User login
Time to retire race- and ethnicity-based carrier screening
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
Babies are dying of syphilis. It’s 100% preventable.
This story was originally published on ProPublica and was co-published with NPR.
When Mai Yang is looking for a patient, she travels light. She dresses deliberately — not too formal, so she won’t be mistaken for a police officer; not too casual, so people will look past her tiny 4-foot-10 stature and youthful face and trust her with sensitive health information. Always, she wears closed-toed shoes, “just in case I need to run.”
Yang carries a stack of cards issued by the Centers for Disease Control and Prevention that show what happens when the Treponema pallidum bacteria invades a patient’s body. There’s a photo of an angry red sore on a penis. There’s one of a tongue, marred by mucus-lined lesions. And there’s one of a newborn baby, its belly, torso and thighs dotted in a rash, its mouth open, as if caught midcry.
It was because of the prospect of one such baby that Yang found herself walking through a homeless encampment on a blazing July day in Huron, Calif., an hour’s drive southwest of her office at the Fresno County Department of Public Health. She was looking for a pregnant woman named Angelica, whose visit to a community clinic had triggered a report to the health department’s sexually transmitted disease program. Angelica had tested positive for syphilis. If she was not treated, her baby could end up like the one in the picture or worse — there was a 40% chance the baby would die.
Ms. Yang knew, though, that if she helped Angelica get treated with three weekly shots of penicillin at least 30 days before she gave birth, it was likely that the infection would be wiped out and her baby would be born without any symptoms at all. Every case of congenital syphilis, when a baby is born with the disease, is avoidable. Each is considered a “sentinel event,” a warning that the public health system is failing.
The alarms are now clamoring. In the United States, more than 129,800 syphilis cases were recorded in 2019, double the case count of five years prior. In the same time period, cases of congenital syphilis quadrupled: 1,870 babies were born with the disease; 128 died. Case counts from 2020 are still being finalized, but the CDC has said that reported cases of congenital syphilis have already exceeded the prior year. Black, Hispanic, and Native American babies are disproportionately at risk.
There was a time, not too long ago, when CDC officials thought they could eliminate the centuries-old scourge from the United States, for adults and babies. But the effort lost steam and cases soon crept up again. Syphilis is not an outlier. The United States goes through what former CDC director Dr. Tom Frieden calls “a deadly cycle of panic and neglect” in which emergencies propel officials to scramble and throw money at a problem — whether that’s Ebola, Zika, or COVID-19. Then, as fear ebbs, so does the attention and motivation to finish the task.
The last fraction of cases can be the hardest to solve, whether that’s eradicating a bug or getting vaccines into arms, yet too often, that’s exactly when political attention gets diverted to the next alarm. The result: The hardest to reach and most vulnerable populations are the ones left suffering, after everyone else looks away.
Ms. Yang first received Angelica’s lab report on June 17. The address listed was a P.O. box, and the phone number belonged to her sister, who said Angelica was living in Huron. That was a piece of luck: Huron is tiny; the city spans just 1.6 square miles. On her first visit, a worker at the Alamo Motel said she knew Angelica and directed Ms. Yang to a nearby homeless encampment. Angelica wasn’t there, so Ms. Yang returned a second time, bringing one of the health department nurses who could serve as an interpreter.
They made their way to the barren patch of land behind Huron Valley Foods, the local grocery store, where people took shelter in makeshift lean-tos composed of cardboard boxes, scrap wood ,and scavenged furniture, draped with sheets that served as ceilings and curtains. Yang stopped outside one of the structures, calling a greeting.
“Hi, I’m from the health department, I’m looking for Angelica.”
The nurse echoed her in Spanish.
Angelica emerged, squinting in the sunlight. Ms. Yang couldn’t tell if she was visibly pregnant yet, as her body was obscured by an oversized shirt. The two women were about the same age: Ms. Yang 26 and Angelica 27. Ms. Yang led her away from the tent, so they could speak privately. Angelica seemed reticent, surprised by the sudden appearance of the two health officers. “You’re not in trouble,” Ms. Yang said, before revealing the results of her blood test.
Angelica had never heard of syphilis.
“Have you been to prenatal care?”
Angelica shook her head. The local clinic had referred her to an obstetrician in Hanford, a 30-minute drive away. She had no car. She also mentioned that she didn’t intend to raise her baby; her two oldest children lived with her mother, and this one likely would, too.
Ms. Yang pulled out the CDC cards, showing them to Angelica and asking if she had experienced any of the symptoms illustrated. No, Angelica said, her lips pursed with disgust.
“Right now you still feel healthy, but this bacteria is still in your body,” Ms. Yang pressed. “You need to get the infection treated to prevent further health complications to yourself and your baby.”
The community clinic was just across the street. “Can we walk you over to the clinic and make sure you get seen so we can get this taken care of?”
Angelica demurred. She said she hadn’t showered for a week and wanted to wash up first. She said she’d go later.
Ms. Yang tried once more to extract a promise: “What time do you think you’ll go?”
“Today, for sure.”
Syphilis is called The Great Imitator: It can look like any number of diseases. In its first stage, the only evidence of infection is a painless sore at the bacteria’s point of entry. Weeks later, as the bacteria multiplies, skin rashes bloom on the palms of the hands and bottoms of the feet. Other traits of this stage include fever, headaches, muscle aches, sore throat, and fatigue. These symptoms eventually disappear and the patient progresses into the latent phase, which betrays no external signs. But if left untreated, after a decade or more, syphilis will reemerge in up to 30% of patients, capable of wreaking horror on a wide range of organ systems. Dr. Marion Sims, president of the American Medical Association in 1876, called it a “terrible scourge, which begins with lamb-like mildness and ends with lion-like rage that ruthlessly destroys everything in its way.”
The corkscrew-shaped bacteria can infiltrate the nervous system at any stage of the infection. Ms. Yang is haunted by her memory of interviewing a young man whose dementia was so severe that he didn’t know why he was in the hospital or how old he was. And regardless of symptoms or stage, the bacteria can penetrate the placenta to infect a fetus. Even in these cases the infection is unpredictable: Many babies are born with normal physical features, but others can have deformed bones or damaged brains, and they can struggle to hear, see, or breathe.
From its earliest days, syphilis has been shrouded in stigma. The first recorded outbreak was in the late 15th century, when Charles VIII led the French army to invade Naples. Italian physicians described French soldiers covered with pustules, dying from a sexually transmitted disease. As the affliction spread, Italians called it the French Disease. The French blamed the Neopolitans. It was also called the German, Polish, or Spanish disease, depending on which neighbor one wanted to blame. Even its name bears the taint of divine judgement: It comes from a 16th-century poem that tells of a shepherd, Syphilus, who offended the god Apollo and was punished with a hideous disease.
By 1937 in America, when former Surgeon General Thomas Parran wrote the book “Shadow on the Land,” he estimated some 680,000 people were under treatment for syphilis; about 60,000 babies were being born annually with congenital syphilis. There was no cure, and the stigma was so strong that public health officials feared even properly documenting cases.
Thanks to Dr. Parran’s ardent advocacy, Congress in 1938 passed the National Venereal Disease Control Act, which created grants for states to set up clinics and support testing and treatment. Other than a short-lived funding effort during World War I, this was the first coordinated federal push to respond to the disease.
Around the same time, the Public Health Service launched an effort to record the natural history of syphilis. Situated in Tuskegee, Ala., the infamous study recruited 600 black men. By the early 1940s, penicillin became widely available and was found to be a reliable cure, but the treatment was withheld from the study participants. Outrage over the ethical violations would cast a stain across syphilis research for decades to come and fuel generations of mistrust in the medical system among Black Americans that continues to this day.
With the introduction of penicillin, cases began to plummet. Twice, the CDC has announced efforts to wipe out the disease — once in the 1960s and again in 1999.
In the latest effort, the CDC announced that the United States had “a unique opportunity to eliminate syphilis within its borders,” thanks to historically low rates, with 80% of counties reporting zero cases. The concentration of cases in the South “identifies communities in which there is a fundamental failure of public health capacity,” the agency noted, adding that elimination — which it defined as fewer than 1,000 cases a year — would “decrease one of our most glaring racial disparities in health.”
Two years after the campaign began, cases started climbing, first among gay men and later, heterosexuals. Cases in women started accelerating in 2013, followed shortly by increasing numbers of babies born with syphilis.The reasons for failure are complex; people relaxed safer sex practices after the advent of potent HIV combination therapies, increased methamphetamine use drove riskier behavior and an explosion of online dating made it hard to track and test sexual partners, according to Dr. Ina Park, medical director of the California Prevention Training Center at the University of California San Francisco.
But federal and state public health efforts were hamstrung from the get-go. In 1999, the CDC said it would need about $35 million to $39 million in new federal funds annually for at least five years to eliminate syphilis. The agency got less than half of what it asked for, according to Jo Valentine, former program coordinator of the CDC’s Syphilis Elimination Effort. As cases rose, the CDC modified its goals in 2006 from 0.4 primary and secondary syphilis cases per 100,000 in population to 2.2 cases per 100,000. By 2013, as elimination seemed less and less viable, the CDC changed its focus to ending congenital syphilis only.
Since then, funding has remained anemic. From 2015 to 2020, the CDC’s budget for preventing sexually transmitted infections grew by 2.2%. Taking inflation into account, that’s a 7.4% reduction in purchasing power. In the same period, cases of syphilis, gonorrhea and chlamydia — the three STDs that have federally funded control programs — increased by nearly 30%.
“We have a long history of nearly eradicating something, then changing our attention, and seeing a resurgence in numbers,” said David Harvey, executive director of the National Coalition of STD Directors. “We have more congenital syphilis cases today in America than we ever had pediatric AIDS at the height of the AIDS epidemic. It’s heartbreaking.”
Adriane Casalotti, chief of government and public affairs at the National Association of County and City Health Officials, warns that the United States should not be surprised to see case counts continue to climb. “The bugs don’t go away,” she said. “They’re just waiting for the next opportunity, when you’re not paying attention.”
Ms. Yang waited until the end of the day, then called the clinic to see if Angelica had gone for her shot. She had not. Ms. Yang would have to block off another half day to visit Huron again, but she had three dozen other cases to deal with.
States in the South and West have seen the highest syphilis rates in recent years. In 2017, 64 babies in Fresno County were born with syphilis at a rate of 440 babies per 100,000 live births — about 19 times the national rate. While the county had managed to lower case counts in the two years that followed, the pandemic threatened to unravel that progress, forcing STD staffers to do COVID-19 contact tracing, pausing field visits to find infected people, and scaring patients from seeking care. Ms. Yang’s colleague handled three cases of stillbirth in 2020; in each, the woman was never diagnosed with syphilis because she feared catching the coronavirus and skipped prenatal care.
Ms. Yang, whose caseload peaked at 70 during a COVID-19 surge, knew she would not be able handle them all as thoroughly as she’d like to. “When I was being mentored by another investigator, he said: ‘You’re not a superhero. You can’t save everybody,’” she said. She prioritizes men who have sex with men, because there’s a higher prevalence of syphilis in that population, and pregnant people, because of the horrific consequences for babies.
The job of a disease intervention specialist isn’t for everyone: It means meeting patients whenever and wherever they are available — in the mop closet of a bus station, in a quiet parking lot — to inform them about the disease, to extract names of sex partners and to encourage treatment. Patients are often reluctant to talk. They can get belligerent, upset that “the government” has their personal information or shattered at the thought that a partner is likely cheating on them. Salaries typically start in the low $40,000s.
Jena Adams, Ms. Yang’s supervisor, has eight investigators working on HIV and syphilis. In the middle of 2020, she lost two and replaced them only recently. “It’s been exhausting,” Ms. Adams said. She has only one specialist who is trained to take blood samples in the field, crucial for guaranteeing that the partners of those who test positive for syphilis also get tested. Ms. Adams wants to get phlebotomy training for the rest of her staff, but it’s $2,000 per person. The department also doesn’t have anyone who can administer penicillin injections in the field; that would have been key when Ms. Yang met Angelica. For a while, a nurse who worked in the tuberculosis program would ride along to give penicillin shots on a volunteer basis. Then he, too, left the health department.
Much of the resources in public health trickle down from the CDC, which distributes money to states, which then parcel it out to counties. The CDC gets its budget from Congress, which tells the agency, by line item, exactly how much money it can spend to fight a disease or virus, in an uncommonly specific manner not seen in many other agencies. The decisions are often politically driven and can be detached from actual health needs.
When the House and Senate appropriations committees meet to decide how much the CDC will get for each line item, they are barraged by lobbyists for individual disease interests. Stephanie Arnold Pang, senior director of policy and government relations at the National Coalition of STD Directors, can pick out the groups by sight: breast cancer wears pink, Alzheimer’s goes in purple, multiple sclerosis comes in orange, HIV in red. STD prevention advocates, like herself, don a green ribbon, but they’re far outnumbered.
And unlike diseases that might already be familiar to lawmakers, or have patient and family spokespeople who can tell their own powerful stories, syphilis doesn’t have many willing poster children. “Congressmen don’t wake up one day and say, ‘Oh hey, there’s congenital syphilis in my jurisdiction.’ You have to raise awareness,” Arnold Pang said. It can be hard jockeying for a meeting. “Some offices might say, ‘I don’t have time for you because we’ve just seen HIV.’ ... Sometimes, it feels like you’re talking into a void.”
The consequences of the political nature of public health funding have become more obvious during the coronavirus pandemic. The 2014 Ebola epidemic was seen as a “global wakeup call” that the world wasn’t prepared for a major pandemic, yet in 2018, the CDC scaled back its epidemic prevention work as money ran out. “If you’ve got to choose between Alzheimer’s research and stopping an outbreak that may not happen? Stopping an outbreak that might not happen doesn’t do well,” said Dr. Frieden, the former CDC director. “The CDC needs to have more money and more flexible money. Otherwise, we’re going to be in this situation long term.”
In May 2021, President Joe Biden’s administration announced it would set aside $7.4 billion over the next five years to hire and train public health workers, including $1.1 billion for more disease intervention specialists like Ms. Yang. Public health officials are thrilled to have the chance to expand their workforce, but some worry the time horizon may be too short. “We’ve seen this movie before, right?” Dr. Frieden said. “Everyone gets concerned when there’s an outbreak, and when that outbreak stops, the headlines stop, and an economic downturn happens, the budget gets cut.”
Fresno’s STD clinic was shuttered in 2010 amid the Great Recession. Many others have vanished since the passage of the Affordable Care Act. Health leaders thought “by magically beefing up the primary care system, that we would do a better job of catching STIs and treating them,” said Mr. Harvey, the executive director of the National Coalition of STD Directors. That hasn’t worked out; people want access to anonymous services, and primary care doctors often don’t have STDs top of mind. The coalition is lobbying Congress for funding to support STD clinical services, proposing a three-year demonstration project funded at $600 million.
It’s one of Ms. Adams’ dreams to see Fresno’s STD clinic restored as it was. “You could come in for an HIV test and get other STDs checked,” she said. “And if a patient is positive, you can give a first injection on the spot.”
On Aug. 12, Ms. Yang set out for Huron again, speeding past groves of almond trees and fields of grapes in the department’s white Chevy Cruze. She brought along a colleague, Jorge Sevilla, who had recently transferred to the STD program from COVID-19 contact tracing. Ms. Yang was anxious to find Angelica again. “She’s probably in her second trimester now,” she said.
They found her outside of a pale yellow house a few blocks from the homeless encampment; the owner was letting her stay in a shed tucked in the corner of the dirt yard. This time, it was evident that she was pregnant. Ms. Yang noted that Angelica was wearing a wig; hair loss is a symptom of syphilis.
“Do you remember me?” Ms. Yang asked.
Angelica nodded. She didn’t seem surprised to see Ms. Yang again. (I came along, and Mr. Sevilla explained who I was and that I was writing about syphilis and the people affected by it. Angelica signed a release for me to report about her case, and she said she had no problem with me writing about her or even using her full name. ProPublica chose to only print her first name.)
“How are you doing? How’s the baby?”
“Bien.”
“So the last time we talked, we were going to have you go to United Healthcare Center to get treatment. Have you gone since?”
Angelica shook her head.
“We brought some gift cards...” Mr. Sevilla started in Spanish. The department uses them as incentives for completing injections. But Angelica was already shaking her head. The nearest Walmart was the next town over.
Ms. Yang turned to her partner. “Tell her: So the reason why we’re coming out here again is because we really need her to go in for treatment. ... We really are concerned for the baby’s health especially since she’s had the infection for quite a while.”
Angelica listened while Mr. Sevilla interpreted, her eyes on the ground. Then she looked up. “Orita?” she asked. Right now?
“I’ll walk with you,” Ms. Yang offered. Angelica shook her head. “She said she wants to shower first before she goes over there,” Mr. Sevilla said.
Ms. Yang made a face. “She said that to me last time.” Ms. Yang offered to wait, but Angelica didn’t want the health officers to linger by the house. She said she would meet them by the clinic in 15 minutes.
Ms. Yang was reluctant to let her go but again had no other option. She and Mr. Sevilla drove to the clinic, then stood on the corner of the parking lot, staring down the road.
Talk to the pediatricians, obstetricians, and families on the front lines of the congenital syphilis surge and it becomes clear why Ms. Yang and others are trying so desperately to prevent cases. Dr. J. B. Cantey, associate professor in pediatrics at UT Health San Antonio, remembers a baby girl born at 25 weeks gestation who weighed a pound and a half. Syphilis had spread through her bones and lungs. She spent five months in the neonatal intensive care unit, breathing through a ventilator, and was still eating through a tube when she was discharged.
Then, there are the miscarriages, the stillbirths and the inconsolable parents. Dr. Irene Stafford, an associate professor and maternal-fetal medicine specialist at UT Health in Houston, cannot forget a patient who came in at 36 weeks for a routine checkup, pregnant with her first child. Dr. Stafford realized that there was no heartbeat. “She could see on my face that something was really wrong,” Dr. Stafford recalled. She had to let the patient know that syphilis had killed her baby. “She was hysterical, just bawling,” Dr. Stafford said. “I’ve seen people’s families ripped apart and I’ve seen beautiful babies die.” Fewer than 10% of patients who experience a stillbirth are tested for syphilis, suggesting that cases are underdiagnosed.
A Texas grandmother named Solidad Odunuga offers a glimpse into what the future could hold for Angelica’s mother, who may wind up raising her baby.
In February of last year, Ms. Odunuga got a call from the Lyndon B. Johnson Hospital in Houston. A nurse told her that her daughter was about to give birth and that child protective services had been called. Ms. Odunuga had lost contact with her daughter, who struggled with homelessness and substance abuse. She arrived in time to see her grandson delivered, premature at 30 weeks old, weighing 2.7 pounds. He tested positive for syphilis.
When a child protective worker asked Ms. Odunuga to take custody of the infant, she felt a wave of dread. “I was in denial,” she recalled. “I did not plan to be a mom again.” The baby’s medical problems were daunting: “Global developmental delays ... concerns for visual impairments ... high risk of cerebral palsy,” read a note from the doctor at the time.
Still, Ms. Odunuga visited her grandson every day for three months, driving to the NICU from her job at the University of Houston. “I’d put him in my shirt to keep him warm and hold him there.” She fell in love. She named him Emmanuel.
Once Emmanuel was discharged, Ms. Odunuga realized she had no choice but to quit her job. While Medicaid covered the costs of Emmanuel’s treatment, it was on her to care for him. From infancy, Emmanuel’s life has been a whirlwind of constant therapy. Today, at 20 months old, Odunuga brings him to physical, occupational, speech, and developmental therapy, each a different appointment on a different day of the week.
Emmanuel has thrived beyond what his doctors predicted, toddling so fast that Ms. Odunuga can’t look away for a minute and beaming as he waves his favorite toy phone. Yet he still suffers from gagging issues, which means Ms. Odunuga can’t feed him any solid foods. Liquid gets into his lungs when he aspirates; it has led to pneumonia three times. Emmanuel has a special stroller that helps keep his head in a position that won’t aggravate his persistent reflux, but Odunuga said she still has to pull over on the side of the road sometimes when she hears him projectile vomiting from the backseat.
The days are endless. Once she puts Emmanuel to bed, Ms. Odunuga starts planning the next day’s appointments. “I’ve had to cry alone, scream out alone,” she said. “Sometimes I wake up and think, Is this real? And then I hear him in the next room.”
Putting aside the challenge of eliminating syphilis entirely, everyone agrees it’s both doable and necessary to prevent newborn cases. “There was a crisis in perinatal HIV almost 30 years ago and people stood up and said this is not OK — it’s not acceptable for babies to be born in that condition. ... [We] brought it down from 1,700 babies born each year with perinatal HIV to less than 40 per year today,” said Virginia Bowen, an epidemiologist at the CDC. “Now here we are with a slightly different condition. We can also stand up and say, ‘This is not acceptable.’” Belarus, Bermuda, Cuba, Malaysia, Thailand, and Sri Lanka are among countries recognized by the World Health Organization for eliminating congenital syphilis.
Success starts with filling gaps across the health care system.
For almost a century, public health experts have advocated for testing pregnant patients more than once for syphilis in order to catch the infection. But policies nationwide still don’t reflect this best practice. Six states have no prenatal screening requirement at all. Even in states that require three tests, public health officials say that many physicians aren’t aware of the requirements. Dr. Stafford, the maternal-fetal medicine specialist in Houston, says she’s tired of hearing her own peers in medicine tell her, “Oh, syphilis is a problem?”
It costs public health departments less than 25 cents a dose to buy penicillin, but for a private practice, it’s more than $1,000, according to Dr. Park of the University of California San Francisco. “There’s no incentive for a private physician to stock a dose that could expire before it’s used, so they often don’t have it. So a woman comes in, they say, ‘We’ll send you to the emergency department or health department to get it,’ then [the patients] don’t show up.”
A vaccine would be invaluable for preventing spread among people at high risk for reinfection. But there is none. Scientists only recently figured out how to grow the bacteria in the lab, prompting grants from the National Institutes of Health to fund research into a vaccine. Dr. Justin Radolf, a researcher at the University of Connecticut School of Medicine, said he hopes his team will have a vaccine candidate by the end of its five-year grant. But it’ll likely take years more to find a manufacturer and run human trials.
Public health agencies also need to recognize that many of the hurdles to getting pregnant people treated involve access to care, economic stability, safe housing and transportation. In Fresno, Ms. Adams has been working on ways her department can collaborate with mental health services. Recently, one of her disease intervention specialists managed to get a pregnant woman treated with penicillin shots and, at the patient’s request, connected her with an addiction treatment center.
Gaining a patient’s cooperation means seeing them as complex humans instead of just a case to solve. “There may be past traumas with the health care system,” said Cynthia Deverson, project manager of the Houston Fetal Infant Morbidity Review. “There’s the fear of being discovered if she’s doing something illegal to survive. ... She may need to be in a certain place at a certain time so she can get something to eat, or maybe it’s the only time of the day that’s safe for her to sleep. They’re not going to tell you that. Yes, they understand there’s a problem, but it’s not an immediate threat, maybe they don’t feel bad yet, so obviously this is not urgent. ...
“What helps to gain trust is consistency,” she said. “Literally, it’s seeing that [disease specialist] constantly, daily. ... The woman can see that you’re not going to harm her, you’re saying, ‘I’m here at this time if you need me.’”
Ms. Yang stood outside the clinic, waiting for Angelica to show up, baking in the 90-degree heat. Her feelings ranged from irritation — Why didn’t she just go? I’d have more energy for other cases — to an appreciation for the parts of Angelica’s story that she didn’t know — She’s in survival mode. I need to be more patient.
Fifteen minutes ticked by, then 20.
“OK,” Ms. Yang announced. “We’re going back.”
She asked Sevilla if he would be OK if they drove Angelica to the clinic; they technically weren’t supposed to because of coronavirus precautions, but Ms. Yang wasn’t sure she could convince Angelica to walk. Mr. Sevilla gave her the thumbs up.
When they pulled up, they saw Angelica sitting in the backyard, chatting with a friend. She now wore a fresh T-shirt and had shoes on her feet. Angelica sat silently in the back seat as Ms. Yang drove to the clinic. A few minutes later, they pulled up to the parking lot.
Finally, Ms. Yang thought. We got her here.
The clinic was packed with people waiting for COVID-19 tests and vaccinations. A worker there had previously told Ms. Yang that a walk-in would be fine, but a receptionist now said they were too busy to treat Angelica. She would have to return.
Ms. Yang felt a surge of frustration, sensing that her hard-fought opportunity was slipping away. She tried to talk to the nurse supervisor, but he wasn’t available. She tried to leave the gift cards at the office to reward Angelica if she came, but the receptionist said she couldn’t hold them. While Ms. Yang negotiated, Mr. Sevilla sat with Angelica in the car, waiting.
Finally, Ms. Yang accepted this was yet another thing she couldn’t control.
She drove Angelica back to the yellow house. As they arrived, she tried once more to impress on her just how important it was to get treated, asking Mr. Sevilla to interpret. “We don’t want it to get any more serious, because she can go blind, she could go deaf, she could lose her baby.”
Angelica already had the door halfway open.
“So on a scale from one to 10, how important is this to get treated?” Ms. Yang asked.
“Ten,” Angelica said. Ms. Yang reminded her of the appointment that afternoon. Then Angelica stepped out and returned to the dusty yard.
Ms. Yang lingered for a moment, watching Angelica go. Then she turned the car back onto the highway and set off toward Fresno, knowing, already, that she’d be back.
Postscript: A reporter visited Huron twice more in the months that followed, including once independently to try to interview Angelica, but she wasn’t in town. Ms. Yang has visited Huron twice more as well — six times in total thus far. In October, a couple of men at the yellow house said Angelica was still in town, still pregnant. Ms. Yang and Mr. Sevilla spent an hour driving around, talking to residents, hoping to catch Angelica. But she was nowhere to be found.
ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
This story was originally published on ProPublica and was co-published with NPR.
When Mai Yang is looking for a patient, she travels light. She dresses deliberately — not too formal, so she won’t be mistaken for a police officer; not too casual, so people will look past her tiny 4-foot-10 stature and youthful face and trust her with sensitive health information. Always, she wears closed-toed shoes, “just in case I need to run.”
Yang carries a stack of cards issued by the Centers for Disease Control and Prevention that show what happens when the Treponema pallidum bacteria invades a patient’s body. There’s a photo of an angry red sore on a penis. There’s one of a tongue, marred by mucus-lined lesions. And there’s one of a newborn baby, its belly, torso and thighs dotted in a rash, its mouth open, as if caught midcry.
It was because of the prospect of one such baby that Yang found herself walking through a homeless encampment on a blazing July day in Huron, Calif., an hour’s drive southwest of her office at the Fresno County Department of Public Health. She was looking for a pregnant woman named Angelica, whose visit to a community clinic had triggered a report to the health department’s sexually transmitted disease program. Angelica had tested positive for syphilis. If she was not treated, her baby could end up like the one in the picture or worse — there was a 40% chance the baby would die.
Ms. Yang knew, though, that if she helped Angelica get treated with three weekly shots of penicillin at least 30 days before she gave birth, it was likely that the infection would be wiped out and her baby would be born without any symptoms at all. Every case of congenital syphilis, when a baby is born with the disease, is avoidable. Each is considered a “sentinel event,” a warning that the public health system is failing.
The alarms are now clamoring. In the United States, more than 129,800 syphilis cases were recorded in 2019, double the case count of five years prior. In the same time period, cases of congenital syphilis quadrupled: 1,870 babies were born with the disease; 128 died. Case counts from 2020 are still being finalized, but the CDC has said that reported cases of congenital syphilis have already exceeded the prior year. Black, Hispanic, and Native American babies are disproportionately at risk.
There was a time, not too long ago, when CDC officials thought they could eliminate the centuries-old scourge from the United States, for adults and babies. But the effort lost steam and cases soon crept up again. Syphilis is not an outlier. The United States goes through what former CDC director Dr. Tom Frieden calls “a deadly cycle of panic and neglect” in which emergencies propel officials to scramble and throw money at a problem — whether that’s Ebola, Zika, or COVID-19. Then, as fear ebbs, so does the attention and motivation to finish the task.
The last fraction of cases can be the hardest to solve, whether that’s eradicating a bug or getting vaccines into arms, yet too often, that’s exactly when political attention gets diverted to the next alarm. The result: The hardest to reach and most vulnerable populations are the ones left suffering, after everyone else looks away.
Ms. Yang first received Angelica’s lab report on June 17. The address listed was a P.O. box, and the phone number belonged to her sister, who said Angelica was living in Huron. That was a piece of luck: Huron is tiny; the city spans just 1.6 square miles. On her first visit, a worker at the Alamo Motel said she knew Angelica and directed Ms. Yang to a nearby homeless encampment. Angelica wasn’t there, so Ms. Yang returned a second time, bringing one of the health department nurses who could serve as an interpreter.
They made their way to the barren patch of land behind Huron Valley Foods, the local grocery store, where people took shelter in makeshift lean-tos composed of cardboard boxes, scrap wood ,and scavenged furniture, draped with sheets that served as ceilings and curtains. Yang stopped outside one of the structures, calling a greeting.
“Hi, I’m from the health department, I’m looking for Angelica.”
The nurse echoed her in Spanish.
Angelica emerged, squinting in the sunlight. Ms. Yang couldn’t tell if she was visibly pregnant yet, as her body was obscured by an oversized shirt. The two women were about the same age: Ms. Yang 26 and Angelica 27. Ms. Yang led her away from the tent, so they could speak privately. Angelica seemed reticent, surprised by the sudden appearance of the two health officers. “You’re not in trouble,” Ms. Yang said, before revealing the results of her blood test.
Angelica had never heard of syphilis.
“Have you been to prenatal care?”
Angelica shook her head. The local clinic had referred her to an obstetrician in Hanford, a 30-minute drive away. She had no car. She also mentioned that she didn’t intend to raise her baby; her two oldest children lived with her mother, and this one likely would, too.
Ms. Yang pulled out the CDC cards, showing them to Angelica and asking if she had experienced any of the symptoms illustrated. No, Angelica said, her lips pursed with disgust.
“Right now you still feel healthy, but this bacteria is still in your body,” Ms. Yang pressed. “You need to get the infection treated to prevent further health complications to yourself and your baby.”
The community clinic was just across the street. “Can we walk you over to the clinic and make sure you get seen so we can get this taken care of?”
Angelica demurred. She said she hadn’t showered for a week and wanted to wash up first. She said she’d go later.
Ms. Yang tried once more to extract a promise: “What time do you think you’ll go?”
“Today, for sure.”
Syphilis is called The Great Imitator: It can look like any number of diseases. In its first stage, the only evidence of infection is a painless sore at the bacteria’s point of entry. Weeks later, as the bacteria multiplies, skin rashes bloom on the palms of the hands and bottoms of the feet. Other traits of this stage include fever, headaches, muscle aches, sore throat, and fatigue. These symptoms eventually disappear and the patient progresses into the latent phase, which betrays no external signs. But if left untreated, after a decade or more, syphilis will reemerge in up to 30% of patients, capable of wreaking horror on a wide range of organ systems. Dr. Marion Sims, president of the American Medical Association in 1876, called it a “terrible scourge, which begins with lamb-like mildness and ends with lion-like rage that ruthlessly destroys everything in its way.”
The corkscrew-shaped bacteria can infiltrate the nervous system at any stage of the infection. Ms. Yang is haunted by her memory of interviewing a young man whose dementia was so severe that he didn’t know why he was in the hospital or how old he was. And regardless of symptoms or stage, the bacteria can penetrate the placenta to infect a fetus. Even in these cases the infection is unpredictable: Many babies are born with normal physical features, but others can have deformed bones or damaged brains, and they can struggle to hear, see, or breathe.
From its earliest days, syphilis has been shrouded in stigma. The first recorded outbreak was in the late 15th century, when Charles VIII led the French army to invade Naples. Italian physicians described French soldiers covered with pustules, dying from a sexually transmitted disease. As the affliction spread, Italians called it the French Disease. The French blamed the Neopolitans. It was also called the German, Polish, or Spanish disease, depending on which neighbor one wanted to blame. Even its name bears the taint of divine judgement: It comes from a 16th-century poem that tells of a shepherd, Syphilus, who offended the god Apollo and was punished with a hideous disease.
By 1937 in America, when former Surgeon General Thomas Parran wrote the book “Shadow on the Land,” he estimated some 680,000 people were under treatment for syphilis; about 60,000 babies were being born annually with congenital syphilis. There was no cure, and the stigma was so strong that public health officials feared even properly documenting cases.
Thanks to Dr. Parran’s ardent advocacy, Congress in 1938 passed the National Venereal Disease Control Act, which created grants for states to set up clinics and support testing and treatment. Other than a short-lived funding effort during World War I, this was the first coordinated federal push to respond to the disease.
Around the same time, the Public Health Service launched an effort to record the natural history of syphilis. Situated in Tuskegee, Ala., the infamous study recruited 600 black men. By the early 1940s, penicillin became widely available and was found to be a reliable cure, but the treatment was withheld from the study participants. Outrage over the ethical violations would cast a stain across syphilis research for decades to come and fuel generations of mistrust in the medical system among Black Americans that continues to this day.
With the introduction of penicillin, cases began to plummet. Twice, the CDC has announced efforts to wipe out the disease — once in the 1960s and again in 1999.
In the latest effort, the CDC announced that the United States had “a unique opportunity to eliminate syphilis within its borders,” thanks to historically low rates, with 80% of counties reporting zero cases. The concentration of cases in the South “identifies communities in which there is a fundamental failure of public health capacity,” the agency noted, adding that elimination — which it defined as fewer than 1,000 cases a year — would “decrease one of our most glaring racial disparities in health.”
Two years after the campaign began, cases started climbing, first among gay men and later, heterosexuals. Cases in women started accelerating in 2013, followed shortly by increasing numbers of babies born with syphilis.The reasons for failure are complex; people relaxed safer sex practices after the advent of potent HIV combination therapies, increased methamphetamine use drove riskier behavior and an explosion of online dating made it hard to track and test sexual partners, according to Dr. Ina Park, medical director of the California Prevention Training Center at the University of California San Francisco.
But federal and state public health efforts were hamstrung from the get-go. In 1999, the CDC said it would need about $35 million to $39 million in new federal funds annually for at least five years to eliminate syphilis. The agency got less than half of what it asked for, according to Jo Valentine, former program coordinator of the CDC’s Syphilis Elimination Effort. As cases rose, the CDC modified its goals in 2006 from 0.4 primary and secondary syphilis cases per 100,000 in population to 2.2 cases per 100,000. By 2013, as elimination seemed less and less viable, the CDC changed its focus to ending congenital syphilis only.
Since then, funding has remained anemic. From 2015 to 2020, the CDC’s budget for preventing sexually transmitted infections grew by 2.2%. Taking inflation into account, that’s a 7.4% reduction in purchasing power. In the same period, cases of syphilis, gonorrhea and chlamydia — the three STDs that have federally funded control programs — increased by nearly 30%.
“We have a long history of nearly eradicating something, then changing our attention, and seeing a resurgence in numbers,” said David Harvey, executive director of the National Coalition of STD Directors. “We have more congenital syphilis cases today in America than we ever had pediatric AIDS at the height of the AIDS epidemic. It’s heartbreaking.”
Adriane Casalotti, chief of government and public affairs at the National Association of County and City Health Officials, warns that the United States should not be surprised to see case counts continue to climb. “The bugs don’t go away,” she said. “They’re just waiting for the next opportunity, when you’re not paying attention.”
Ms. Yang waited until the end of the day, then called the clinic to see if Angelica had gone for her shot. She had not. Ms. Yang would have to block off another half day to visit Huron again, but she had three dozen other cases to deal with.
States in the South and West have seen the highest syphilis rates in recent years. In 2017, 64 babies in Fresno County were born with syphilis at a rate of 440 babies per 100,000 live births — about 19 times the national rate. While the county had managed to lower case counts in the two years that followed, the pandemic threatened to unravel that progress, forcing STD staffers to do COVID-19 contact tracing, pausing field visits to find infected people, and scaring patients from seeking care. Ms. Yang’s colleague handled three cases of stillbirth in 2020; in each, the woman was never diagnosed with syphilis because she feared catching the coronavirus and skipped prenatal care.
Ms. Yang, whose caseload peaked at 70 during a COVID-19 surge, knew she would not be able handle them all as thoroughly as she’d like to. “When I was being mentored by another investigator, he said: ‘You’re not a superhero. You can’t save everybody,’” she said. She prioritizes men who have sex with men, because there’s a higher prevalence of syphilis in that population, and pregnant people, because of the horrific consequences for babies.
The job of a disease intervention specialist isn’t for everyone: It means meeting patients whenever and wherever they are available — in the mop closet of a bus station, in a quiet parking lot — to inform them about the disease, to extract names of sex partners and to encourage treatment. Patients are often reluctant to talk. They can get belligerent, upset that “the government” has their personal information or shattered at the thought that a partner is likely cheating on them. Salaries typically start in the low $40,000s.
Jena Adams, Ms. Yang’s supervisor, has eight investigators working on HIV and syphilis. In the middle of 2020, she lost two and replaced them only recently. “It’s been exhausting,” Ms. Adams said. She has only one specialist who is trained to take blood samples in the field, crucial for guaranteeing that the partners of those who test positive for syphilis also get tested. Ms. Adams wants to get phlebotomy training for the rest of her staff, but it’s $2,000 per person. The department also doesn’t have anyone who can administer penicillin injections in the field; that would have been key when Ms. Yang met Angelica. For a while, a nurse who worked in the tuberculosis program would ride along to give penicillin shots on a volunteer basis. Then he, too, left the health department.
Much of the resources in public health trickle down from the CDC, which distributes money to states, which then parcel it out to counties. The CDC gets its budget from Congress, which tells the agency, by line item, exactly how much money it can spend to fight a disease or virus, in an uncommonly specific manner not seen in many other agencies. The decisions are often politically driven and can be detached from actual health needs.
When the House and Senate appropriations committees meet to decide how much the CDC will get for each line item, they are barraged by lobbyists for individual disease interests. Stephanie Arnold Pang, senior director of policy and government relations at the National Coalition of STD Directors, can pick out the groups by sight: breast cancer wears pink, Alzheimer’s goes in purple, multiple sclerosis comes in orange, HIV in red. STD prevention advocates, like herself, don a green ribbon, but they’re far outnumbered.
And unlike diseases that might already be familiar to lawmakers, or have patient and family spokespeople who can tell their own powerful stories, syphilis doesn’t have many willing poster children. “Congressmen don’t wake up one day and say, ‘Oh hey, there’s congenital syphilis in my jurisdiction.’ You have to raise awareness,” Arnold Pang said. It can be hard jockeying for a meeting. “Some offices might say, ‘I don’t have time for you because we’ve just seen HIV.’ ... Sometimes, it feels like you’re talking into a void.”
The consequences of the political nature of public health funding have become more obvious during the coronavirus pandemic. The 2014 Ebola epidemic was seen as a “global wakeup call” that the world wasn’t prepared for a major pandemic, yet in 2018, the CDC scaled back its epidemic prevention work as money ran out. “If you’ve got to choose between Alzheimer’s research and stopping an outbreak that may not happen? Stopping an outbreak that might not happen doesn’t do well,” said Dr. Frieden, the former CDC director. “The CDC needs to have more money and more flexible money. Otherwise, we’re going to be in this situation long term.”
In May 2021, President Joe Biden’s administration announced it would set aside $7.4 billion over the next five years to hire and train public health workers, including $1.1 billion for more disease intervention specialists like Ms. Yang. Public health officials are thrilled to have the chance to expand their workforce, but some worry the time horizon may be too short. “We’ve seen this movie before, right?” Dr. Frieden said. “Everyone gets concerned when there’s an outbreak, and when that outbreak stops, the headlines stop, and an economic downturn happens, the budget gets cut.”
Fresno’s STD clinic was shuttered in 2010 amid the Great Recession. Many others have vanished since the passage of the Affordable Care Act. Health leaders thought “by magically beefing up the primary care system, that we would do a better job of catching STIs and treating them,” said Mr. Harvey, the executive director of the National Coalition of STD Directors. That hasn’t worked out; people want access to anonymous services, and primary care doctors often don’t have STDs top of mind. The coalition is lobbying Congress for funding to support STD clinical services, proposing a three-year demonstration project funded at $600 million.
It’s one of Ms. Adams’ dreams to see Fresno’s STD clinic restored as it was. “You could come in for an HIV test and get other STDs checked,” she said. “And if a patient is positive, you can give a first injection on the spot.”
On Aug. 12, Ms. Yang set out for Huron again, speeding past groves of almond trees and fields of grapes in the department’s white Chevy Cruze. She brought along a colleague, Jorge Sevilla, who had recently transferred to the STD program from COVID-19 contact tracing. Ms. Yang was anxious to find Angelica again. “She’s probably in her second trimester now,” she said.
They found her outside of a pale yellow house a few blocks from the homeless encampment; the owner was letting her stay in a shed tucked in the corner of the dirt yard. This time, it was evident that she was pregnant. Ms. Yang noted that Angelica was wearing a wig; hair loss is a symptom of syphilis.
“Do you remember me?” Ms. Yang asked.
Angelica nodded. She didn’t seem surprised to see Ms. Yang again. (I came along, and Mr. Sevilla explained who I was and that I was writing about syphilis and the people affected by it. Angelica signed a release for me to report about her case, and she said she had no problem with me writing about her or even using her full name. ProPublica chose to only print her first name.)
“How are you doing? How’s the baby?”
“Bien.”
“So the last time we talked, we were going to have you go to United Healthcare Center to get treatment. Have you gone since?”
Angelica shook her head.
“We brought some gift cards...” Mr. Sevilla started in Spanish. The department uses them as incentives for completing injections. But Angelica was already shaking her head. The nearest Walmart was the next town over.
Ms. Yang turned to her partner. “Tell her: So the reason why we’re coming out here again is because we really need her to go in for treatment. ... We really are concerned for the baby’s health especially since she’s had the infection for quite a while.”
Angelica listened while Mr. Sevilla interpreted, her eyes on the ground. Then she looked up. “Orita?” she asked. Right now?
“I’ll walk with you,” Ms. Yang offered. Angelica shook her head. “She said she wants to shower first before she goes over there,” Mr. Sevilla said.
Ms. Yang made a face. “She said that to me last time.” Ms. Yang offered to wait, but Angelica didn’t want the health officers to linger by the house. She said she would meet them by the clinic in 15 minutes.
Ms. Yang was reluctant to let her go but again had no other option. She and Mr. Sevilla drove to the clinic, then stood on the corner of the parking lot, staring down the road.
Talk to the pediatricians, obstetricians, and families on the front lines of the congenital syphilis surge and it becomes clear why Ms. Yang and others are trying so desperately to prevent cases. Dr. J. B. Cantey, associate professor in pediatrics at UT Health San Antonio, remembers a baby girl born at 25 weeks gestation who weighed a pound and a half. Syphilis had spread through her bones and lungs. She spent five months in the neonatal intensive care unit, breathing through a ventilator, and was still eating through a tube when she was discharged.
Then, there are the miscarriages, the stillbirths and the inconsolable parents. Dr. Irene Stafford, an associate professor and maternal-fetal medicine specialist at UT Health in Houston, cannot forget a patient who came in at 36 weeks for a routine checkup, pregnant with her first child. Dr. Stafford realized that there was no heartbeat. “She could see on my face that something was really wrong,” Dr. Stafford recalled. She had to let the patient know that syphilis had killed her baby. “She was hysterical, just bawling,” Dr. Stafford said. “I’ve seen people’s families ripped apart and I’ve seen beautiful babies die.” Fewer than 10% of patients who experience a stillbirth are tested for syphilis, suggesting that cases are underdiagnosed.
A Texas grandmother named Solidad Odunuga offers a glimpse into what the future could hold for Angelica’s mother, who may wind up raising her baby.
In February of last year, Ms. Odunuga got a call from the Lyndon B. Johnson Hospital in Houston. A nurse told her that her daughter was about to give birth and that child protective services had been called. Ms. Odunuga had lost contact with her daughter, who struggled with homelessness and substance abuse. She arrived in time to see her grandson delivered, premature at 30 weeks old, weighing 2.7 pounds. He tested positive for syphilis.
When a child protective worker asked Ms. Odunuga to take custody of the infant, she felt a wave of dread. “I was in denial,” she recalled. “I did not plan to be a mom again.” The baby’s medical problems were daunting: “Global developmental delays ... concerns for visual impairments ... high risk of cerebral palsy,” read a note from the doctor at the time.
Still, Ms. Odunuga visited her grandson every day for three months, driving to the NICU from her job at the University of Houston. “I’d put him in my shirt to keep him warm and hold him there.” She fell in love. She named him Emmanuel.
Once Emmanuel was discharged, Ms. Odunuga realized she had no choice but to quit her job. While Medicaid covered the costs of Emmanuel’s treatment, it was on her to care for him. From infancy, Emmanuel’s life has been a whirlwind of constant therapy. Today, at 20 months old, Odunuga brings him to physical, occupational, speech, and developmental therapy, each a different appointment on a different day of the week.
Emmanuel has thrived beyond what his doctors predicted, toddling so fast that Ms. Odunuga can’t look away for a minute and beaming as he waves his favorite toy phone. Yet he still suffers from gagging issues, which means Ms. Odunuga can’t feed him any solid foods. Liquid gets into his lungs when he aspirates; it has led to pneumonia three times. Emmanuel has a special stroller that helps keep his head in a position that won’t aggravate his persistent reflux, but Odunuga said she still has to pull over on the side of the road sometimes when she hears him projectile vomiting from the backseat.
The days are endless. Once she puts Emmanuel to bed, Ms. Odunuga starts planning the next day’s appointments. “I’ve had to cry alone, scream out alone,” she said. “Sometimes I wake up and think, Is this real? And then I hear him in the next room.”
Putting aside the challenge of eliminating syphilis entirely, everyone agrees it’s both doable and necessary to prevent newborn cases. “There was a crisis in perinatal HIV almost 30 years ago and people stood up and said this is not OK — it’s not acceptable for babies to be born in that condition. ... [We] brought it down from 1,700 babies born each year with perinatal HIV to less than 40 per year today,” said Virginia Bowen, an epidemiologist at the CDC. “Now here we are with a slightly different condition. We can also stand up and say, ‘This is not acceptable.’” Belarus, Bermuda, Cuba, Malaysia, Thailand, and Sri Lanka are among countries recognized by the World Health Organization for eliminating congenital syphilis.
Success starts with filling gaps across the health care system.
For almost a century, public health experts have advocated for testing pregnant patients more than once for syphilis in order to catch the infection. But policies nationwide still don’t reflect this best practice. Six states have no prenatal screening requirement at all. Even in states that require three tests, public health officials say that many physicians aren’t aware of the requirements. Dr. Stafford, the maternal-fetal medicine specialist in Houston, says she’s tired of hearing her own peers in medicine tell her, “Oh, syphilis is a problem?”
It costs public health departments less than 25 cents a dose to buy penicillin, but for a private practice, it’s more than $1,000, according to Dr. Park of the University of California San Francisco. “There’s no incentive for a private physician to stock a dose that could expire before it’s used, so they often don’t have it. So a woman comes in, they say, ‘We’ll send you to the emergency department or health department to get it,’ then [the patients] don’t show up.”
A vaccine would be invaluable for preventing spread among people at high risk for reinfection. But there is none. Scientists only recently figured out how to grow the bacteria in the lab, prompting grants from the National Institutes of Health to fund research into a vaccine. Dr. Justin Radolf, a researcher at the University of Connecticut School of Medicine, said he hopes his team will have a vaccine candidate by the end of its five-year grant. But it’ll likely take years more to find a manufacturer and run human trials.
Public health agencies also need to recognize that many of the hurdles to getting pregnant people treated involve access to care, economic stability, safe housing and transportation. In Fresno, Ms. Adams has been working on ways her department can collaborate with mental health services. Recently, one of her disease intervention specialists managed to get a pregnant woman treated with penicillin shots and, at the patient’s request, connected her with an addiction treatment center.
Gaining a patient’s cooperation means seeing them as complex humans instead of just a case to solve. “There may be past traumas with the health care system,” said Cynthia Deverson, project manager of the Houston Fetal Infant Morbidity Review. “There’s the fear of being discovered if she’s doing something illegal to survive. ... She may need to be in a certain place at a certain time so she can get something to eat, or maybe it’s the only time of the day that’s safe for her to sleep. They’re not going to tell you that. Yes, they understand there’s a problem, but it’s not an immediate threat, maybe they don’t feel bad yet, so obviously this is not urgent. ...
“What helps to gain trust is consistency,” she said. “Literally, it’s seeing that [disease specialist] constantly, daily. ... The woman can see that you’re not going to harm her, you’re saying, ‘I’m here at this time if you need me.’”
Ms. Yang stood outside the clinic, waiting for Angelica to show up, baking in the 90-degree heat. Her feelings ranged from irritation — Why didn’t she just go? I’d have more energy for other cases — to an appreciation for the parts of Angelica’s story that she didn’t know — She’s in survival mode. I need to be more patient.
Fifteen minutes ticked by, then 20.
“OK,” Ms. Yang announced. “We’re going back.”
She asked Sevilla if he would be OK if they drove Angelica to the clinic; they technically weren’t supposed to because of coronavirus precautions, but Ms. Yang wasn’t sure she could convince Angelica to walk. Mr. Sevilla gave her the thumbs up.
When they pulled up, they saw Angelica sitting in the backyard, chatting with a friend. She now wore a fresh T-shirt and had shoes on her feet. Angelica sat silently in the back seat as Ms. Yang drove to the clinic. A few minutes later, they pulled up to the parking lot.
Finally, Ms. Yang thought. We got her here.
The clinic was packed with people waiting for COVID-19 tests and vaccinations. A worker there had previously told Ms. Yang that a walk-in would be fine, but a receptionist now said they were too busy to treat Angelica. She would have to return.
Ms. Yang felt a surge of frustration, sensing that her hard-fought opportunity was slipping away. She tried to talk to the nurse supervisor, but he wasn’t available. She tried to leave the gift cards at the office to reward Angelica if she came, but the receptionist said she couldn’t hold them. While Ms. Yang negotiated, Mr. Sevilla sat with Angelica in the car, waiting.
Finally, Ms. Yang accepted this was yet another thing she couldn’t control.
She drove Angelica back to the yellow house. As they arrived, she tried once more to impress on her just how important it was to get treated, asking Mr. Sevilla to interpret. “We don’t want it to get any more serious, because she can go blind, she could go deaf, she could lose her baby.”
Angelica already had the door halfway open.
“So on a scale from one to 10, how important is this to get treated?” Ms. Yang asked.
“Ten,” Angelica said. Ms. Yang reminded her of the appointment that afternoon. Then Angelica stepped out and returned to the dusty yard.
Ms. Yang lingered for a moment, watching Angelica go. Then she turned the car back onto the highway and set off toward Fresno, knowing, already, that she’d be back.
Postscript: A reporter visited Huron twice more in the months that followed, including once independently to try to interview Angelica, but she wasn’t in town. Ms. Yang has visited Huron twice more as well — six times in total thus far. In October, a couple of men at the yellow house said Angelica was still in town, still pregnant. Ms. Yang and Mr. Sevilla spent an hour driving around, talking to residents, hoping to catch Angelica. But she was nowhere to be found.
ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
This story was originally published on ProPublica and was co-published with NPR.
When Mai Yang is looking for a patient, she travels light. She dresses deliberately — not too formal, so she won’t be mistaken for a police officer; not too casual, so people will look past her tiny 4-foot-10 stature and youthful face and trust her with sensitive health information. Always, she wears closed-toed shoes, “just in case I need to run.”
Yang carries a stack of cards issued by the Centers for Disease Control and Prevention that show what happens when the Treponema pallidum bacteria invades a patient’s body. There’s a photo of an angry red sore on a penis. There’s one of a tongue, marred by mucus-lined lesions. And there’s one of a newborn baby, its belly, torso and thighs dotted in a rash, its mouth open, as if caught midcry.
It was because of the prospect of one such baby that Yang found herself walking through a homeless encampment on a blazing July day in Huron, Calif., an hour’s drive southwest of her office at the Fresno County Department of Public Health. She was looking for a pregnant woman named Angelica, whose visit to a community clinic had triggered a report to the health department’s sexually transmitted disease program. Angelica had tested positive for syphilis. If she was not treated, her baby could end up like the one in the picture or worse — there was a 40% chance the baby would die.
Ms. Yang knew, though, that if she helped Angelica get treated with three weekly shots of penicillin at least 30 days before she gave birth, it was likely that the infection would be wiped out and her baby would be born without any symptoms at all. Every case of congenital syphilis, when a baby is born with the disease, is avoidable. Each is considered a “sentinel event,” a warning that the public health system is failing.
The alarms are now clamoring. In the United States, more than 129,800 syphilis cases were recorded in 2019, double the case count of five years prior. In the same time period, cases of congenital syphilis quadrupled: 1,870 babies were born with the disease; 128 died. Case counts from 2020 are still being finalized, but the CDC has said that reported cases of congenital syphilis have already exceeded the prior year. Black, Hispanic, and Native American babies are disproportionately at risk.
There was a time, not too long ago, when CDC officials thought they could eliminate the centuries-old scourge from the United States, for adults and babies. But the effort lost steam and cases soon crept up again. Syphilis is not an outlier. The United States goes through what former CDC director Dr. Tom Frieden calls “a deadly cycle of panic and neglect” in which emergencies propel officials to scramble and throw money at a problem — whether that’s Ebola, Zika, or COVID-19. Then, as fear ebbs, so does the attention and motivation to finish the task.
The last fraction of cases can be the hardest to solve, whether that’s eradicating a bug or getting vaccines into arms, yet too often, that’s exactly when political attention gets diverted to the next alarm. The result: The hardest to reach and most vulnerable populations are the ones left suffering, after everyone else looks away.
Ms. Yang first received Angelica’s lab report on June 17. The address listed was a P.O. box, and the phone number belonged to her sister, who said Angelica was living in Huron. That was a piece of luck: Huron is tiny; the city spans just 1.6 square miles. On her first visit, a worker at the Alamo Motel said she knew Angelica and directed Ms. Yang to a nearby homeless encampment. Angelica wasn’t there, so Ms. Yang returned a second time, bringing one of the health department nurses who could serve as an interpreter.
They made their way to the barren patch of land behind Huron Valley Foods, the local grocery store, where people took shelter in makeshift lean-tos composed of cardboard boxes, scrap wood ,and scavenged furniture, draped with sheets that served as ceilings and curtains. Yang stopped outside one of the structures, calling a greeting.
“Hi, I’m from the health department, I’m looking for Angelica.”
The nurse echoed her in Spanish.
Angelica emerged, squinting in the sunlight. Ms. Yang couldn’t tell if she was visibly pregnant yet, as her body was obscured by an oversized shirt. The two women were about the same age: Ms. Yang 26 and Angelica 27. Ms. Yang led her away from the tent, so they could speak privately. Angelica seemed reticent, surprised by the sudden appearance of the two health officers. “You’re not in trouble,” Ms. Yang said, before revealing the results of her blood test.
Angelica had never heard of syphilis.
“Have you been to prenatal care?”
Angelica shook her head. The local clinic had referred her to an obstetrician in Hanford, a 30-minute drive away. She had no car. She also mentioned that she didn’t intend to raise her baby; her two oldest children lived with her mother, and this one likely would, too.
Ms. Yang pulled out the CDC cards, showing them to Angelica and asking if she had experienced any of the symptoms illustrated. No, Angelica said, her lips pursed with disgust.
“Right now you still feel healthy, but this bacteria is still in your body,” Ms. Yang pressed. “You need to get the infection treated to prevent further health complications to yourself and your baby.”
The community clinic was just across the street. “Can we walk you over to the clinic and make sure you get seen so we can get this taken care of?”
Angelica demurred. She said she hadn’t showered for a week and wanted to wash up first. She said she’d go later.
Ms. Yang tried once more to extract a promise: “What time do you think you’ll go?”
“Today, for sure.”
Syphilis is called The Great Imitator: It can look like any number of diseases. In its first stage, the only evidence of infection is a painless sore at the bacteria’s point of entry. Weeks later, as the bacteria multiplies, skin rashes bloom on the palms of the hands and bottoms of the feet. Other traits of this stage include fever, headaches, muscle aches, sore throat, and fatigue. These symptoms eventually disappear and the patient progresses into the latent phase, which betrays no external signs. But if left untreated, after a decade or more, syphilis will reemerge in up to 30% of patients, capable of wreaking horror on a wide range of organ systems. Dr. Marion Sims, president of the American Medical Association in 1876, called it a “terrible scourge, which begins with lamb-like mildness and ends with lion-like rage that ruthlessly destroys everything in its way.”
The corkscrew-shaped bacteria can infiltrate the nervous system at any stage of the infection. Ms. Yang is haunted by her memory of interviewing a young man whose dementia was so severe that he didn’t know why he was in the hospital or how old he was. And regardless of symptoms or stage, the bacteria can penetrate the placenta to infect a fetus. Even in these cases the infection is unpredictable: Many babies are born with normal physical features, but others can have deformed bones or damaged brains, and they can struggle to hear, see, or breathe.
From its earliest days, syphilis has been shrouded in stigma. The first recorded outbreak was in the late 15th century, when Charles VIII led the French army to invade Naples. Italian physicians described French soldiers covered with pustules, dying from a sexually transmitted disease. As the affliction spread, Italians called it the French Disease. The French blamed the Neopolitans. It was also called the German, Polish, or Spanish disease, depending on which neighbor one wanted to blame. Even its name bears the taint of divine judgement: It comes from a 16th-century poem that tells of a shepherd, Syphilus, who offended the god Apollo and was punished with a hideous disease.
By 1937 in America, when former Surgeon General Thomas Parran wrote the book “Shadow on the Land,” he estimated some 680,000 people were under treatment for syphilis; about 60,000 babies were being born annually with congenital syphilis. There was no cure, and the stigma was so strong that public health officials feared even properly documenting cases.
Thanks to Dr. Parran’s ardent advocacy, Congress in 1938 passed the National Venereal Disease Control Act, which created grants for states to set up clinics and support testing and treatment. Other than a short-lived funding effort during World War I, this was the first coordinated federal push to respond to the disease.
Around the same time, the Public Health Service launched an effort to record the natural history of syphilis. Situated in Tuskegee, Ala., the infamous study recruited 600 black men. By the early 1940s, penicillin became widely available and was found to be a reliable cure, but the treatment was withheld from the study participants. Outrage over the ethical violations would cast a stain across syphilis research for decades to come and fuel generations of mistrust in the medical system among Black Americans that continues to this day.
With the introduction of penicillin, cases began to plummet. Twice, the CDC has announced efforts to wipe out the disease — once in the 1960s and again in 1999.
In the latest effort, the CDC announced that the United States had “a unique opportunity to eliminate syphilis within its borders,” thanks to historically low rates, with 80% of counties reporting zero cases. The concentration of cases in the South “identifies communities in which there is a fundamental failure of public health capacity,” the agency noted, adding that elimination — which it defined as fewer than 1,000 cases a year — would “decrease one of our most glaring racial disparities in health.”
Two years after the campaign began, cases started climbing, first among gay men and later, heterosexuals. Cases in women started accelerating in 2013, followed shortly by increasing numbers of babies born with syphilis.The reasons for failure are complex; people relaxed safer sex practices after the advent of potent HIV combination therapies, increased methamphetamine use drove riskier behavior and an explosion of online dating made it hard to track and test sexual partners, according to Dr. Ina Park, medical director of the California Prevention Training Center at the University of California San Francisco.
But federal and state public health efforts were hamstrung from the get-go. In 1999, the CDC said it would need about $35 million to $39 million in new federal funds annually for at least five years to eliminate syphilis. The agency got less than half of what it asked for, according to Jo Valentine, former program coordinator of the CDC’s Syphilis Elimination Effort. As cases rose, the CDC modified its goals in 2006 from 0.4 primary and secondary syphilis cases per 100,000 in population to 2.2 cases per 100,000. By 2013, as elimination seemed less and less viable, the CDC changed its focus to ending congenital syphilis only.
Since then, funding has remained anemic. From 2015 to 2020, the CDC’s budget for preventing sexually transmitted infections grew by 2.2%. Taking inflation into account, that’s a 7.4% reduction in purchasing power. In the same period, cases of syphilis, gonorrhea and chlamydia — the three STDs that have federally funded control programs — increased by nearly 30%.
“We have a long history of nearly eradicating something, then changing our attention, and seeing a resurgence in numbers,” said David Harvey, executive director of the National Coalition of STD Directors. “We have more congenital syphilis cases today in America than we ever had pediatric AIDS at the height of the AIDS epidemic. It’s heartbreaking.”
Adriane Casalotti, chief of government and public affairs at the National Association of County and City Health Officials, warns that the United States should not be surprised to see case counts continue to climb. “The bugs don’t go away,” she said. “They’re just waiting for the next opportunity, when you’re not paying attention.”
Ms. Yang waited until the end of the day, then called the clinic to see if Angelica had gone for her shot. She had not. Ms. Yang would have to block off another half day to visit Huron again, but she had three dozen other cases to deal with.
States in the South and West have seen the highest syphilis rates in recent years. In 2017, 64 babies in Fresno County were born with syphilis at a rate of 440 babies per 100,000 live births — about 19 times the national rate. While the county had managed to lower case counts in the two years that followed, the pandemic threatened to unravel that progress, forcing STD staffers to do COVID-19 contact tracing, pausing field visits to find infected people, and scaring patients from seeking care. Ms. Yang’s colleague handled three cases of stillbirth in 2020; in each, the woman was never diagnosed with syphilis because she feared catching the coronavirus and skipped prenatal care.
Ms. Yang, whose caseload peaked at 70 during a COVID-19 surge, knew she would not be able handle them all as thoroughly as she’d like to. “When I was being mentored by another investigator, he said: ‘You’re not a superhero. You can’t save everybody,’” she said. She prioritizes men who have sex with men, because there’s a higher prevalence of syphilis in that population, and pregnant people, because of the horrific consequences for babies.
The job of a disease intervention specialist isn’t for everyone: It means meeting patients whenever and wherever they are available — in the mop closet of a bus station, in a quiet parking lot — to inform them about the disease, to extract names of sex partners and to encourage treatment. Patients are often reluctant to talk. They can get belligerent, upset that “the government” has their personal information or shattered at the thought that a partner is likely cheating on them. Salaries typically start in the low $40,000s.
Jena Adams, Ms. Yang’s supervisor, has eight investigators working on HIV and syphilis. In the middle of 2020, she lost two and replaced them only recently. “It’s been exhausting,” Ms. Adams said. She has only one specialist who is trained to take blood samples in the field, crucial for guaranteeing that the partners of those who test positive for syphilis also get tested. Ms. Adams wants to get phlebotomy training for the rest of her staff, but it’s $2,000 per person. The department also doesn’t have anyone who can administer penicillin injections in the field; that would have been key when Ms. Yang met Angelica. For a while, a nurse who worked in the tuberculosis program would ride along to give penicillin shots on a volunteer basis. Then he, too, left the health department.
Much of the resources in public health trickle down from the CDC, which distributes money to states, which then parcel it out to counties. The CDC gets its budget from Congress, which tells the agency, by line item, exactly how much money it can spend to fight a disease or virus, in an uncommonly specific manner not seen in many other agencies. The decisions are often politically driven and can be detached from actual health needs.
When the House and Senate appropriations committees meet to decide how much the CDC will get for each line item, they are barraged by lobbyists for individual disease interests. Stephanie Arnold Pang, senior director of policy and government relations at the National Coalition of STD Directors, can pick out the groups by sight: breast cancer wears pink, Alzheimer’s goes in purple, multiple sclerosis comes in orange, HIV in red. STD prevention advocates, like herself, don a green ribbon, but they’re far outnumbered.
And unlike diseases that might already be familiar to lawmakers, or have patient and family spokespeople who can tell their own powerful stories, syphilis doesn’t have many willing poster children. “Congressmen don’t wake up one day and say, ‘Oh hey, there’s congenital syphilis in my jurisdiction.’ You have to raise awareness,” Arnold Pang said. It can be hard jockeying for a meeting. “Some offices might say, ‘I don’t have time for you because we’ve just seen HIV.’ ... Sometimes, it feels like you’re talking into a void.”
The consequences of the political nature of public health funding have become more obvious during the coronavirus pandemic. The 2014 Ebola epidemic was seen as a “global wakeup call” that the world wasn’t prepared for a major pandemic, yet in 2018, the CDC scaled back its epidemic prevention work as money ran out. “If you’ve got to choose between Alzheimer’s research and stopping an outbreak that may not happen? Stopping an outbreak that might not happen doesn’t do well,” said Dr. Frieden, the former CDC director. “The CDC needs to have more money and more flexible money. Otherwise, we’re going to be in this situation long term.”
In May 2021, President Joe Biden’s administration announced it would set aside $7.4 billion over the next five years to hire and train public health workers, including $1.1 billion for more disease intervention specialists like Ms. Yang. Public health officials are thrilled to have the chance to expand their workforce, but some worry the time horizon may be too short. “We’ve seen this movie before, right?” Dr. Frieden said. “Everyone gets concerned when there’s an outbreak, and when that outbreak stops, the headlines stop, and an economic downturn happens, the budget gets cut.”
Fresno’s STD clinic was shuttered in 2010 amid the Great Recession. Many others have vanished since the passage of the Affordable Care Act. Health leaders thought “by magically beefing up the primary care system, that we would do a better job of catching STIs and treating them,” said Mr. Harvey, the executive director of the National Coalition of STD Directors. That hasn’t worked out; people want access to anonymous services, and primary care doctors often don’t have STDs top of mind. The coalition is lobbying Congress for funding to support STD clinical services, proposing a three-year demonstration project funded at $600 million.
It’s one of Ms. Adams’ dreams to see Fresno’s STD clinic restored as it was. “You could come in for an HIV test and get other STDs checked,” she said. “And if a patient is positive, you can give a first injection on the spot.”
On Aug. 12, Ms. Yang set out for Huron again, speeding past groves of almond trees and fields of grapes in the department’s white Chevy Cruze. She brought along a colleague, Jorge Sevilla, who had recently transferred to the STD program from COVID-19 contact tracing. Ms. Yang was anxious to find Angelica again. “She’s probably in her second trimester now,” she said.
They found her outside of a pale yellow house a few blocks from the homeless encampment; the owner was letting her stay in a shed tucked in the corner of the dirt yard. This time, it was evident that she was pregnant. Ms. Yang noted that Angelica was wearing a wig; hair loss is a symptom of syphilis.
“Do you remember me?” Ms. Yang asked.
Angelica nodded. She didn’t seem surprised to see Ms. Yang again. (I came along, and Mr. Sevilla explained who I was and that I was writing about syphilis and the people affected by it. Angelica signed a release for me to report about her case, and she said she had no problem with me writing about her or even using her full name. ProPublica chose to only print her first name.)
“How are you doing? How’s the baby?”
“Bien.”
“So the last time we talked, we were going to have you go to United Healthcare Center to get treatment. Have you gone since?”
Angelica shook her head.
“We brought some gift cards...” Mr. Sevilla started in Spanish. The department uses them as incentives for completing injections. But Angelica was already shaking her head. The nearest Walmart was the next town over.
Ms. Yang turned to her partner. “Tell her: So the reason why we’re coming out here again is because we really need her to go in for treatment. ... We really are concerned for the baby’s health especially since she’s had the infection for quite a while.”
Angelica listened while Mr. Sevilla interpreted, her eyes on the ground. Then she looked up. “Orita?” she asked. Right now?
“I’ll walk with you,” Ms. Yang offered. Angelica shook her head. “She said she wants to shower first before she goes over there,” Mr. Sevilla said.
Ms. Yang made a face. “She said that to me last time.” Ms. Yang offered to wait, but Angelica didn’t want the health officers to linger by the house. She said she would meet them by the clinic in 15 minutes.
Ms. Yang was reluctant to let her go but again had no other option. She and Mr. Sevilla drove to the clinic, then stood on the corner of the parking lot, staring down the road.
Talk to the pediatricians, obstetricians, and families on the front lines of the congenital syphilis surge and it becomes clear why Ms. Yang and others are trying so desperately to prevent cases. Dr. J. B. Cantey, associate professor in pediatrics at UT Health San Antonio, remembers a baby girl born at 25 weeks gestation who weighed a pound and a half. Syphilis had spread through her bones and lungs. She spent five months in the neonatal intensive care unit, breathing through a ventilator, and was still eating through a tube when she was discharged.
Then, there are the miscarriages, the stillbirths and the inconsolable parents. Dr. Irene Stafford, an associate professor and maternal-fetal medicine specialist at UT Health in Houston, cannot forget a patient who came in at 36 weeks for a routine checkup, pregnant with her first child. Dr. Stafford realized that there was no heartbeat. “She could see on my face that something was really wrong,” Dr. Stafford recalled. She had to let the patient know that syphilis had killed her baby. “She was hysterical, just bawling,” Dr. Stafford said. “I’ve seen people’s families ripped apart and I’ve seen beautiful babies die.” Fewer than 10% of patients who experience a stillbirth are tested for syphilis, suggesting that cases are underdiagnosed.
A Texas grandmother named Solidad Odunuga offers a glimpse into what the future could hold for Angelica’s mother, who may wind up raising her baby.
In February of last year, Ms. Odunuga got a call from the Lyndon B. Johnson Hospital in Houston. A nurse told her that her daughter was about to give birth and that child protective services had been called. Ms. Odunuga had lost contact with her daughter, who struggled with homelessness and substance abuse. She arrived in time to see her grandson delivered, premature at 30 weeks old, weighing 2.7 pounds. He tested positive for syphilis.
When a child protective worker asked Ms. Odunuga to take custody of the infant, she felt a wave of dread. “I was in denial,” she recalled. “I did not plan to be a mom again.” The baby’s medical problems were daunting: “Global developmental delays ... concerns for visual impairments ... high risk of cerebral palsy,” read a note from the doctor at the time.
Still, Ms. Odunuga visited her grandson every day for three months, driving to the NICU from her job at the University of Houston. “I’d put him in my shirt to keep him warm and hold him there.” She fell in love. She named him Emmanuel.
Once Emmanuel was discharged, Ms. Odunuga realized she had no choice but to quit her job. While Medicaid covered the costs of Emmanuel’s treatment, it was on her to care for him. From infancy, Emmanuel’s life has been a whirlwind of constant therapy. Today, at 20 months old, Odunuga brings him to physical, occupational, speech, and developmental therapy, each a different appointment on a different day of the week.
Emmanuel has thrived beyond what his doctors predicted, toddling so fast that Ms. Odunuga can’t look away for a minute and beaming as he waves his favorite toy phone. Yet he still suffers from gagging issues, which means Ms. Odunuga can’t feed him any solid foods. Liquid gets into his lungs when he aspirates; it has led to pneumonia three times. Emmanuel has a special stroller that helps keep his head in a position that won’t aggravate his persistent reflux, but Odunuga said she still has to pull over on the side of the road sometimes when she hears him projectile vomiting from the backseat.
The days are endless. Once she puts Emmanuel to bed, Ms. Odunuga starts planning the next day’s appointments. “I’ve had to cry alone, scream out alone,” she said. “Sometimes I wake up and think, Is this real? And then I hear him in the next room.”
Putting aside the challenge of eliminating syphilis entirely, everyone agrees it’s both doable and necessary to prevent newborn cases. “There was a crisis in perinatal HIV almost 30 years ago and people stood up and said this is not OK — it’s not acceptable for babies to be born in that condition. ... [We] brought it down from 1,700 babies born each year with perinatal HIV to less than 40 per year today,” said Virginia Bowen, an epidemiologist at the CDC. “Now here we are with a slightly different condition. We can also stand up and say, ‘This is not acceptable.’” Belarus, Bermuda, Cuba, Malaysia, Thailand, and Sri Lanka are among countries recognized by the World Health Organization for eliminating congenital syphilis.
Success starts with filling gaps across the health care system.
For almost a century, public health experts have advocated for testing pregnant patients more than once for syphilis in order to catch the infection. But policies nationwide still don’t reflect this best practice. Six states have no prenatal screening requirement at all. Even in states that require three tests, public health officials say that many physicians aren’t aware of the requirements. Dr. Stafford, the maternal-fetal medicine specialist in Houston, says she’s tired of hearing her own peers in medicine tell her, “Oh, syphilis is a problem?”
It costs public health departments less than 25 cents a dose to buy penicillin, but for a private practice, it’s more than $1,000, according to Dr. Park of the University of California San Francisco. “There’s no incentive for a private physician to stock a dose that could expire before it’s used, so they often don’t have it. So a woman comes in, they say, ‘We’ll send you to the emergency department or health department to get it,’ then [the patients] don’t show up.”
A vaccine would be invaluable for preventing spread among people at high risk for reinfection. But there is none. Scientists only recently figured out how to grow the bacteria in the lab, prompting grants from the National Institutes of Health to fund research into a vaccine. Dr. Justin Radolf, a researcher at the University of Connecticut School of Medicine, said he hopes his team will have a vaccine candidate by the end of its five-year grant. But it’ll likely take years more to find a manufacturer and run human trials.
Public health agencies also need to recognize that many of the hurdles to getting pregnant people treated involve access to care, economic stability, safe housing and transportation. In Fresno, Ms. Adams has been working on ways her department can collaborate with mental health services. Recently, one of her disease intervention specialists managed to get a pregnant woman treated with penicillin shots and, at the patient’s request, connected her with an addiction treatment center.
Gaining a patient’s cooperation means seeing them as complex humans instead of just a case to solve. “There may be past traumas with the health care system,” said Cynthia Deverson, project manager of the Houston Fetal Infant Morbidity Review. “There’s the fear of being discovered if she’s doing something illegal to survive. ... She may need to be in a certain place at a certain time so she can get something to eat, or maybe it’s the only time of the day that’s safe for her to sleep. They’re not going to tell you that. Yes, they understand there’s a problem, but it’s not an immediate threat, maybe they don’t feel bad yet, so obviously this is not urgent. ...
“What helps to gain trust is consistency,” she said. “Literally, it’s seeing that [disease specialist] constantly, daily. ... The woman can see that you’re not going to harm her, you’re saying, ‘I’m here at this time if you need me.’”
Ms. Yang stood outside the clinic, waiting for Angelica to show up, baking in the 90-degree heat. Her feelings ranged from irritation — Why didn’t she just go? I’d have more energy for other cases — to an appreciation for the parts of Angelica’s story that she didn’t know — She’s in survival mode. I need to be more patient.
Fifteen minutes ticked by, then 20.
“OK,” Ms. Yang announced. “We’re going back.”
She asked Sevilla if he would be OK if they drove Angelica to the clinic; they technically weren’t supposed to because of coronavirus precautions, but Ms. Yang wasn’t sure she could convince Angelica to walk. Mr. Sevilla gave her the thumbs up.
When they pulled up, they saw Angelica sitting in the backyard, chatting with a friend. She now wore a fresh T-shirt and had shoes on her feet. Angelica sat silently in the back seat as Ms. Yang drove to the clinic. A few minutes later, they pulled up to the parking lot.
Finally, Ms. Yang thought. We got her here.
The clinic was packed with people waiting for COVID-19 tests and vaccinations. A worker there had previously told Ms. Yang that a walk-in would be fine, but a receptionist now said they were too busy to treat Angelica. She would have to return.
Ms. Yang felt a surge of frustration, sensing that her hard-fought opportunity was slipping away. She tried to talk to the nurse supervisor, but he wasn’t available. She tried to leave the gift cards at the office to reward Angelica if she came, but the receptionist said she couldn’t hold them. While Ms. Yang negotiated, Mr. Sevilla sat with Angelica in the car, waiting.
Finally, Ms. Yang accepted this was yet another thing she couldn’t control.
She drove Angelica back to the yellow house. As they arrived, she tried once more to impress on her just how important it was to get treated, asking Mr. Sevilla to interpret. “We don’t want it to get any more serious, because she can go blind, she could go deaf, she could lose her baby.”
Angelica already had the door halfway open.
“So on a scale from one to 10, how important is this to get treated?” Ms. Yang asked.
“Ten,” Angelica said. Ms. Yang reminded her of the appointment that afternoon. Then Angelica stepped out and returned to the dusty yard.
Ms. Yang lingered for a moment, watching Angelica go. Then she turned the car back onto the highway and set off toward Fresno, knowing, already, that she’d be back.
Postscript: A reporter visited Huron twice more in the months that followed, including once independently to try to interview Angelica, but she wasn’t in town. Ms. Yang has visited Huron twice more as well — six times in total thus far. In October, a couple of men at the yellow house said Angelica was still in town, still pregnant. Ms. Yang and Mr. Sevilla spent an hour driving around, talking to residents, hoping to catch Angelica. But she was nowhere to be found.
ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Health care unaffordability common for pregnant/postpartum women
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
FROM JAMA NETWORK OPEN
Higher odds for preterm, C-section births seen in women with PsA
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stress, depression during pregnancy can harm child
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
Unvaccinated pregnant women have more severe COVID
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
Risk-based antenatal type-and-screen blood testing safe and economical
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
FROM OBSTETRICS & GYNECOLOGY
How 100 years of insulin have changed pregnancy for women with type 1 diabetes
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%
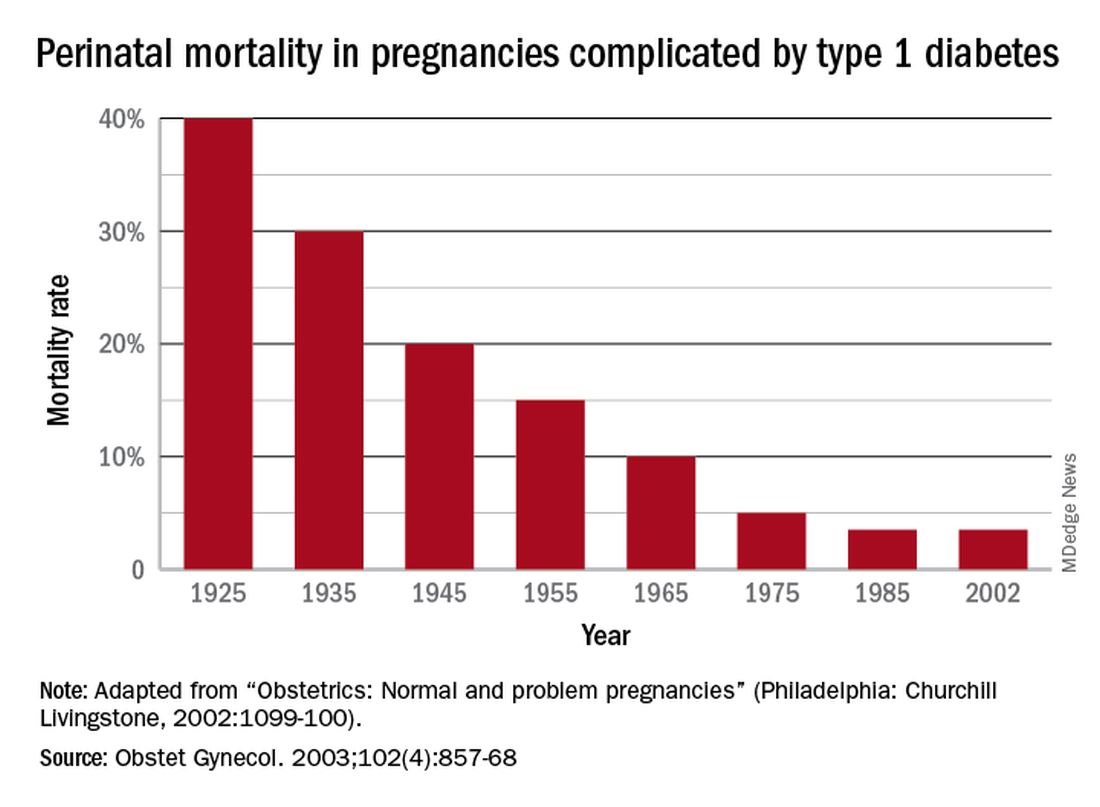
In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.
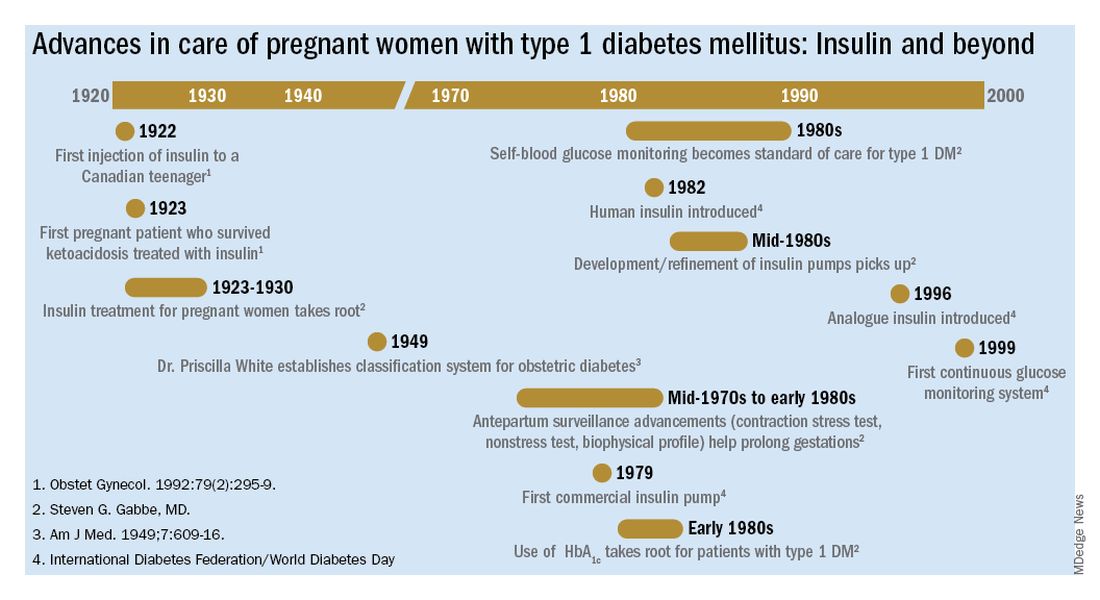
Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Insulin in pregnancy: A look back at history for Diabetes Awareness Month
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Your patient’s medication label lacks human safety information: What now?
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.











