User login
My Most Unusual Case: Cesarean Scar Ectopic Pregnancy
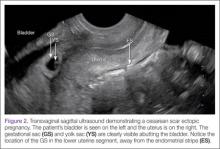
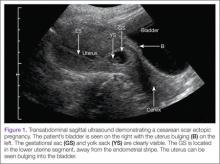
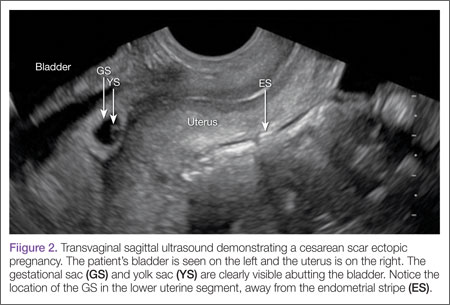
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
Case Studies in Toxicology: A Common Procedure, an Uncommon Complication
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 2: Pathophysiology and causation
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
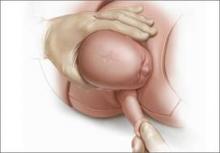
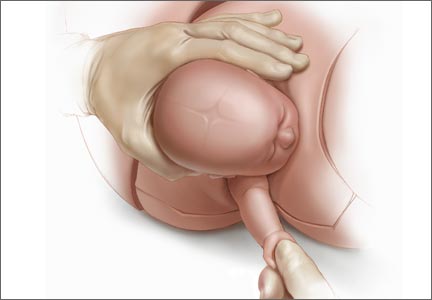
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Tackle the challenging shoulder dystocia emergency by practicing delivery of the posterior arm
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________
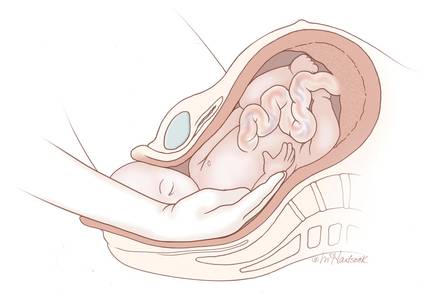
| 
| |
| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________

| 
| |

| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________

| 
| |

| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
Women who were better informed chose fewer prenatal genetic tests
Pregnant women who used an interactive computer program to help them decide whether to undergo prenatal testing were 55% less likely to choose invasive testing than women who received standard prenatal care, even when they were offered free testing, researchers reported online Sept. 23 in JAMA.
Women who used the computer program also were more likely to correctly estimate the risks of amniocentesis-related miscarriage and fetal trisomy 21, said Miriam Kuppermann, Ph.D., of the University of California, San Francisco, and her associates. “
If validated in additional populations, this approach may result in more informed and preference-based prenatal testing decision making and fewer women undergoing testing,” the investigators said.
The multicenter study included 710 English- or Spanish-speaking women who were at 20 weeks’ gestation or less.
A bilingual actress narrated the computer program, emulating a “warm and knowledgeable friend” and emphasizing “the personal nature of prenatal testing decisions,” said the researchers.
The control group received prenatal care based on current guidelines, and did not receive financial support for prenatal tests beyond their own insurance plans, the investigators added (JAMA 2014 Sept. 23 [doi:10.1001/jama.2014.11479]).
Medical record reviews showed that the intervention group was significantly less likely to pursue amniocentesis or chorionic villi sampling than was the control group (5.9% vs. 12.3%; odds ratio, 0.45; 95% confidence interval, 0.25 to 0.80). In addition, computer program users were more than three times as likely to forego testing altogether and more than twice as likely to elect screening alone than to choose screening followed by invasive testing.
Although the computer program did not address cell-free DNA testing, the findings should extend to these tests because they cover the same conditions, the researchers said.
The intervention group also was more likely to correctly estimate the risk of amniocentesis-related miscarriage (73.8% vs. 59.0%; OR, 1.95 [95% CI, 1.39-2.75]) and the age-adjusted chance of fetal trisomy 21 (58.7% vs. 46.1%; OR, 1.66 [95% CI, 1.22-2.28]) than the control group, Dr. Kuppermann and her associates reported.
The National Institutes of Health and the March of Dimes Foundation funded the study. Dr. Kuppermann reported receiving research support from Ariosa Diagnostics, Verinata Health, and Natera. Two coauthors reported receiving funding or stock options from Verinata Health, Natera, Ariosa Diagnostics, and Cellscape. The authors disclosed no other financial conflicts.
“The finding that women who were fully informed about various prenatal testing options were less likely to undertake invasive testing is important, and contradicts the notion that more information is always desired. It is possible that the nature of prenatal testing is different than other health care decisions, but the public may be increasingly aware that the numerous medical advances of the last decade also have created greater complexity in decision making. This finding also suggests that prenatal genetic testing decisions require a complex calculus that considers the timing of the testing, the certainty of the results, and the risks of invasive genetic testing during pregnancy.
“Most women are somewhere between wanting the most sophisticated testing and declining all testing. While seeking reassurance through prenatal testing, they acknowledge that difficult information may be revealed. Many admit not knowing exactly what decisions might be made based on receiving difficult information until they have it. For these women, providing information about testing options, with scenarios that illustrate the risks and benefits of each test as well as assisting with values clarification, is important before embarking on any particular testing strategy,” according to Dr. Siobhan M. Dolan.
Dr. Dolan is a professor of obstetrics and gynecology specializing in reproductive genetics at the Albert Einstein College of Medicine in New York. She gave her comments in an editorial to Dr. Kuppermann’s report (JAMA 2014;312:1203-5). She reported no relevant financial conflicts.
“The finding that women who were fully informed about various prenatal testing options were less likely to undertake invasive testing is important, and contradicts the notion that more information is always desired. It is possible that the nature of prenatal testing is different than other health care decisions, but the public may be increasingly aware that the numerous medical advances of the last decade also have created greater complexity in decision making. This finding also suggests that prenatal genetic testing decisions require a complex calculus that considers the timing of the testing, the certainty of the results, and the risks of invasive genetic testing during pregnancy.
“Most women are somewhere between wanting the most sophisticated testing and declining all testing. While seeking reassurance through prenatal testing, they acknowledge that difficult information may be revealed. Many admit not knowing exactly what decisions might be made based on receiving difficult information until they have it. For these women, providing information about testing options, with scenarios that illustrate the risks and benefits of each test as well as assisting with values clarification, is important before embarking on any particular testing strategy,” according to Dr. Siobhan M. Dolan.
Dr. Dolan is a professor of obstetrics and gynecology specializing in reproductive genetics at the Albert Einstein College of Medicine in New York. She gave her comments in an editorial to Dr. Kuppermann’s report (JAMA 2014;312:1203-5). She reported no relevant financial conflicts.
“The finding that women who were fully informed about various prenatal testing options were less likely to undertake invasive testing is important, and contradicts the notion that more information is always desired. It is possible that the nature of prenatal testing is different than other health care decisions, but the public may be increasingly aware that the numerous medical advances of the last decade also have created greater complexity in decision making. This finding also suggests that prenatal genetic testing decisions require a complex calculus that considers the timing of the testing, the certainty of the results, and the risks of invasive genetic testing during pregnancy.
“Most women are somewhere between wanting the most sophisticated testing and declining all testing. While seeking reassurance through prenatal testing, they acknowledge that difficult information may be revealed. Many admit not knowing exactly what decisions might be made based on receiving difficult information until they have it. For these women, providing information about testing options, with scenarios that illustrate the risks and benefits of each test as well as assisting with values clarification, is important before embarking on any particular testing strategy,” according to Dr. Siobhan M. Dolan.
Dr. Dolan is a professor of obstetrics and gynecology specializing in reproductive genetics at the Albert Einstein College of Medicine in New York. She gave her comments in an editorial to Dr. Kuppermann’s report (JAMA 2014;312:1203-5). She reported no relevant financial conflicts.
Pregnant women who used an interactive computer program to help them decide whether to undergo prenatal testing were 55% less likely to choose invasive testing than women who received standard prenatal care, even when they were offered free testing, researchers reported online Sept. 23 in JAMA.
Women who used the computer program also were more likely to correctly estimate the risks of amniocentesis-related miscarriage and fetal trisomy 21, said Miriam Kuppermann, Ph.D., of the University of California, San Francisco, and her associates. “
If validated in additional populations, this approach may result in more informed and preference-based prenatal testing decision making and fewer women undergoing testing,” the investigators said.
The multicenter study included 710 English- or Spanish-speaking women who were at 20 weeks’ gestation or less.
A bilingual actress narrated the computer program, emulating a “warm and knowledgeable friend” and emphasizing “the personal nature of prenatal testing decisions,” said the researchers.
The control group received prenatal care based on current guidelines, and did not receive financial support for prenatal tests beyond their own insurance plans, the investigators added (JAMA 2014 Sept. 23 [doi:10.1001/jama.2014.11479]).
Medical record reviews showed that the intervention group was significantly less likely to pursue amniocentesis or chorionic villi sampling than was the control group (5.9% vs. 12.3%; odds ratio, 0.45; 95% confidence interval, 0.25 to 0.80). In addition, computer program users were more than three times as likely to forego testing altogether and more than twice as likely to elect screening alone than to choose screening followed by invasive testing.
Although the computer program did not address cell-free DNA testing, the findings should extend to these tests because they cover the same conditions, the researchers said.
The intervention group also was more likely to correctly estimate the risk of amniocentesis-related miscarriage (73.8% vs. 59.0%; OR, 1.95 [95% CI, 1.39-2.75]) and the age-adjusted chance of fetal trisomy 21 (58.7% vs. 46.1%; OR, 1.66 [95% CI, 1.22-2.28]) than the control group, Dr. Kuppermann and her associates reported.
The National Institutes of Health and the March of Dimes Foundation funded the study. Dr. Kuppermann reported receiving research support from Ariosa Diagnostics, Verinata Health, and Natera. Two coauthors reported receiving funding or stock options from Verinata Health, Natera, Ariosa Diagnostics, and Cellscape. The authors disclosed no other financial conflicts.
Pregnant women who used an interactive computer program to help them decide whether to undergo prenatal testing were 55% less likely to choose invasive testing than women who received standard prenatal care, even when they were offered free testing, researchers reported online Sept. 23 in JAMA.
Women who used the computer program also were more likely to correctly estimate the risks of amniocentesis-related miscarriage and fetal trisomy 21, said Miriam Kuppermann, Ph.D., of the University of California, San Francisco, and her associates. “
If validated in additional populations, this approach may result in more informed and preference-based prenatal testing decision making and fewer women undergoing testing,” the investigators said.
The multicenter study included 710 English- or Spanish-speaking women who were at 20 weeks’ gestation or less.
A bilingual actress narrated the computer program, emulating a “warm and knowledgeable friend” and emphasizing “the personal nature of prenatal testing decisions,” said the researchers.
The control group received prenatal care based on current guidelines, and did not receive financial support for prenatal tests beyond their own insurance plans, the investigators added (JAMA 2014 Sept. 23 [doi:10.1001/jama.2014.11479]).
Medical record reviews showed that the intervention group was significantly less likely to pursue amniocentesis or chorionic villi sampling than was the control group (5.9% vs. 12.3%; odds ratio, 0.45; 95% confidence interval, 0.25 to 0.80). In addition, computer program users were more than three times as likely to forego testing altogether and more than twice as likely to elect screening alone than to choose screening followed by invasive testing.
Although the computer program did not address cell-free DNA testing, the findings should extend to these tests because they cover the same conditions, the researchers said.
The intervention group also was more likely to correctly estimate the risk of amniocentesis-related miscarriage (73.8% vs. 59.0%; OR, 1.95 [95% CI, 1.39-2.75]) and the age-adjusted chance of fetal trisomy 21 (58.7% vs. 46.1%; OR, 1.66 [95% CI, 1.22-2.28]) than the control group, Dr. Kuppermann and her associates reported.
The National Institutes of Health and the March of Dimes Foundation funded the study. Dr. Kuppermann reported receiving research support from Ariosa Diagnostics, Verinata Health, and Natera. Two coauthors reported receiving funding or stock options from Verinata Health, Natera, Ariosa Diagnostics, and Cellscape. The authors disclosed no other financial conflicts.
FROM JAMA
Key clinical point: Pregnant women who received interactive computerized guidance on prenatal testing were better informed, but less likely to choose invasive testing than women who received typical prenatal care.
Major finding: Women who received computerized guidance on prenatal testing were 55% less likely to choose invasive testing than women who received usual care, even when tests were offered without cost.
Data source: A randomized multicenter trial of 710 pregnant women in the San Francisco Bay area.
Disclosures: The National Institutes of Health and the March of Dimes Foundation funded the study. Dr. Kuppermann reported past research support from Ariosa Diagnostics, Verinata Health, and Natera. Two coauthors reported advisory or financial relationships with Verinata Health, Natera, Ariosa Diagnostics, and Cellscape. The authors disclosed no other financial conflicts.
Dr. Robert L. Barbieri’s Editor’s Picks September 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
VIDEO: Experts offer top tips for flu season 2014-2015
WASHINGTON – Options and opportunity are the keys to navigating the 2014-2015 flu season, according to a panel of experts at a press conference sponsored by the National Foundation for Infectious Diseases.
“The easier we make it for people to get vaccinated, the more likely they are to get vaccinated,” said CDC Director Thomas Frieden, who received his flu shot at the press conference.
In interviews at the conference, Dr. Frieden, Dr. Paul A. Offit of the Children’s Hospital of Philadelphia; Dr. Laura E. Riley of Massachusetts General Hospital, Boston; and Dr. William Schaffner of Vanderbilt University, Nashville, Tenn., discussed making the most of opportunities to vaccinate patients, offering reassurance about vaccine safety (especially for pregnant women), setting an example in your practice by getting vaccinated yourself, and ensuring that everyone who works in your office receives a flu vaccine as well.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Options and opportunity are the keys to navigating the 2014-2015 flu season, according to a panel of experts at a press conference sponsored by the National Foundation for Infectious Diseases.
“The easier we make it for people to get vaccinated, the more likely they are to get vaccinated,” said CDC Director Thomas Frieden, who received his flu shot at the press conference.
In interviews at the conference, Dr. Frieden, Dr. Paul A. Offit of the Children’s Hospital of Philadelphia; Dr. Laura E. Riley of Massachusetts General Hospital, Boston; and Dr. William Schaffner of Vanderbilt University, Nashville, Tenn., discussed making the most of opportunities to vaccinate patients, offering reassurance about vaccine safety (especially for pregnant women), setting an example in your practice by getting vaccinated yourself, and ensuring that everyone who works in your office receives a flu vaccine as well.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Options and opportunity are the keys to navigating the 2014-2015 flu season, according to a panel of experts at a press conference sponsored by the National Foundation for Infectious Diseases.
“The easier we make it for people to get vaccinated, the more likely they are to get vaccinated,” said CDC Director Thomas Frieden, who received his flu shot at the press conference.
In interviews at the conference, Dr. Frieden, Dr. Paul A. Offit of the Children’s Hospital of Philadelphia; Dr. Laura E. Riley of Massachusetts General Hospital, Boston; and Dr. William Schaffner of Vanderbilt University, Nashville, Tenn., discussed making the most of opportunities to vaccinate patients, offering reassurance about vaccine safety (especially for pregnant women), setting an example in your practice by getting vaccinated yourself, and ensuring that everyone who works in your office receives a flu vaccine as well.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Mechanical heart valves create high pregnancy risk
BARCELONA – Women with mechanical heart valves who become pregnant face a very-high-risk pregnancy, with a 58% rate of an uncomplicated pregnancy resulting in a live birth, according to international registry data collected since 2007.
Pregnant women at high risk because of a mechanical heart valve need management by a multidisciplinary team at a referral center that focuses on these cases, a type of care that many of these women do not receive today, Dr. Jolien W. Roos-Hesselink said at the annual congress of the European Society of Cardiology.
“We believe management of these women needs to be better structured and organized,” said Dr. Roos-Hesselink, professor and head of the department of congenital cardiology at Erasmus Medical Center in Rotterdam, the Netherlands. “Pregnancy is a time of risk for any woman with structural heart disease, but for those with a mechanical valve are really high-risk patients,” she said in an interview.
“Most of these women are now cared for by a general cardiologist. They need a specialist in obstetric cardiology,” as well as care from other experts with experience in the types of complications these woman develop, said Dr. Roger J.C. Hall, professor of cardiology at Norfolk and Norwich (U.K.) University Hospital.
The data also showed that physicians around the world used any one of seven different anticoagulant regimens during these pregnancies, a strikingly high number that highlights uncertainty about which regimen is best, although heparin use during the first trimester was linked with a higher rate of valve thrombosis. The various regimens use different combinations of periods of treatment with unfractionated heparin, low-molecular-weight heparin, or a vitamin K antagonist drug during the first trimester, during weeks 14-36, and during the last weeks of pregnancy.
“We found large differences in management among different countries, physicians, and among individual patients. All the regimens have advantages and disadvantages” and are based on expert opinion with no prospect for a randomized, controlled trial, said Dr. Roos-Hesselink. For now, the numbers of women receiving each of the seven regimens remains too small for statistical analysis, but the researchers hope that eventually larger numbers may start to reveal which regimens work best, said Dr. Hall.
However, the available data showed two clear trends: Treatment with vitamin K antagonists was tied to an increased rate of miscarriages, and treatment with heparin during the first trimester was associated with an increased rate of valve thrombosis, noted Dr. Roos-Hesselink.
One other notable finding was that the risks faced by women with mechanical heart valves far exceeded the risk seen in women with tissue valves, and in women with other forms of mechanical heart disease but no valve prostheses. Adolescents and young women who need a heart valve should be part of a shared decision process that reviews the pros and cons of a mechanical and tissue valve, said Dr. Roos-Hesselink and Dr. Hall. A tissue valve is less durable, and so typically requires replacement sooner than does a mechanical valve. But in the current study, the pregnancy loss and complication rates among the 134 women with a tissue valve roughly matched the rates among the 2,620 women with no valve prosthesis, while the rates among 212 women with a mechanical valve ran much higher.
The Registry of Pregnancy and Cardiac Diseases began in 2007 through an initiative of two interest groups of the European Society of Cardiology, the valve group and the congenital heart disease group (Eur. Heart J. 2013;34:657-65). By September 2014, the registry had enrolled more than 3,600 pregnancies. The current report focused on the first 2,966 women enrolled, with an average age of 29 years. Slightly more than half the enrolled women had congenital heart disease, slightly fewer than a third had valvular heart disease (usually because of rheumatic heart disease), 7% had cardiomyopathy, and smaller number of women had other etiologies.
Maternal mortality averaged 1.4% for women with mechanical valves, 1.5% for those with tissue valves, and 0.2% for everyone else. Miscarriage rates were 16% for mothers with mechanical valves and 2% for everyone else, including those with tissues valves. Fetal mortality was 3% among women with mechanical valves and less than 1% for everyone else. Thrombotic and hemorrhagic events occurred in about 29% of women with mechanical valves, compared with less than 6% in everyone else. Ten of the women with mechanical valves (5%) developed valve thrombosis. The live birth rate was 80% for women with mechanical valves and 95% or better for everyone else.
Dr. Roos-Hesselink and Dr. Hall had no disclosures.
On Twitter @mitchelzoler
Dr. Hossein Almassi, FCCP, comments: One of the toughest decisions in valvular heart surgery is the selection of a valve prosthesis for a young female. Historically, age has been the major deciding factor in selecting a mechanical valve over a tissue valve. The current report is a testament to this difficulty, showing a lack of a standardized anticoagulation regimen and a much higher rate of pregnancy loss and complications with mechanical heart valves. Availability of transcatheter valve technology may make the tissue valves more attractive for this group of patients. Clearly, the care for these women would be best provided by a multidisciplinary team of specialists.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: One of the toughest decisions in valvular heart surgery is the selection of a valve prosthesis for a young female. Historically, age has been the major deciding factor in selecting a mechanical valve over a tissue valve. The current report is a testament to this difficulty, showing a lack of a standardized anticoagulation regimen and a much higher rate of pregnancy loss and complications with mechanical heart valves. Availability of transcatheter valve technology may make the tissue valves more attractive for this group of patients. Clearly, the care for these women would be best provided by a multidisciplinary team of specialists.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: One of the toughest decisions in valvular heart surgery is the selection of a valve prosthesis for a young female. Historically, age has been the major deciding factor in selecting a mechanical valve over a tissue valve. The current report is a testament to this difficulty, showing a lack of a standardized anticoagulation regimen and a much higher rate of pregnancy loss and complications with mechanical heart valves. Availability of transcatheter valve technology may make the tissue valves more attractive for this group of patients. Clearly, the care for these women would be best provided by a multidisciplinary team of specialists.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
BARCELONA – Women with mechanical heart valves who become pregnant face a very-high-risk pregnancy, with a 58% rate of an uncomplicated pregnancy resulting in a live birth, according to international registry data collected since 2007.
Pregnant women at high risk because of a mechanical heart valve need management by a multidisciplinary team at a referral center that focuses on these cases, a type of care that many of these women do not receive today, Dr. Jolien W. Roos-Hesselink said at the annual congress of the European Society of Cardiology.
“We believe management of these women needs to be better structured and organized,” said Dr. Roos-Hesselink, professor and head of the department of congenital cardiology at Erasmus Medical Center in Rotterdam, the Netherlands. “Pregnancy is a time of risk for any woman with structural heart disease, but for those with a mechanical valve are really high-risk patients,” she said in an interview.
“Most of these women are now cared for by a general cardiologist. They need a specialist in obstetric cardiology,” as well as care from other experts with experience in the types of complications these woman develop, said Dr. Roger J.C. Hall, professor of cardiology at Norfolk and Norwich (U.K.) University Hospital.
The data also showed that physicians around the world used any one of seven different anticoagulant regimens during these pregnancies, a strikingly high number that highlights uncertainty about which regimen is best, although heparin use during the first trimester was linked with a higher rate of valve thrombosis. The various regimens use different combinations of periods of treatment with unfractionated heparin, low-molecular-weight heparin, or a vitamin K antagonist drug during the first trimester, during weeks 14-36, and during the last weeks of pregnancy.
“We found large differences in management among different countries, physicians, and among individual patients. All the regimens have advantages and disadvantages” and are based on expert opinion with no prospect for a randomized, controlled trial, said Dr. Roos-Hesselink. For now, the numbers of women receiving each of the seven regimens remains too small for statistical analysis, but the researchers hope that eventually larger numbers may start to reveal which regimens work best, said Dr. Hall.
However, the available data showed two clear trends: Treatment with vitamin K antagonists was tied to an increased rate of miscarriages, and treatment with heparin during the first trimester was associated with an increased rate of valve thrombosis, noted Dr. Roos-Hesselink.
One other notable finding was that the risks faced by women with mechanical heart valves far exceeded the risk seen in women with tissue valves, and in women with other forms of mechanical heart disease but no valve prostheses. Adolescents and young women who need a heart valve should be part of a shared decision process that reviews the pros and cons of a mechanical and tissue valve, said Dr. Roos-Hesselink and Dr. Hall. A tissue valve is less durable, and so typically requires replacement sooner than does a mechanical valve. But in the current study, the pregnancy loss and complication rates among the 134 women with a tissue valve roughly matched the rates among the 2,620 women with no valve prosthesis, while the rates among 212 women with a mechanical valve ran much higher.
The Registry of Pregnancy and Cardiac Diseases began in 2007 through an initiative of two interest groups of the European Society of Cardiology, the valve group and the congenital heart disease group (Eur. Heart J. 2013;34:657-65). By September 2014, the registry had enrolled more than 3,600 pregnancies. The current report focused on the first 2,966 women enrolled, with an average age of 29 years. Slightly more than half the enrolled women had congenital heart disease, slightly fewer than a third had valvular heart disease (usually because of rheumatic heart disease), 7% had cardiomyopathy, and smaller number of women had other etiologies.
Maternal mortality averaged 1.4% for women with mechanical valves, 1.5% for those with tissue valves, and 0.2% for everyone else. Miscarriage rates were 16% for mothers with mechanical valves and 2% for everyone else, including those with tissues valves. Fetal mortality was 3% among women with mechanical valves and less than 1% for everyone else. Thrombotic and hemorrhagic events occurred in about 29% of women with mechanical valves, compared with less than 6% in everyone else. Ten of the women with mechanical valves (5%) developed valve thrombosis. The live birth rate was 80% for women with mechanical valves and 95% or better for everyone else.
Dr. Roos-Hesselink and Dr. Hall had no disclosures.
On Twitter @mitchelzoler
BARCELONA – Women with mechanical heart valves who become pregnant face a very-high-risk pregnancy, with a 58% rate of an uncomplicated pregnancy resulting in a live birth, according to international registry data collected since 2007.
Pregnant women at high risk because of a mechanical heart valve need management by a multidisciplinary team at a referral center that focuses on these cases, a type of care that many of these women do not receive today, Dr. Jolien W. Roos-Hesselink said at the annual congress of the European Society of Cardiology.
“We believe management of these women needs to be better structured and organized,” said Dr. Roos-Hesselink, professor and head of the department of congenital cardiology at Erasmus Medical Center in Rotterdam, the Netherlands. “Pregnancy is a time of risk for any woman with structural heart disease, but for those with a mechanical valve are really high-risk patients,” she said in an interview.
“Most of these women are now cared for by a general cardiologist. They need a specialist in obstetric cardiology,” as well as care from other experts with experience in the types of complications these woman develop, said Dr. Roger J.C. Hall, professor of cardiology at Norfolk and Norwich (U.K.) University Hospital.
The data also showed that physicians around the world used any one of seven different anticoagulant regimens during these pregnancies, a strikingly high number that highlights uncertainty about which regimen is best, although heparin use during the first trimester was linked with a higher rate of valve thrombosis. The various regimens use different combinations of periods of treatment with unfractionated heparin, low-molecular-weight heparin, or a vitamin K antagonist drug during the first trimester, during weeks 14-36, and during the last weeks of pregnancy.
“We found large differences in management among different countries, physicians, and among individual patients. All the regimens have advantages and disadvantages” and are based on expert opinion with no prospect for a randomized, controlled trial, said Dr. Roos-Hesselink. For now, the numbers of women receiving each of the seven regimens remains too small for statistical analysis, but the researchers hope that eventually larger numbers may start to reveal which regimens work best, said Dr. Hall.
However, the available data showed two clear trends: Treatment with vitamin K antagonists was tied to an increased rate of miscarriages, and treatment with heparin during the first trimester was associated with an increased rate of valve thrombosis, noted Dr. Roos-Hesselink.
One other notable finding was that the risks faced by women with mechanical heart valves far exceeded the risk seen in women with tissue valves, and in women with other forms of mechanical heart disease but no valve prostheses. Adolescents and young women who need a heart valve should be part of a shared decision process that reviews the pros and cons of a mechanical and tissue valve, said Dr. Roos-Hesselink and Dr. Hall. A tissue valve is less durable, and so typically requires replacement sooner than does a mechanical valve. But in the current study, the pregnancy loss and complication rates among the 134 women with a tissue valve roughly matched the rates among the 2,620 women with no valve prosthesis, while the rates among 212 women with a mechanical valve ran much higher.
The Registry of Pregnancy and Cardiac Diseases began in 2007 through an initiative of two interest groups of the European Society of Cardiology, the valve group and the congenital heart disease group (Eur. Heart J. 2013;34:657-65). By September 2014, the registry had enrolled more than 3,600 pregnancies. The current report focused on the first 2,966 women enrolled, with an average age of 29 years. Slightly more than half the enrolled women had congenital heart disease, slightly fewer than a third had valvular heart disease (usually because of rheumatic heart disease), 7% had cardiomyopathy, and smaller number of women had other etiologies.
Maternal mortality averaged 1.4% for women with mechanical valves, 1.5% for those with tissue valves, and 0.2% for everyone else. Miscarriage rates were 16% for mothers with mechanical valves and 2% for everyone else, including those with tissues valves. Fetal mortality was 3% among women with mechanical valves and less than 1% for everyone else. Thrombotic and hemorrhagic events occurred in about 29% of women with mechanical valves, compared with less than 6% in everyone else. Ten of the women with mechanical valves (5%) developed valve thrombosis. The live birth rate was 80% for women with mechanical valves and 95% or better for everyone else.
Dr. Roos-Hesselink and Dr. Hall had no disclosures.
On Twitter @mitchelzoler
AT ESC 2014
Key clinical point: Pregnant women with mechanical heart valves have a very high rate of pregnancy complications and miscarriages.
Major finding: The rate of live births without complications was 58% in women with a mechanical heart valve.
Data source: ROPAC registry of 2,966 pregnant women with structural heart disease from 132 centers in 48 countries.
Disclosures: Dr. Roos-Hesselink and Dr. Hall had no disclosures.
States with higher malpractice rates have more cesarean deliveries and fewer vaginal deliveries
Over the last 20 years, litigation rates, malpractice coverage costs, and the number of cesarean deliveries have increased significantly. In fact, many ObGyns have dropped their obstetric practices because of the increased cost of malpractice insurance, say researchers from Johns Hopkins and Brown, and three states (New York, Florida, and Virginia) now have birth injury funds to help deflect the burden of malpractice insurance and litigation costs.1
What is the existing state-level relationship between malpractice rates and the mode of delivery, including cesarean delivery (CD) and vaginal delivery (VD), as well as operative vaginal delivery (OVD)? And how do the state-run injury funds affect this relationship, asked Clark T. Johnson, MD, MPH, and Erika F. Werner, MD.
To answer their question, the investigators collected 8 years of data on mode of delivery (from the Centers for Disease Control and Prevention National Vital Statistics System) and malpractice rates for ObGyns in each US state (from the 2011 Medical Liability Monitor Rate Survey). The researchers used linear regression modeling to analyze their 2003−2010 data.1
They found that states with higher malpractice rates had higher CD delivery rates and lower VD rates than states with lower malpractice rates, and that higher malpractice rates also correlated with lower rates of OVD, including the use of forceps and vacuum extraction. Overall, the change in malpractice rates between 2003 and 2010 did not correlate with the rates of OVD.1
Those states with the highest average annual malpractice insurance rate (> $120,000) to cover claims with a cap of $1 million/$3 million in 2010, were Florida, New York, and Connecticut. Illinois, Pennsylvania, New Jersey, and Maryland had an average annual rate of $100,000 to $120,000.
Those states with the lowest average annual malpractice insurance rate (0$ to $40,000) were North Dakota, South Dakota, Nebraska, Minnesota, Iowa, and Wisconsin.
In the 1980s, medical malpractice insurance rates soared and insurance companies were faced with eliminating coverage to obstetricians. In 1987, the Virginia General Assembly developed the Virginia Birth-Related Neurological Injury Compensation Program. This no-fault system ensures lifetime care to eligible participants, and has successfully stabilized the malpractice environment in the state.2 Birth-related neurologic injury funds were also created in Florida in 19883 and in New York in 2011.4
The three states with birth injury funds showed a decrease in malpractice rates, a slower rate of CD-rate increase, and an OVD-rate decrease. However, the researchers note that while developing a state birth injury fund is seen as a trend that might reduce malpractice rates, there were not enough data to demonstrate significance.1
1. Johnson CT, Werner EF. The nationwide relationship between malpractice rates and rates of vaginal and cesarean delivery. Poster presented at: American College of Obstetricians and Gynecologists 62nd Annual Clinical Meeting; April 26–30, 2014; Chicago, IL. Obstet Gynecol.
2. Medical Society of Virginia. Virginia birth injury fund (BIF) FAQs. http://www.msv.org/MainMenuCategories/MemberCenter/FAQs/Virginia-Birth-Injury-Fund-BIF-FAQs.aspx. Accessed July 30, 2014.
3. What is NICA? Florida Birth-Related Neurological Injury Compensation Association (NICA) Web site. https://www.nica.com/what-is-nica.html. Accessed July 30, 2014.
4. Medical Indemnity Fund frequently asked questions. New York State Department of Financial Services Web site. http://www.dfs.ny.gov/insurance/mif/mif_faqs.htm. Updated February 13, 2012. Accessed July 30, 2014.
Over the last 20 years, litigation rates, malpractice coverage costs, and the number of cesarean deliveries have increased significantly. In fact, many ObGyns have dropped their obstetric practices because of the increased cost of malpractice insurance, say researchers from Johns Hopkins and Brown, and three states (New York, Florida, and Virginia) now have birth injury funds to help deflect the burden of malpractice insurance and litigation costs.1
What is the existing state-level relationship between malpractice rates and the mode of delivery, including cesarean delivery (CD) and vaginal delivery (VD), as well as operative vaginal delivery (OVD)? And how do the state-run injury funds affect this relationship, asked Clark T. Johnson, MD, MPH, and Erika F. Werner, MD.
To answer their question, the investigators collected 8 years of data on mode of delivery (from the Centers for Disease Control and Prevention National Vital Statistics System) and malpractice rates for ObGyns in each US state (from the 2011 Medical Liability Monitor Rate Survey). The researchers used linear regression modeling to analyze their 2003−2010 data.1
They found that states with higher malpractice rates had higher CD delivery rates and lower VD rates than states with lower malpractice rates, and that higher malpractice rates also correlated with lower rates of OVD, including the use of forceps and vacuum extraction. Overall, the change in malpractice rates between 2003 and 2010 did not correlate with the rates of OVD.1
Those states with the highest average annual malpractice insurance rate (> $120,000) to cover claims with a cap of $1 million/$3 million in 2010, were Florida, New York, and Connecticut. Illinois, Pennsylvania, New Jersey, and Maryland had an average annual rate of $100,000 to $120,000.
Those states with the lowest average annual malpractice insurance rate (0$ to $40,000) were North Dakota, South Dakota, Nebraska, Minnesota, Iowa, and Wisconsin.
In the 1980s, medical malpractice insurance rates soared and insurance companies were faced with eliminating coverage to obstetricians. In 1987, the Virginia General Assembly developed the Virginia Birth-Related Neurological Injury Compensation Program. This no-fault system ensures lifetime care to eligible participants, and has successfully stabilized the malpractice environment in the state.2 Birth-related neurologic injury funds were also created in Florida in 19883 and in New York in 2011.4
The three states with birth injury funds showed a decrease in malpractice rates, a slower rate of CD-rate increase, and an OVD-rate decrease. However, the researchers note that while developing a state birth injury fund is seen as a trend that might reduce malpractice rates, there were not enough data to demonstrate significance.1
Over the last 20 years, litigation rates, malpractice coverage costs, and the number of cesarean deliveries have increased significantly. In fact, many ObGyns have dropped their obstetric practices because of the increased cost of malpractice insurance, say researchers from Johns Hopkins and Brown, and three states (New York, Florida, and Virginia) now have birth injury funds to help deflect the burden of malpractice insurance and litigation costs.1
What is the existing state-level relationship between malpractice rates and the mode of delivery, including cesarean delivery (CD) and vaginal delivery (VD), as well as operative vaginal delivery (OVD)? And how do the state-run injury funds affect this relationship, asked Clark T. Johnson, MD, MPH, and Erika F. Werner, MD.
To answer their question, the investigators collected 8 years of data on mode of delivery (from the Centers for Disease Control and Prevention National Vital Statistics System) and malpractice rates for ObGyns in each US state (from the 2011 Medical Liability Monitor Rate Survey). The researchers used linear regression modeling to analyze their 2003−2010 data.1
They found that states with higher malpractice rates had higher CD delivery rates and lower VD rates than states with lower malpractice rates, and that higher malpractice rates also correlated with lower rates of OVD, including the use of forceps and vacuum extraction. Overall, the change in malpractice rates between 2003 and 2010 did not correlate with the rates of OVD.1
Those states with the highest average annual malpractice insurance rate (> $120,000) to cover claims with a cap of $1 million/$3 million in 2010, were Florida, New York, and Connecticut. Illinois, Pennsylvania, New Jersey, and Maryland had an average annual rate of $100,000 to $120,000.
Those states with the lowest average annual malpractice insurance rate (0$ to $40,000) were North Dakota, South Dakota, Nebraska, Minnesota, Iowa, and Wisconsin.
In the 1980s, medical malpractice insurance rates soared and insurance companies were faced with eliminating coverage to obstetricians. In 1987, the Virginia General Assembly developed the Virginia Birth-Related Neurological Injury Compensation Program. This no-fault system ensures lifetime care to eligible participants, and has successfully stabilized the malpractice environment in the state.2 Birth-related neurologic injury funds were also created in Florida in 19883 and in New York in 2011.4
The three states with birth injury funds showed a decrease in malpractice rates, a slower rate of CD-rate increase, and an OVD-rate decrease. However, the researchers note that while developing a state birth injury fund is seen as a trend that might reduce malpractice rates, there were not enough data to demonstrate significance.1
1. Johnson CT, Werner EF. The nationwide relationship between malpractice rates and rates of vaginal and cesarean delivery. Poster presented at: American College of Obstetricians and Gynecologists 62nd Annual Clinical Meeting; April 26–30, 2014; Chicago, IL. Obstet Gynecol.
2. Medical Society of Virginia. Virginia birth injury fund (BIF) FAQs. http://www.msv.org/MainMenuCategories/MemberCenter/FAQs/Virginia-Birth-Injury-Fund-BIF-FAQs.aspx. Accessed July 30, 2014.
3. What is NICA? Florida Birth-Related Neurological Injury Compensation Association (NICA) Web site. https://www.nica.com/what-is-nica.html. Accessed July 30, 2014.
4. Medical Indemnity Fund frequently asked questions. New York State Department of Financial Services Web site. http://www.dfs.ny.gov/insurance/mif/mif_faqs.htm. Updated February 13, 2012. Accessed July 30, 2014.
1. Johnson CT, Werner EF. The nationwide relationship between malpractice rates and rates of vaginal and cesarean delivery. Poster presented at: American College of Obstetricians and Gynecologists 62nd Annual Clinical Meeting; April 26–30, 2014; Chicago, IL. Obstet Gynecol.
2. Medical Society of Virginia. Virginia birth injury fund (BIF) FAQs. http://www.msv.org/MainMenuCategories/MemberCenter/FAQs/Virginia-Birth-Injury-Fund-BIF-FAQs.aspx. Accessed July 30, 2014.
3. What is NICA? Florida Birth-Related Neurological Injury Compensation Association (NICA) Web site. https://www.nica.com/what-is-nica.html. Accessed July 30, 2014.
4. Medical Indemnity Fund frequently asked questions. New York State Department of Financial Services Web site. http://www.dfs.ny.gov/insurance/mif/mif_faqs.htm. Updated February 13, 2012. Accessed July 30, 2014.
USPSTF recommends low-dose aspirin for preeclampsia prevention
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
FROM ANNALS OF INTERNAL MEDICINE