User login
Gestational diabetes may increase child’s risk of glucose intolerance
Obese children may have a higher risk of developing type 2 diabetes if their mothers had gestational diabetes during pregnancy, according to a recent study.
"The ever growing number of women with gestational diabetes (18%) suggests that the future will be filled with children with early diabetes at a rate that far exceeds the current prevalence," wrote Tara Holder of Yale University, New Haven, Conn., and her associates in Diabetologia.
"Offspring of GDM [gestational diabetes mellitus] mothers ought to be screened for impaired glucose tolerance and/or impaired fasting glucose, and preventive and therapeutic strategies should be considered before the development of full clinical manifestation of diabetes," the researchers reported online (Diabetologia 2014 Aug. 29 [doi: 10.1007/s00125-014-3345-2]).
The investigators conducted an oral glucose tolerance test to establish normal glucose tolerance among 210 obese teens who had not been exposed to GDM and 45 obese teens who had been exposed. Then they conducted another OGTT at an average follow-up of 2.8 years later.
A fasting glucose level of less than 5.55 mmol/L and a 2-hour glucose level of less than 7.77 mmol/L were defined as normal glucose tolerance. A fasting glucose of 5.55-6.88 mmol/L was considered impaired, and a fasting glucose greater than 6.88 mmol/ L or a 2-hour glucose greater than 11.05 mmol/L was designated type 2 diabetes.
At follow-up, 91.4% of the teens not exposed to GDM had normal glucose tolerance, compared with 68.9% of the teens exposed to GDM. Therefore, 8.6% of those not exposed to GDM and 31.1% of those exposed to GDM had developed either impaired glucose tolerance or type 2 diabetes.
The research and researchers were supported by the National Center for Advancing Translational Science, the Yale Diabetes Endocrinology Research Center, the European Society of Pediatric Endocrinology, the American Heart Association, the Stephen Morse Diabetes Research Foundation, the National Institutes of Health, and the American Diabetes Association. The authors had no disclosures.
The study by Holder et al. confirms previous findings that a child’s exposure to maternal GDM can predispose him or her to developing impaired glucose tolerance or type 2 diabetes later in life. Seminal work by David Pettitt and Peter Bennett, who studied the Pima Indians in Arizona, showed that a child born to a mother without GDM and a child born to the same mother with GDM had different susceptibilities to developing metabolic disease. They found that the child exposed to GDM had a higher likelihood of developing diabetes.
The findings of Holder et al. reinforce the idea that maternal health can greatly influence the long-term health of her offspring. It is conceivable that diabetes may "imprint" information onto the islet cells of the developing fetus, thereby resulting in the reduced beta-cell function and reduced insulin sensitivity observed by the investigators. Although it remains to be elucidated, it is not unlikely that there are diabetes susceptibility genes, which women may pass on to their offspring. If so, this could explain why only some children of GDM mothers develop impaired glucose tolerance or type 2 diabetes mellitus while others do not.

|
|
Counseling children of diabetic mothers on the importance of a healthy lifestyle, maintaining an ideal weight, consuming a balanced diet, and getting enough physical exercise could reduce their risk of future metabolic disease. Because the exposure to maternal hyperglycemia cannot be reversed, it is vital that children of GDM mothers take steps needed to reduce their risks of developing diabetes.
Dr. E. Albert Reece, M.D., Ph.D., M.B.A., is the Vice President for Medical Affairs at the University of Maryland, Dean of the School of Medicine, and the John Z. and Akiko K. Bowers Distinguished Professor in Obstetrics and Gynecology. He made these comments in an interview. Dr. Reece had no relevant financial disclosures.
The study by Holder et al. confirms previous findings that a child’s exposure to maternal GDM can predispose him or her to developing impaired glucose tolerance or type 2 diabetes later in life. Seminal work by David Pettitt and Peter Bennett, who studied the Pima Indians in Arizona, showed that a child born to a mother without GDM and a child born to the same mother with GDM had different susceptibilities to developing metabolic disease. They found that the child exposed to GDM had a higher likelihood of developing diabetes.
The findings of Holder et al. reinforce the idea that maternal health can greatly influence the long-term health of her offspring. It is conceivable that diabetes may "imprint" information onto the islet cells of the developing fetus, thereby resulting in the reduced beta-cell function and reduced insulin sensitivity observed by the investigators. Although it remains to be elucidated, it is not unlikely that there are diabetes susceptibility genes, which women may pass on to their offspring. If so, this could explain why only some children of GDM mothers develop impaired glucose tolerance or type 2 diabetes mellitus while others do not.

|
|
Counseling children of diabetic mothers on the importance of a healthy lifestyle, maintaining an ideal weight, consuming a balanced diet, and getting enough physical exercise could reduce their risk of future metabolic disease. Because the exposure to maternal hyperglycemia cannot be reversed, it is vital that children of GDM mothers take steps needed to reduce their risks of developing diabetes.
Dr. E. Albert Reece, M.D., Ph.D., M.B.A., is the Vice President for Medical Affairs at the University of Maryland, Dean of the School of Medicine, and the John Z. and Akiko K. Bowers Distinguished Professor in Obstetrics and Gynecology. He made these comments in an interview. Dr. Reece had no relevant financial disclosures.
The study by Holder et al. confirms previous findings that a child’s exposure to maternal GDM can predispose him or her to developing impaired glucose tolerance or type 2 diabetes later in life. Seminal work by David Pettitt and Peter Bennett, who studied the Pima Indians in Arizona, showed that a child born to a mother without GDM and a child born to the same mother with GDM had different susceptibilities to developing metabolic disease. They found that the child exposed to GDM had a higher likelihood of developing diabetes.
The findings of Holder et al. reinforce the idea that maternal health can greatly influence the long-term health of her offspring. It is conceivable that diabetes may "imprint" information onto the islet cells of the developing fetus, thereby resulting in the reduced beta-cell function and reduced insulin sensitivity observed by the investigators. Although it remains to be elucidated, it is not unlikely that there are diabetes susceptibility genes, which women may pass on to their offspring. If so, this could explain why only some children of GDM mothers develop impaired glucose tolerance or type 2 diabetes mellitus while others do not.

|
|
Counseling children of diabetic mothers on the importance of a healthy lifestyle, maintaining an ideal weight, consuming a balanced diet, and getting enough physical exercise could reduce their risk of future metabolic disease. Because the exposure to maternal hyperglycemia cannot be reversed, it is vital that children of GDM mothers take steps needed to reduce their risks of developing diabetes.
Dr. E. Albert Reece, M.D., Ph.D., M.B.A., is the Vice President for Medical Affairs at the University of Maryland, Dean of the School of Medicine, and the John Z. and Akiko K. Bowers Distinguished Professor in Obstetrics and Gynecology. He made these comments in an interview. Dr. Reece had no relevant financial disclosures.
Obese children may have a higher risk of developing type 2 diabetes if their mothers had gestational diabetes during pregnancy, according to a recent study.
"The ever growing number of women with gestational diabetes (18%) suggests that the future will be filled with children with early diabetes at a rate that far exceeds the current prevalence," wrote Tara Holder of Yale University, New Haven, Conn., and her associates in Diabetologia.
"Offspring of GDM [gestational diabetes mellitus] mothers ought to be screened for impaired glucose tolerance and/or impaired fasting glucose, and preventive and therapeutic strategies should be considered before the development of full clinical manifestation of diabetes," the researchers reported online (Diabetologia 2014 Aug. 29 [doi: 10.1007/s00125-014-3345-2]).
The investigators conducted an oral glucose tolerance test to establish normal glucose tolerance among 210 obese teens who had not been exposed to GDM and 45 obese teens who had been exposed. Then they conducted another OGTT at an average follow-up of 2.8 years later.
A fasting glucose level of less than 5.55 mmol/L and a 2-hour glucose level of less than 7.77 mmol/L were defined as normal glucose tolerance. A fasting glucose of 5.55-6.88 mmol/L was considered impaired, and a fasting glucose greater than 6.88 mmol/ L or a 2-hour glucose greater than 11.05 mmol/L was designated type 2 diabetes.
At follow-up, 91.4% of the teens not exposed to GDM had normal glucose tolerance, compared with 68.9% of the teens exposed to GDM. Therefore, 8.6% of those not exposed to GDM and 31.1% of those exposed to GDM had developed either impaired glucose tolerance or type 2 diabetes.
The research and researchers were supported by the National Center for Advancing Translational Science, the Yale Diabetes Endocrinology Research Center, the European Society of Pediatric Endocrinology, the American Heart Association, the Stephen Morse Diabetes Research Foundation, the National Institutes of Health, and the American Diabetes Association. The authors had no disclosures.
Obese children may have a higher risk of developing type 2 diabetes if their mothers had gestational diabetes during pregnancy, according to a recent study.
"The ever growing number of women with gestational diabetes (18%) suggests that the future will be filled with children with early diabetes at a rate that far exceeds the current prevalence," wrote Tara Holder of Yale University, New Haven, Conn., and her associates in Diabetologia.
"Offspring of GDM [gestational diabetes mellitus] mothers ought to be screened for impaired glucose tolerance and/or impaired fasting glucose, and preventive and therapeutic strategies should be considered before the development of full clinical manifestation of diabetes," the researchers reported online (Diabetologia 2014 Aug. 29 [doi: 10.1007/s00125-014-3345-2]).
The investigators conducted an oral glucose tolerance test to establish normal glucose tolerance among 210 obese teens who had not been exposed to GDM and 45 obese teens who had been exposed. Then they conducted another OGTT at an average follow-up of 2.8 years later.
A fasting glucose level of less than 5.55 mmol/L and a 2-hour glucose level of less than 7.77 mmol/L were defined as normal glucose tolerance. A fasting glucose of 5.55-6.88 mmol/L was considered impaired, and a fasting glucose greater than 6.88 mmol/ L or a 2-hour glucose greater than 11.05 mmol/L was designated type 2 diabetes.
At follow-up, 91.4% of the teens not exposed to GDM had normal glucose tolerance, compared with 68.9% of the teens exposed to GDM. Therefore, 8.6% of those not exposed to GDM and 31.1% of those exposed to GDM had developed either impaired glucose tolerance or type 2 diabetes.
The research and researchers were supported by the National Center for Advancing Translational Science, the Yale Diabetes Endocrinology Research Center, the European Society of Pediatric Endocrinology, the American Heart Association, the Stephen Morse Diabetes Research Foundation, the National Institutes of Health, and the American Diabetes Association. The authors had no disclosures.
FROM DIABETOLOGIA
Key clinical point: Children whose mothers had gestational diabetes should be screened for impaired glucose tolerance and preventive strategies taught to forestall type 2 diabetes.
Major finding: A total of 31.1% of obese teens exposed to GDM had developed either impaired glucose tolerance or type 2 diabetes at follow-up, compared with 8.6% of those not exposed to GDM.
Data source: The findings are based on a cohort study of 255 obese adolescents followed for a mean 2.8 years.
Disclosures: The research and researchers were supported by the National Center for Advancing Translational Science, the Yale Diabetes Endocrinology Research Center, the European Society of Pediatric Endocrinology, the American Heart Association, the Stephen Morse Diabetes Research Foundation, the National Institutes of Health, and the American Diabetes Association. The authors had no disclosures.
Was fetus’ wrist injured during cesarean delivery?
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
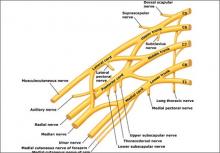
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 1: Can it be predicted?
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Does PTSD during pregnancy increase the likelihood of preterm birth?
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Farewell to indigo carmine
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
Law discourages drug-addicted pregnant women from seeking care
The enactment of a Tennessee law that criminalizes substance abuse by pregnant women has made patients increasingly hesitant to pursue treatment for their drug addictions, according to Dr. Jessica Young, an obstetrician/gynecologist at Vanderbilt University Medical Center in Nashville, Tenn.
Tennessee’s law, which became effective in July, defines illegal narcotic use by pregnant women as assault, depending on whether the child is born addicted to or is harmed by the narcotic. A 26-year-old woman was among the first to be arrested in mid-July under the law.
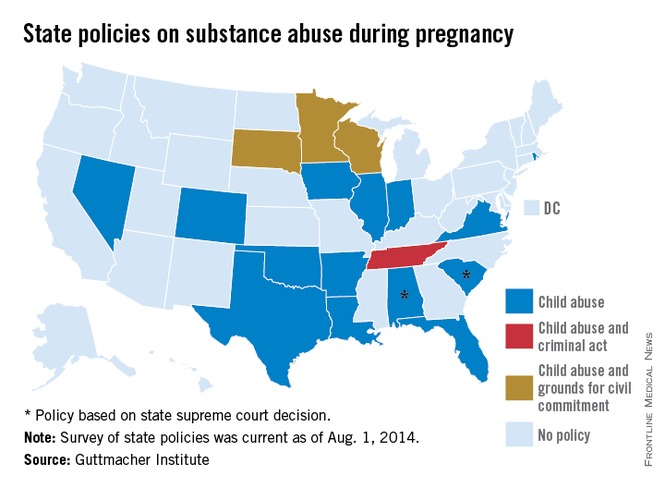
"Since the new law was passed, women . . . often express a fatalistic attitude of, ‘Even if I do the right thing, I will be arrested or have my baby taken away,’ " said Dr. Young, who leads the Vanderbilt Drug Dependency Clinic for pregnant women.
The concern is that women will hide their addictions or self-detox at home, she said. "I think it will require some negative outcomes for this law to be struck down."
Physician associations, including the American Medical Association and ACOG, have long maintained that criminal punishment is not the answer to curbing drug abuse by pregnant women and that such laws create further harm to children. Fear of being reported to police was a primary reason women who abused drugs did not seek prenatal care, a recent study found (Am. J. Obstet. Gynecol. 2009;200:412.e1-10).
Laws that mandate criminal penalties against pregnant women who use drugs can significantly harm the physician-patient relationship, according to Dr. John M. Thorp, professor of obstetrics and gynecology at the University of North Carolina and director of women’s primary health care for UNC Healthcare, both in Chapel Hill.
These laws "put the physician or the clinic or hospital in the role of reporting the crime, which is completely the antithesis of wanting patients to be able to tell physicians what’s wrong with them and being able to offer treatment instead of punishment," Dr. Thorp said in an interview. "These laws are a real setback to the concept that substance abuse addiction is a disease, [and they are] another barrier for women seeking treatment."
Tennessee is the only state to consider drug abuse by pregnant women to be "assault." The law states that a woman may be prosecuted for assault if her child is born addicted or harmed by the drug and for homicide if the child dies from drug exposure. It is an affirmative defense to prosecution if the woman is actively enrolled in an addiction recovery program before the baby is born, remains in the program after delivery, and successfully completes the program.
The American Civil Liberties Union of Tennessee plans to challenge the law in court.
The Tennessee Medical Association opposed the law, but successfully pushed for amendments that would lessen the punitive damages against women, Dave Chaney, TMA director of communications, said in an interview. The medical association successfully lobbied to lower the charging level in the bill to a misdemeanor and helped secure a stipulation that women who enter a treatment program be punished only by a fine.
Various states pursue criminal charges against pregnant women who use narcotics. Eighteen states consider substance abuse during pregnancy to be "child abuse," and three other states consider substance abuse during pregnancy to be grounds for civil commitment, according to an August 2014 analysis by the Guttmacher Institute. Fifteen states require physicians to report suspected prenatal drug abuse to authorities, and four states require health providers to test for prenatal drug exposure if they suspect substance abuse.
Other states could soon follow in Tennessee’s footsteps, Elizabeth Nash, Guttmacher Institute state issues manager, said in an interview. "Legislatures see what’s happening in other states and decide whether it may be right for them."
Meanwhile, a newly proposed federal bill could assist states in addressing the issue of drug-addicted pregnant mothers and babies and drive more effective responses to the crisis. The Protecting Our Infants Act, introduced July 31 by Rep. Mitch McConnell (R-Ky.), aims to better identify and treat opioid use by pregnant women and neonatal abstinence syndrome (NAS) in newborns. The legislation is backed by ACOG.
"Currently, states vary widely in how they mandate and administer screening, diagnosis, and treatment of pregnant women using opioids," ACOG President John C. Jennings said in a statement. The federal "legislation will help identify, compile, and disseminate best practices developed by medical professional organizations, including ACOG, and identify any gaps in best practices that may require additional research or analysis."
The proposed legislation would facilitate the research and dissemination of evidence-informed recommendations for addressing maternal addiction and NAS and provide for NAS studies. The measure also would encourage the Centers for Disease Control and Prevention to work with states to improve the availability and quality of data to more effectively respond to drug-addicted pregnant women. At this article’s deadline, the proposed act was in the Committee on Health, Education, Labor and Pensions.
"My legislation is no silver bullet, but it will help ensure that our public health system is better equipped to prevent and treat opiate addiction in mothers and their newborn children," Mr. McConnell said in a statement. "Together, we can overcome this tragic problem."
Developing resources that promote better medical care for addicted women and their children is a more effective answer than punitive laws, added Hendree Jones, Ph.D., a professor of obstetrics and gynecology at the university and executive director of the UNC Horizons programs. Horizons provides substance abuse treatment and child care to women who are pregnant or parenting young children.
"Incarcerating women to reduce the prevalence of drug use during pregnancy is a misguided solution to the issue," Dr. Jones said in an interview. "Increasing access to high-quality treatment would be a much more fruitful approach."
On Twitter @legal_med
The enactment of a Tennessee law that criminalizes substance abuse by pregnant women has made patients increasingly hesitant to pursue treatment for their drug addictions, according to Dr. Jessica Young, an obstetrician/gynecologist at Vanderbilt University Medical Center in Nashville, Tenn.
Tennessee’s law, which became effective in July, defines illegal narcotic use by pregnant women as assault, depending on whether the child is born addicted to or is harmed by the narcotic. A 26-year-old woman was among the first to be arrested in mid-July under the law.

"Since the new law was passed, women . . . often express a fatalistic attitude of, ‘Even if I do the right thing, I will be arrested or have my baby taken away,’ " said Dr. Young, who leads the Vanderbilt Drug Dependency Clinic for pregnant women.
The concern is that women will hide their addictions or self-detox at home, she said. "I think it will require some negative outcomes for this law to be struck down."
Physician associations, including the American Medical Association and ACOG, have long maintained that criminal punishment is not the answer to curbing drug abuse by pregnant women and that such laws create further harm to children. Fear of being reported to police was a primary reason women who abused drugs did not seek prenatal care, a recent study found (Am. J. Obstet. Gynecol. 2009;200:412.e1-10).
Laws that mandate criminal penalties against pregnant women who use drugs can significantly harm the physician-patient relationship, according to Dr. John M. Thorp, professor of obstetrics and gynecology at the University of North Carolina and director of women’s primary health care for UNC Healthcare, both in Chapel Hill.
These laws "put the physician or the clinic or hospital in the role of reporting the crime, which is completely the antithesis of wanting patients to be able to tell physicians what’s wrong with them and being able to offer treatment instead of punishment," Dr. Thorp said in an interview. "These laws are a real setback to the concept that substance abuse addiction is a disease, [and they are] another barrier for women seeking treatment."
Tennessee is the only state to consider drug abuse by pregnant women to be "assault." The law states that a woman may be prosecuted for assault if her child is born addicted or harmed by the drug and for homicide if the child dies from drug exposure. It is an affirmative defense to prosecution if the woman is actively enrolled in an addiction recovery program before the baby is born, remains in the program after delivery, and successfully completes the program.
The American Civil Liberties Union of Tennessee plans to challenge the law in court.
The Tennessee Medical Association opposed the law, but successfully pushed for amendments that would lessen the punitive damages against women, Dave Chaney, TMA director of communications, said in an interview. The medical association successfully lobbied to lower the charging level in the bill to a misdemeanor and helped secure a stipulation that women who enter a treatment program be punished only by a fine.
Various states pursue criminal charges against pregnant women who use narcotics. Eighteen states consider substance abuse during pregnancy to be "child abuse," and three other states consider substance abuse during pregnancy to be grounds for civil commitment, according to an August 2014 analysis by the Guttmacher Institute. Fifteen states require physicians to report suspected prenatal drug abuse to authorities, and four states require health providers to test for prenatal drug exposure if they suspect substance abuse.
Other states could soon follow in Tennessee’s footsteps, Elizabeth Nash, Guttmacher Institute state issues manager, said in an interview. "Legislatures see what’s happening in other states and decide whether it may be right for them."
Meanwhile, a newly proposed federal bill could assist states in addressing the issue of drug-addicted pregnant mothers and babies and drive more effective responses to the crisis. The Protecting Our Infants Act, introduced July 31 by Rep. Mitch McConnell (R-Ky.), aims to better identify and treat opioid use by pregnant women and neonatal abstinence syndrome (NAS) in newborns. The legislation is backed by ACOG.
"Currently, states vary widely in how they mandate and administer screening, diagnosis, and treatment of pregnant women using opioids," ACOG President John C. Jennings said in a statement. The federal "legislation will help identify, compile, and disseminate best practices developed by medical professional organizations, including ACOG, and identify any gaps in best practices that may require additional research or analysis."
The proposed legislation would facilitate the research and dissemination of evidence-informed recommendations for addressing maternal addiction and NAS and provide for NAS studies. The measure also would encourage the Centers for Disease Control and Prevention to work with states to improve the availability and quality of data to more effectively respond to drug-addicted pregnant women. At this article’s deadline, the proposed act was in the Committee on Health, Education, Labor and Pensions.
"My legislation is no silver bullet, but it will help ensure that our public health system is better equipped to prevent and treat opiate addiction in mothers and their newborn children," Mr. McConnell said in a statement. "Together, we can overcome this tragic problem."
Developing resources that promote better medical care for addicted women and their children is a more effective answer than punitive laws, added Hendree Jones, Ph.D., a professor of obstetrics and gynecology at the university and executive director of the UNC Horizons programs. Horizons provides substance abuse treatment and child care to women who are pregnant or parenting young children.
"Incarcerating women to reduce the prevalence of drug use during pregnancy is a misguided solution to the issue," Dr. Jones said in an interview. "Increasing access to high-quality treatment would be a much more fruitful approach."
On Twitter @legal_med
The enactment of a Tennessee law that criminalizes substance abuse by pregnant women has made patients increasingly hesitant to pursue treatment for their drug addictions, according to Dr. Jessica Young, an obstetrician/gynecologist at Vanderbilt University Medical Center in Nashville, Tenn.
Tennessee’s law, which became effective in July, defines illegal narcotic use by pregnant women as assault, depending on whether the child is born addicted to or is harmed by the narcotic. A 26-year-old woman was among the first to be arrested in mid-July under the law.

"Since the new law was passed, women . . . often express a fatalistic attitude of, ‘Even if I do the right thing, I will be arrested or have my baby taken away,’ " said Dr. Young, who leads the Vanderbilt Drug Dependency Clinic for pregnant women.
The concern is that women will hide their addictions or self-detox at home, she said. "I think it will require some negative outcomes for this law to be struck down."
Physician associations, including the American Medical Association and ACOG, have long maintained that criminal punishment is not the answer to curbing drug abuse by pregnant women and that such laws create further harm to children. Fear of being reported to police was a primary reason women who abused drugs did not seek prenatal care, a recent study found (Am. J. Obstet. Gynecol. 2009;200:412.e1-10).
Laws that mandate criminal penalties against pregnant women who use drugs can significantly harm the physician-patient relationship, according to Dr. John M. Thorp, professor of obstetrics and gynecology at the University of North Carolina and director of women’s primary health care for UNC Healthcare, both in Chapel Hill.
These laws "put the physician or the clinic or hospital in the role of reporting the crime, which is completely the antithesis of wanting patients to be able to tell physicians what’s wrong with them and being able to offer treatment instead of punishment," Dr. Thorp said in an interview. "These laws are a real setback to the concept that substance abuse addiction is a disease, [and they are] another barrier for women seeking treatment."
Tennessee is the only state to consider drug abuse by pregnant women to be "assault." The law states that a woman may be prosecuted for assault if her child is born addicted or harmed by the drug and for homicide if the child dies from drug exposure. It is an affirmative defense to prosecution if the woman is actively enrolled in an addiction recovery program before the baby is born, remains in the program after delivery, and successfully completes the program.
The American Civil Liberties Union of Tennessee plans to challenge the law in court.
The Tennessee Medical Association opposed the law, but successfully pushed for amendments that would lessen the punitive damages against women, Dave Chaney, TMA director of communications, said in an interview. The medical association successfully lobbied to lower the charging level in the bill to a misdemeanor and helped secure a stipulation that women who enter a treatment program be punished only by a fine.
Various states pursue criminal charges against pregnant women who use narcotics. Eighteen states consider substance abuse during pregnancy to be "child abuse," and three other states consider substance abuse during pregnancy to be grounds for civil commitment, according to an August 2014 analysis by the Guttmacher Institute. Fifteen states require physicians to report suspected prenatal drug abuse to authorities, and four states require health providers to test for prenatal drug exposure if they suspect substance abuse.
Other states could soon follow in Tennessee’s footsteps, Elizabeth Nash, Guttmacher Institute state issues manager, said in an interview. "Legislatures see what’s happening in other states and decide whether it may be right for them."
Meanwhile, a newly proposed federal bill could assist states in addressing the issue of drug-addicted pregnant mothers and babies and drive more effective responses to the crisis. The Protecting Our Infants Act, introduced July 31 by Rep. Mitch McConnell (R-Ky.), aims to better identify and treat opioid use by pregnant women and neonatal abstinence syndrome (NAS) in newborns. The legislation is backed by ACOG.
"Currently, states vary widely in how they mandate and administer screening, diagnosis, and treatment of pregnant women using opioids," ACOG President John C. Jennings said in a statement. The federal "legislation will help identify, compile, and disseminate best practices developed by medical professional organizations, including ACOG, and identify any gaps in best practices that may require additional research or analysis."
The proposed legislation would facilitate the research and dissemination of evidence-informed recommendations for addressing maternal addiction and NAS and provide for NAS studies. The measure also would encourage the Centers for Disease Control and Prevention to work with states to improve the availability and quality of data to more effectively respond to drug-addicted pregnant women. At this article’s deadline, the proposed act was in the Committee on Health, Education, Labor and Pensions.
"My legislation is no silver bullet, but it will help ensure that our public health system is better equipped to prevent and treat opiate addiction in mothers and their newborn children," Mr. McConnell said in a statement. "Together, we can overcome this tragic problem."
Developing resources that promote better medical care for addicted women and their children is a more effective answer than punitive laws, added Hendree Jones, Ph.D., a professor of obstetrics and gynecology at the university and executive director of the UNC Horizons programs. Horizons provides substance abuse treatment and child care to women who are pregnant or parenting young children.
"Incarcerating women to reduce the prevalence of drug use during pregnancy is a misguided solution to the issue," Dr. Jones said in an interview. "Increasing access to high-quality treatment would be a much more fruitful approach."
On Twitter @legal_med
Gestational diabetes and the Barker Hypothesis
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).
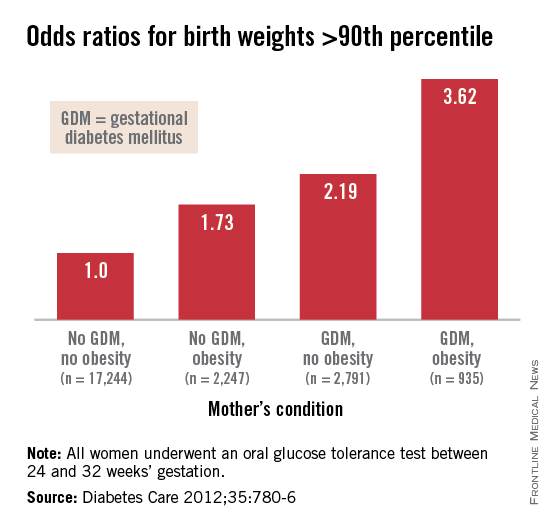
While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.
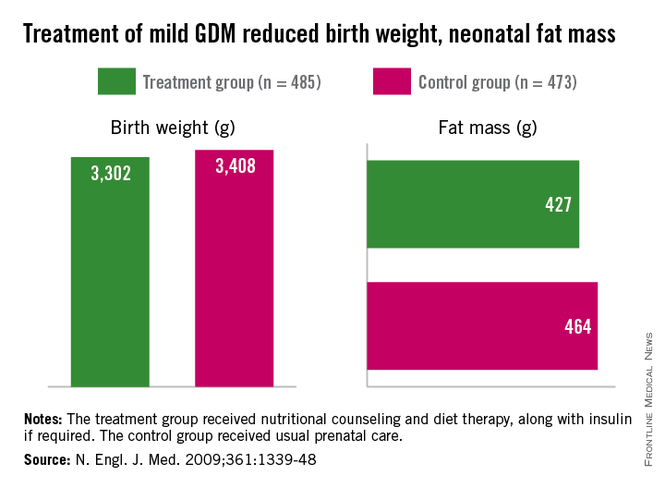
Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.
Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
The fetal origins hypothesis
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
Need for more data
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
Estrogen does not cause breast cancer
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,