widely used in pediatric settings.
That is the good news. But the flip side of the identification of those women whose scores on the EPDS suggest significant depressive symptoms is that the number of these patients who, following identification, receive referrals for adequate treatment that gets them well is unfortunately low. This “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression (Cox E. et al. J Clin Psychiatry. 2016 Sep;77[9]:1189-1200). This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well with the available treatments at hand?
Recently, a widely read and circulated article was published in The Wall Street Journal about the challenges associated with navigating care resources for women suffering from PPD. In that article, it was made clear, based on clinical vignette after clinical vignette from postpartum women across America, that neither obstetricians, mental health professionals, nor pediatricians are the “clinical home” for women suffering from postpartum mood and anxiety disorders. The article painfully highlights the system-wide failure to coordinate mental health care for women suffering from postpartum psychiatric illness.
Within a day of the publication of The Wall Street Journal article, the Food and Drug Administration approved zuranolone (Zurzuvae; Sage Therapeutics; Cambridge, Mass.) for the treatment of PPD following the review of two studies demonstrating the superiority of the new medicine over placebo. Women who were enrolled met criteria for major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders criteria beginning in no earlier than the third trimester of pregnancy or later than 4 weeks of delivery. The two studies included a combined sample size of approximately 350 patients suffering from severe PPD. In the studies, women received either 50 mg or 40 mg of zuranolone, or placebo for 14 days. Treatment was associated with a significant change in the Hamilton Depression Rating Scale at day 15, and treatment response was maintained at day 42, which was 4 weeks after the last dose of study medication.
Zuranolone is a neuroactive steroid, which is taken orally, unlike brexanolone (Zulresso; Sage Therapeutics; Cambridge, Mass.), which requires intravenous administration. Zuranolone will be commercially available based on estimates around the fourth quarter of 2023. The most common side effects are drowsiness, dizziness, and sedation, and the FDA label will have a boxed warning about zuranolone’s potential to impact a person’s driving ability, and performance of potentially hazardous activities.
It is noteworthy that while this new medication received FDA approval for the PPD indication, it did not receive FDA approval for the treatment of major depressive disorder (MDD), and the agency issued a Complete Response Letter to the manufacturers noting their application did not provide substantial evidence of effectiveness in MDD. The FDA said in the Complete Response Letter that an additional study or studies will be needed; the manufacturers are currently evaluating next steps.
Where zuranolone fits into the treatment algorithm for severe PPD
Many clinicians who support women with PPD will wonder, upon hearing this news, where zuranolone fits into the treatment algorithm for severe postpartum major depression. Some relevant issues that may determine the answer are the following:
Cost. The cost of brexanolone was substantial, at $34,000 per year, and was viewed by some as a limiting factor in terms of its very limited uptake. As of this column’s publication, zuranolone’s manufacturer has not stated how much the medication will cost.
Breastfeeding. Unlike selective serotonin reuptake inhibitors, which have been demonstrated to be effective for the treatment of PPD and safe during pregnancy and lactation, we have sparse data on the safety of zuranolone for women who wish to breastfeed. It is also unclear whether women eligible for zuranolone would, based on the limited data on safety in lactation, choose deferral of breastfeeding for 14 days in exchange for treatment.
Duration of treatment. While zuranolone was studied in the context of 14 days of acute treatment, then out to day 42, we have no published data on what happens on the other side of this brief interval. As a simple example, in a patient with a history of recurrent major depression previously treated with antidepressants, but where antidepressants were perhaps deferred during pregnancy, is PPD to be treated with zuranolone for 14 days? Or, hypothetically, should it be followed by empiric antidepressant treatment at day 14? Alternatively, are patient and clinician supposed to wait until recurrence occurs before pursuing adjunctive antidepressant therapy whether it is pharmacologic, nonpharmacologic, or both?
Treatment in patients with bipolar disorder. It is also unclear whether treatment with zuranolone applies to other populations of postpartum women. Certainly, for women with bipolar depression, which is common in postpartum women given the vulnerability of bipolar women to new onset of depression or postpartum depressive relapse of underlying disorder, we simply have no data regarding where zuranolone might fit in with respect to this group of patients.
The answers to these questions may help to determine whether zuranolone, a new antidepressant with efficacy, quick time to onset, and a novel mechanism of action is a “game changer.” The article in The Wall Street Journal provided me with some optimism, as it gave PPD and the issues surrounding PPD the attention it deserves in a major periodical. As a new treatment, it may help alleviate suffering at a critical time for patients and their families. We are inching closer to mitigation of stigma associated with this common illness.
Thinking back across the last 3 decades of my treating women suffering from PPD, I have reflected on what has gotten these patients well. I concluded that , along with family and community-based support groups, as well as a culture that reduces stigma and by so doing lessens the toll of this important and too frequently incompletely-treated illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. The Center for Women’s Mental Health at MGH was a non-enrolling site for the pivotal phase 3 SKYLARK trial evaluating zuranolone. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
Postpartum depression (PPD) remains the most common complication in modern obstetrics, and a leading cause of postpartum mortality in the first year of life. The last 15 years have brought considerable progress with respect to adoption of systematic screening for PPD across America. Screening for PPD, most often using the Edinburgh Postnatal Depression Scale (EPDS), has become part of routine obstetrical care, and is also widely used in pediatric settings.
That is the good news. But the flip side of the identification of those women whose scores on the EPDS suggest significant depressive symptoms is that the number of these patients who, following identification, receive referrals for adequate treatment that gets them well is unfortunately low. This “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression (Cox E. et al. J Clin Psychiatry. 2016 Sep;77[9]:1189-1200). This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well with the available treatments at hand?
Recently, a widely read and circulated article was published in The Wall Street Journal about the challenges associated with navigating care resources for women suffering from PPD. In that article, it was made clear, based on clinical vignette after clinical vignette from postpartum women across America, that neither obstetricians, mental health professionals, nor pediatricians are the “clinical home” for women suffering from postpartum mood and anxiety disorders. The article painfully highlights the system-wide failure to coordinate mental health care for women suffering from postpartum psychiatric illness.
Within a day of the publication of The Wall Street Journal article, the Food and Drug Administration approved zuranolone (Zurzuvae; Sage Therapeutics; Cambridge, Mass.) for the treatment of PPD following the review of two studies demonstrating the superiority of the new medicine over placebo. Women who were enrolled met criteria for major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders criteria beginning in no earlier than the third trimester of pregnancy or later than 4 weeks of delivery. The two studies included a combined sample size of approximately 350 patients suffering from severe PPD. In the studies, women received either 50 mg or 40 mg of zuranolone, or placebo for 14 days. Treatment was associated with a significant change in the Hamilton Depression Rating Scale at day 15, and treatment response was maintained at day 42, which was 4 weeks after the last dose of study medication.
Zuranolone is a neuroactive steroid, which is taken orally, unlike brexanolone (Zulresso; Sage Therapeutics; Cambridge, Mass.), which requires intravenous administration. Zuranolone will be commercially available based on estimates around the fourth quarter of 2023. The most common side effects are drowsiness, dizziness, and sedation, and the FDA label will have a boxed warning about zuranolone’s potential to impact a person’s driving ability, and performance of potentially hazardous activities.
It is noteworthy that while this new medication received FDA approval for the PPD indication, it did not receive FDA approval for the treatment of major depressive disorder (MDD), and the agency issued a Complete Response Letter to the manufacturers noting their application did not provide substantial evidence of effectiveness in MDD. The FDA said in the Complete Response Letter that an additional study or studies will be needed; the manufacturers are currently evaluating next steps.
Where zuranolone fits into the treatment algorithm for severe PPD
Many clinicians who support women with PPD will wonder, upon hearing this news, where zuranolone fits into the treatment algorithm for severe postpartum major depression. Some relevant issues that may determine the answer are the following:
Cost. The cost of brexanolone was substantial, at $34,000 per year, and was viewed by some as a limiting factor in terms of its very limited uptake. As of this column’s publication, zuranolone’s manufacturer has not stated how much the medication will cost.
Breastfeeding. Unlike selective serotonin reuptake inhibitors, which have been demonstrated to be effective for the treatment of PPD and safe during pregnancy and lactation, we have sparse data on the safety of zuranolone for women who wish to breastfeed. It is also unclear whether women eligible for zuranolone would, based on the limited data on safety in lactation, choose deferral of breastfeeding for 14 days in exchange for treatment.
Duration of treatment. While zuranolone was studied in the context of 14 days of acute treatment, then out to day 42, we have no published data on what happens on the other side of this brief interval. As a simple example, in a patient with a history of recurrent major depression previously treated with antidepressants, but where antidepressants were perhaps deferred during pregnancy, is PPD to be treated with zuranolone for 14 days? Or, hypothetically, should it be followed by empiric antidepressant treatment at day 14? Alternatively, are patient and clinician supposed to wait until recurrence occurs before pursuing adjunctive antidepressant therapy whether it is pharmacologic, nonpharmacologic, or both?
Treatment in patients with bipolar disorder. It is also unclear whether treatment with zuranolone applies to other populations of postpartum women. Certainly, for women with bipolar depression, which is common in postpartum women given the vulnerability of bipolar women to new onset of depression or postpartum depressive relapse of underlying disorder, we simply have no data regarding where zuranolone might fit in with respect to this group of patients.
The answers to these questions may help to determine whether zuranolone, a new antidepressant with efficacy, quick time to onset, and a novel mechanism of action is a “game changer.” The article in The Wall Street Journal provided me with some optimism, as it gave PPD and the issues surrounding PPD the attention it deserves in a major periodical. As a new treatment, it may help alleviate suffering at a critical time for patients and their families. We are inching closer to mitigation of stigma associated with this common illness.
Thinking back across the last 3 decades of my treating women suffering from PPD, I have reflected on what has gotten these patients well. I concluded that , along with family and community-based support groups, as well as a culture that reduces stigma and by so doing lessens the toll of this important and too frequently incompletely-treated illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. The Center for Women’s Mental Health at MGH was a non-enrolling site for the pivotal phase 3 SKYLARK trial evaluating zuranolone. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
Postpartum depression (PPD) remains the most common complication in modern obstetrics, and a leading cause of postpartum mortality in the first year of life. The last 15 years have brought considerable progress with respect to adoption of systematic screening for PPD across America. Screening for PPD, most often using the Edinburgh Postnatal Depression Scale (EPDS), has become part of routine obstetrical care, and is also widely used in pediatric settings.
That is the good news. But the flip side of the identification of those women whose scores on the EPDS suggest significant depressive symptoms is that the number of these patients who, following identification, receive referrals for adequate treatment that gets them well is unfortunately low. This “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression (Cox E. et al. J Clin Psychiatry. 2016 Sep;77[9]:1189-1200). This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well with the available treatments at hand?
Recently, a widely read and circulated article was published in The Wall Street Journal about the challenges associated with navigating care resources for women suffering from PPD. In that article, it was made clear, based on clinical vignette after clinical vignette from postpartum women across America, that neither obstetricians, mental health professionals, nor pediatricians are the “clinical home” for women suffering from postpartum mood and anxiety disorders. The article painfully highlights the system-wide failure to coordinate mental health care for women suffering from postpartum psychiatric illness.
Within a day of the publication of The Wall Street Journal article, the Food and Drug Administration approved zuranolone (Zurzuvae; Sage Therapeutics; Cambridge, Mass.) for the treatment of PPD following the review of two studies demonstrating the superiority of the new medicine over placebo. Women who were enrolled met criteria for major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders criteria beginning in no earlier than the third trimester of pregnancy or later than 4 weeks of delivery. The two studies included a combined sample size of approximately 350 patients suffering from severe PPD. In the studies, women received either 50 mg or 40 mg of zuranolone, or placebo for 14 days. Treatment was associated with a significant change in the Hamilton Depression Rating Scale at day 15, and treatment response was maintained at day 42, which was 4 weeks after the last dose of study medication.
Zuranolone is a neuroactive steroid, which is taken orally, unlike brexanolone (Zulresso; Sage Therapeutics; Cambridge, Mass.), which requires intravenous administration. Zuranolone will be commercially available based on estimates around the fourth quarter of 2023. The most common side effects are drowsiness, dizziness, and sedation, and the FDA label will have a boxed warning about zuranolone’s potential to impact a person’s driving ability, and performance of potentially hazardous activities.
It is noteworthy that while this new medication received FDA approval for the PPD indication, it did not receive FDA approval for the treatment of major depressive disorder (MDD), and the agency issued a Complete Response Letter to the manufacturers noting their application did not provide substantial evidence of effectiveness in MDD. The FDA said in the Complete Response Letter that an additional study or studies will be needed; the manufacturers are currently evaluating next steps.
Where zuranolone fits into the treatment algorithm for severe PPD
Many clinicians who support women with PPD will wonder, upon hearing this news, where zuranolone fits into the treatment algorithm for severe postpartum major depression. Some relevant issues that may determine the answer are the following:
Cost. The cost of brexanolone was substantial, at $34,000 per year, and was viewed by some as a limiting factor in terms of its very limited uptake. As of this column’s publication, zuranolone’s manufacturer has not stated how much the medication will cost.
Breastfeeding. Unlike selective serotonin reuptake inhibitors, which have been demonstrated to be effective for the treatment of PPD and safe during pregnancy and lactation, we have sparse data on the safety of zuranolone for women who wish to breastfeed. It is also unclear whether women eligible for zuranolone would, based on the limited data on safety in lactation, choose deferral of breastfeeding for 14 days in exchange for treatment.
Duration of treatment. While zuranolone was studied in the context of 14 days of acute treatment, then out to day 42, we have no published data on what happens on the other side of this brief interval. As a simple example, in a patient with a history of recurrent major depression previously treated with antidepressants, but where antidepressants were perhaps deferred during pregnancy, is PPD to be treated with zuranolone for 14 days? Or, hypothetically, should it be followed by empiric antidepressant treatment at day 14? Alternatively, are patient and clinician supposed to wait until recurrence occurs before pursuing adjunctive antidepressant therapy whether it is pharmacologic, nonpharmacologic, or both?
Treatment in patients with bipolar disorder. It is also unclear whether treatment with zuranolone applies to other populations of postpartum women. Certainly, for women with bipolar depression, which is common in postpartum women given the vulnerability of bipolar women to new onset of depression or postpartum depressive relapse of underlying disorder, we simply have no data regarding where zuranolone might fit in with respect to this group of patients.
The answers to these questions may help to determine whether zuranolone, a new antidepressant with efficacy, quick time to onset, and a novel mechanism of action is a “game changer.” The article in The Wall Street Journal provided me with some optimism, as it gave PPD and the issues surrounding PPD the attention it deserves in a major periodical. As a new treatment, it may help alleviate suffering at a critical time for patients and their families. We are inching closer to mitigation of stigma associated with this common illness.
Thinking back across the last 3 decades of my treating women suffering from PPD, I have reflected on what has gotten these patients well. I concluded that , along with family and community-based support groups, as well as a culture that reduces stigma and by so doing lessens the toll of this important and too frequently incompletely-treated illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. The Center for Women’s Mental Health at MGH was a non-enrolling site for the pivotal phase 3 SKYLARK trial evaluating zuranolone. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
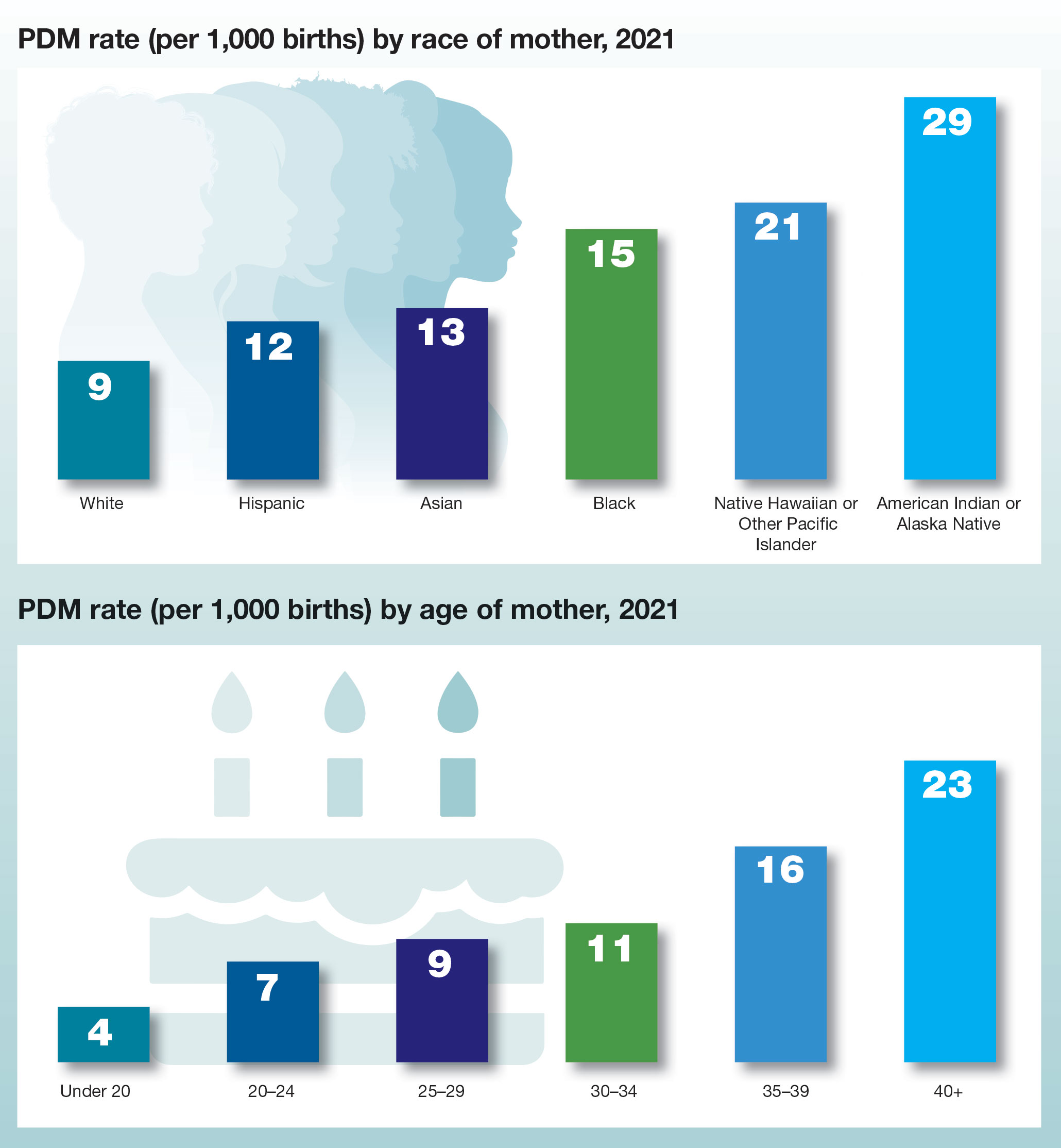
Postpartum depression risk increased among sexual minority women
However, with sexual orientation highly underdocumented among women giving birth, understanding of the prevalence is lacking.
“To our knowledge, this cohort study was the first to examine perinatal depression screening and symptom endorsement among sexual minority women in a major medical center in the U.S.,” reported the authors of the study published in JAMA Psychiatry.
The results “highlight the need for investigations that include strategies for measuring sexual orientation because reliance on medical record review has substantial limitations with regard to the research questions and the validity of the data,” they noted.
Clinical guidelines recommend universal perinatal depression screening at obstetric and pediatric well-infant visits; however, there are significant gaps in data on the issue when it comes to sexual minority women.
To assess the prevalence of sexual minority people giving birth and compare perinatal depression screening rates and scores with those of heterosexual cisgender women, the authors conducted a review of medical records of 18,243 female patients who gave birth at a large, diverse, university-based medical center in Chicago between January and December of 2019.
Of the patients, 57.3% of whom were non-Hispanic White, 1.5% (280) had documentation of their sexual orientation, or sexual minority status.
The results show that those identified as being in sexual minorities, including lesbian, bisexual, queer, pansexual or asexual, were more likely than were heterosexual women to be more engaged in their care – they were more likely to have attended at least one prenatal visit (20.0% vs. 13.7%; P = .002) and at least one postpartum care visit (18.6% vs. 12.8%; P = .004), and more likely to be screened for depression during postpartum care (odds ratio, 1.77; P = .002).
Sexual minority women were also significantly more likely to screen positive for depression during the postpartum period than were heterosexual women (odds ratio, 2.38; P = .03); however, all other comparisons were not significantly different.
The finding regarding postpartum depression was consistent with recent literature, including a systematic review indicating that the stress of being in a sexual minority may be heightened during the postpartum period, the authors noted.
Reasons for the heightened stress may include “being perceived as inadequate parents, heteronormativity in perinatal care, such as intake forms asking for information about the child’s father, and lack of familial social support due to nonacceptance of the parents’ sexual orientation,” the researchers explained.
The rate of only 1.5% of people giving birth who identified as a sexual minority was significantly lower than expected, and much lower that the 17% reported in a recent nationally representative sample of women, first author Leiszle Lapping-Carr, PhD, director of the sexual and relationship health program, department of psychiatry and behavioral sciences, Northwestern University, Chicago, said in an interview.
“I did not expect a rate as low at 1.5%,” she said. “I anticipated it would not be as high as the 17%, but this was quite low. I think one primary reason is that women are not interested in disclosing their sexual orientation to their ob.gyns. if they don’t have to.”
Furthermore, Dr. Lapping-Carr said, “most medical systems do not have an easy way to document sexual orientation or gender identity, and even if it exists many physicians are unaware of the process.”
On a broader level, the lower rates may be indicative of a lack of acknowledgment of sexual minorities in the ob.gyn. setting, Dr. Lapping-Carr added.
“There is a heteronormative bias implicit in most obstetrics clinics, in which pregnant people are automatically gendered as women and assumed to be heterosexual, especially if they present with a male partner,” she said.
Because of those factors, even if a pregnant person discloses sexual identity, that person may request that it not be documented in the chart, she noted.
The higher rates of postpartum depression are consistent with higher rates of mental illness that are reported in general among sexual minority women, pregnant or not, including depression, anxiety, higher rates of substance abuse, stressful life events, and intimate partner violence, compared with heterosexual women, the authors noted.
Develop more supportive systems
To address postpartum depression among sexual minority women, Dr. Lapping-Carr suggested that clinicians generally start by avoiding language and behaviors that could suggest the potential bias that sexual minority patients can face.
“The main change [in treatment] that would likely be helpful for postpartum depression treatment is removing heteronormative language, e.g., not referring to partners as ‘fathers,’ ” she said.
Also, patients may benefit from “discussion of issues of relevance to people with sexual minority identities, such as the process of adoption for female non-birthing partners,” Dr. Lapping-Carr added.
“Starting to create spaces that are inclusive and welcoming for people of all identities will go a long way in increasing your patient’s trust in you,” she said.
While there is a lack of published data regarding increases in rates of sexual minority patients who are giving birth, societal trends suggest the rates may likely be on the rise, Dr. Lapping-Carr said.
“We do know that among adolescents, endorsement of sexual and gender minority identities is much higher than in previous generations, so it would follow that the proportion of birthing people with sexual and gender minority identities would also increase,” she said.
Commenting on the study, K. Ashley Brandt, DO, obstetrics section chief and medical director of Gender Affirming Surgery at Reading Hospital, in West Reading, Pa., noted that limitations include a lack of information about the bigger picture of patients’ risk factors.
“There is no documentation of other risks factors, including rates of depression in the antenatal period, which is higher in LGBTQ individuals and also a risk factor for postpartum depression,” Dr. Brandt told this news organization.
She agreed, however, that patients may be reluctant to report their sexual minority status on the record – but such issues are often addressed.
“I believe that obstetricians do ask this question far more than other providers, but it may not be easily captured in medical records, and patients may also hesitate to disclose sexual practices and sexual orientation due to fear of medical discrimination, which is still extremely prevalent,” Dr. Brandt said.
The study underscores, however, that “same-sex parents are a reality that providers will face,” she said. “They have unique social determinants for health that often go undocumented and unaddressed, which could contribute to higher rates of depression in the postpartum period.”
Factors that may be ignored or undocumented, such as sexual minorities’ religious beliefs or social and familial support, can play significant roles in health care outcomes, Dr. Brandt added.
“Providers need to find ways to better educate themselves about LGBTQ individuals and develop more supportive systems to ensure patients feel safe in disclosing their identities.”
The authors and Dr. Brandt had no disclosures to report.
However, with sexual orientation highly underdocumented among women giving birth, understanding of the prevalence is lacking.
“To our knowledge, this cohort study was the first to examine perinatal depression screening and symptom endorsement among sexual minority women in a major medical center in the U.S.,” reported the authors of the study published in JAMA Psychiatry.
The results “highlight the need for investigations that include strategies for measuring sexual orientation because reliance on medical record review has substantial limitations with regard to the research questions and the validity of the data,” they noted.
Clinical guidelines recommend universal perinatal depression screening at obstetric and pediatric well-infant visits; however, there are significant gaps in data on the issue when it comes to sexual minority women.
To assess the prevalence of sexual minority people giving birth and compare perinatal depression screening rates and scores with those of heterosexual cisgender women, the authors conducted a review of medical records of 18,243 female patients who gave birth at a large, diverse, university-based medical center in Chicago between January and December of 2019.
Of the patients, 57.3% of whom were non-Hispanic White, 1.5% (280) had documentation of their sexual orientation, or sexual minority status.
The results show that those identified as being in sexual minorities, including lesbian, bisexual, queer, pansexual or asexual, were more likely than were heterosexual women to be more engaged in their care – they were more likely to have attended at least one prenatal visit (20.0% vs. 13.7%; P = .002) and at least one postpartum care visit (18.6% vs. 12.8%; P = .004), and more likely to be screened for depression during postpartum care (odds ratio, 1.77; P = .002).
Sexual minority women were also significantly more likely to screen positive for depression during the postpartum period than were heterosexual women (odds ratio, 2.38; P = .03); however, all other comparisons were not significantly different.
The finding regarding postpartum depression was consistent with recent literature, including a systematic review indicating that the stress of being in a sexual minority may be heightened during the postpartum period, the authors noted.
Reasons for the heightened stress may include “being perceived as inadequate parents, heteronormativity in perinatal care, such as intake forms asking for information about the child’s father, and lack of familial social support due to nonacceptance of the parents’ sexual orientation,” the researchers explained.
The rate of only 1.5% of people giving birth who identified as a sexual minority was significantly lower than expected, and much lower that the 17% reported in a recent nationally representative sample of women, first author Leiszle Lapping-Carr, PhD, director of the sexual and relationship health program, department of psychiatry and behavioral sciences, Northwestern University, Chicago, said in an interview.
“I did not expect a rate as low at 1.5%,” she said. “I anticipated it would not be as high as the 17%, but this was quite low. I think one primary reason is that women are not interested in disclosing their sexual orientation to their ob.gyns. if they don’t have to.”
Furthermore, Dr. Lapping-Carr said, “most medical systems do not have an easy way to document sexual orientation or gender identity, and even if it exists many physicians are unaware of the process.”
On a broader level, the lower rates may be indicative of a lack of acknowledgment of sexual minorities in the ob.gyn. setting, Dr. Lapping-Carr added.
“There is a heteronormative bias implicit in most obstetrics clinics, in which pregnant people are automatically gendered as women and assumed to be heterosexual, especially if they present with a male partner,” she said.
Because of those factors, even if a pregnant person discloses sexual identity, that person may request that it not be documented in the chart, she noted.
The higher rates of postpartum depression are consistent with higher rates of mental illness that are reported in general among sexual minority women, pregnant or not, including depression, anxiety, higher rates of substance abuse, stressful life events, and intimate partner violence, compared with heterosexual women, the authors noted.
Develop more supportive systems
To address postpartum depression among sexual minority women, Dr. Lapping-Carr suggested that clinicians generally start by avoiding language and behaviors that could suggest the potential bias that sexual minority patients can face.
“The main change [in treatment] that would likely be helpful for postpartum depression treatment is removing heteronormative language, e.g., not referring to partners as ‘fathers,’ ” she said.
Also, patients may benefit from “discussion of issues of relevance to people with sexual minority identities, such as the process of adoption for female non-birthing partners,” Dr. Lapping-Carr added.
“Starting to create spaces that are inclusive and welcoming for people of all identities will go a long way in increasing your patient’s trust in you,” she said.
While there is a lack of published data regarding increases in rates of sexual minority patients who are giving birth, societal trends suggest the rates may likely be on the rise, Dr. Lapping-Carr said.
“We do know that among adolescents, endorsement of sexual and gender minority identities is much higher than in previous generations, so it would follow that the proportion of birthing people with sexual and gender minority identities would also increase,” she said.
Commenting on the study, K. Ashley Brandt, DO, obstetrics section chief and medical director of Gender Affirming Surgery at Reading Hospital, in West Reading, Pa., noted that limitations include a lack of information about the bigger picture of patients’ risk factors.
“There is no documentation of other risks factors, including rates of depression in the antenatal period, which is higher in LGBTQ individuals and also a risk factor for postpartum depression,” Dr. Brandt told this news organization.
She agreed, however, that patients may be reluctant to report their sexual minority status on the record – but such issues are often addressed.
“I believe that obstetricians do ask this question far more than other providers, but it may not be easily captured in medical records, and patients may also hesitate to disclose sexual practices and sexual orientation due to fear of medical discrimination, which is still extremely prevalent,” Dr. Brandt said.
The study underscores, however, that “same-sex parents are a reality that providers will face,” she said. “They have unique social determinants for health that often go undocumented and unaddressed, which could contribute to higher rates of depression in the postpartum period.”
Factors that may be ignored or undocumented, such as sexual minorities’ religious beliefs or social and familial support, can play significant roles in health care outcomes, Dr. Brandt added.
“Providers need to find ways to better educate themselves about LGBTQ individuals and develop more supportive systems to ensure patients feel safe in disclosing their identities.”
The authors and Dr. Brandt had no disclosures to report.
However, with sexual orientation highly underdocumented among women giving birth, understanding of the prevalence is lacking.
“To our knowledge, this cohort study was the first to examine perinatal depression screening and symptom endorsement among sexual minority women in a major medical center in the U.S.,” reported the authors of the study published in JAMA Psychiatry.
The results “highlight the need for investigations that include strategies for measuring sexual orientation because reliance on medical record review has substantial limitations with regard to the research questions and the validity of the data,” they noted.
Clinical guidelines recommend universal perinatal depression screening at obstetric and pediatric well-infant visits; however, there are significant gaps in data on the issue when it comes to sexual minority women.
To assess the prevalence of sexual minority people giving birth and compare perinatal depression screening rates and scores with those of heterosexual cisgender women, the authors conducted a review of medical records of 18,243 female patients who gave birth at a large, diverse, university-based medical center in Chicago between January and December of 2019.
Of the patients, 57.3% of whom were non-Hispanic White, 1.5% (280) had documentation of their sexual orientation, or sexual minority status.
The results show that those identified as being in sexual minorities, including lesbian, bisexual, queer, pansexual or asexual, were more likely than were heterosexual women to be more engaged in their care – they were more likely to have attended at least one prenatal visit (20.0% vs. 13.7%; P = .002) and at least one postpartum care visit (18.6% vs. 12.8%; P = .004), and more likely to be screened for depression during postpartum care (odds ratio, 1.77; P = .002).
Sexual minority women were also significantly more likely to screen positive for depression during the postpartum period than were heterosexual women (odds ratio, 2.38; P = .03); however, all other comparisons were not significantly different.
The finding regarding postpartum depression was consistent with recent literature, including a systematic review indicating that the stress of being in a sexual minority may be heightened during the postpartum period, the authors noted.
Reasons for the heightened stress may include “being perceived as inadequate parents, heteronormativity in perinatal care, such as intake forms asking for information about the child’s father, and lack of familial social support due to nonacceptance of the parents’ sexual orientation,” the researchers explained.
The rate of only 1.5% of people giving birth who identified as a sexual minority was significantly lower than expected, and much lower that the 17% reported in a recent nationally representative sample of women, first author Leiszle Lapping-Carr, PhD, director of the sexual and relationship health program, department of psychiatry and behavioral sciences, Northwestern University, Chicago, said in an interview.
“I did not expect a rate as low at 1.5%,” she said. “I anticipated it would not be as high as the 17%, but this was quite low. I think one primary reason is that women are not interested in disclosing their sexual orientation to their ob.gyns. if they don’t have to.”
Furthermore, Dr. Lapping-Carr said, “most medical systems do not have an easy way to document sexual orientation or gender identity, and even if it exists many physicians are unaware of the process.”
On a broader level, the lower rates may be indicative of a lack of acknowledgment of sexual minorities in the ob.gyn. setting, Dr. Lapping-Carr added.
“There is a heteronormative bias implicit in most obstetrics clinics, in which pregnant people are automatically gendered as women and assumed to be heterosexual, especially if they present with a male partner,” she said.
Because of those factors, even if a pregnant person discloses sexual identity, that person may request that it not be documented in the chart, she noted.
The higher rates of postpartum depression are consistent with higher rates of mental illness that are reported in general among sexual minority women, pregnant or not, including depression, anxiety, higher rates of substance abuse, stressful life events, and intimate partner violence, compared with heterosexual women, the authors noted.
Develop more supportive systems
To address postpartum depression among sexual minority women, Dr. Lapping-Carr suggested that clinicians generally start by avoiding language and behaviors that could suggest the potential bias that sexual minority patients can face.
“The main change [in treatment] that would likely be helpful for postpartum depression treatment is removing heteronormative language, e.g., not referring to partners as ‘fathers,’ ” she said.
Also, patients may benefit from “discussion of issues of relevance to people with sexual minority identities, such as the process of adoption for female non-birthing partners,” Dr. Lapping-Carr added.
“Starting to create spaces that are inclusive and welcoming for people of all identities will go a long way in increasing your patient’s trust in you,” she said.
While there is a lack of published data regarding increases in rates of sexual minority patients who are giving birth, societal trends suggest the rates may likely be on the rise, Dr. Lapping-Carr said.
“We do know that among adolescents, endorsement of sexual and gender minority identities is much higher than in previous generations, so it would follow that the proportion of birthing people with sexual and gender minority identities would also increase,” she said.
Commenting on the study, K. Ashley Brandt, DO, obstetrics section chief and medical director of Gender Affirming Surgery at Reading Hospital, in West Reading, Pa., noted that limitations include a lack of information about the bigger picture of patients’ risk factors.
“There is no documentation of other risks factors, including rates of depression in the antenatal period, which is higher in LGBTQ individuals and also a risk factor for postpartum depression,” Dr. Brandt told this news organization.
She agreed, however, that patients may be reluctant to report their sexual minority status on the record – but such issues are often addressed.
“I believe that obstetricians do ask this question far more than other providers, but it may not be easily captured in medical records, and patients may also hesitate to disclose sexual practices and sexual orientation due to fear of medical discrimination, which is still extremely prevalent,” Dr. Brandt said.
The study underscores, however, that “same-sex parents are a reality that providers will face,” she said. “They have unique social determinants for health that often go undocumented and unaddressed, which could contribute to higher rates of depression in the postpartum period.”
Factors that may be ignored or undocumented, such as sexual minorities’ religious beliefs or social and familial support, can play significant roles in health care outcomes, Dr. Brandt added.
“Providers need to find ways to better educate themselves about LGBTQ individuals and develop more supportive systems to ensure patients feel safe in disclosing their identities.”
The authors and Dr. Brandt had no disclosures to report.
FROM JAMA PSYCHIATRY
Managing intrahepatic cholestasis of pregnancy
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
Experts highlight benefits and offer caveats for first postpartum depression pill
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ontario case shows potential supplement risk for consumers
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Unveiling the potential of prediction models in obstetrics
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
Dural-puncture epidural drives faster conversion to cesarean anesthesia
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
FDA approves first pill for postpartum depression
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
Trends in prepregnancy diabetes rates in the United States, 2016 -2021