User login
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
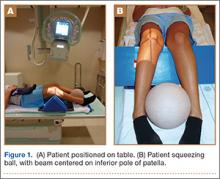
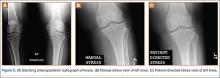
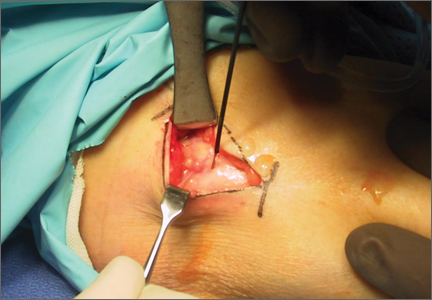
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Isolated Brachialis Muscle Atrophy
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
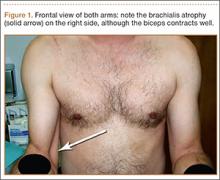
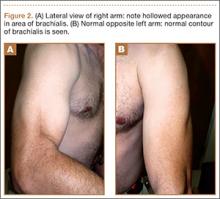
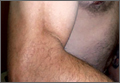
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Concomitant Ulnar Styloid Fracture and Distal Radius Fracture Portend Poorer Outcome
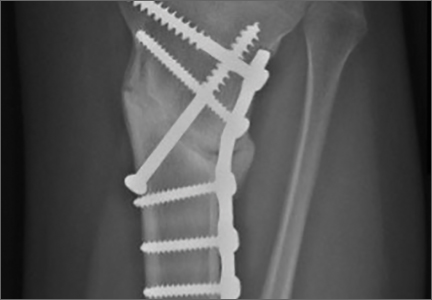
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Radiofrequency Microtenotomy for Elbow Epicondylitis: Midterm Results
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Pigmented Villonodular Synovitis of the Hip: A Systematic Review
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Giant Bone Island of the Tibia in a Child
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
Compartment Syndrome in Children: Diagnosis and Management
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
Complete Closing Wedge Osteotomy for Correction of Blount Disease (Tibia Vara): A Technique
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
The Changing Face of Pediatric Orthopedics
In my 16 years of practice, there has been tremendous change in the field of pediatric orthopedics in both demographics and scope of practice. Because of scientific and technological advances, efforts of the Pediatric Orthopaedic Society of North America (POSNA), and a changing workforce, the nature of pediatric orthopedics is changing dramatically and will continue to do so.
In the late 1990s, a “typical” pediatric orthopedic surgeon was treating fractures, developmental dysplasia of the hip, clubfeet, and other congenital deformities. Surgery for adolescent idiopathic scoliosis was moving toward anterior instrumentation and correction of the spine. The concepts of early-onset scoliosis and thoracic insufficiency syndrome were in their infancy. Children with anterior cruciate ligament tears were treated with braces until skeletal maturity, often leading to life-altering meniscal pathology. Essential medical treatments for genetic conditions, including bisphosphonates for osteogenesis imperfecta and corticosteroids for Duchenne muscular dystrophy, were considered experimental.
The field itself also was at a crossroads. In 1993, there were 410 active members in POSNA (vs 653 in 2014), and the vast majority were male.1 In the late 1990s, there were approximately 30 pediatric fellowship spots and 10 fellows being trained per year. Simultaneously, approximately 20 to 30 active POSNA members were retiring annually, leading to a projected shortage of pediatric orthopedic surgeons.1 A 2007 American Orthopaedic Association survey found that 59% of members believed that pediatric orthopedics was the most underserved specialty for a variety of reasons, including perceived lower reimbursement, higher volume of nonoperative treatment, and lifestyle issues (such as on-call burden).2
Owing in part to efforts of POSNA in resident/fellow education and mentorship, the practice of pediatric orthopedics in 2016 is dramatically different from a decade ago. The number of fellowship programs has increased to 44 programs, offering a total of 71 fellowship spots, of which 60 were filled by US applicants in 2014. Interestingly, the current active membership of POSNA is 19% female, and the 2014 fellowship class was 34% female. This is in contrast to the 4.4% of all AAOS members who are female. If current trends continue, POSNA could be 40% female by 2025 as senior, predominantly male members retire.1
Pediatric orthopedic practice in 2016 is also dramatically different owing to the development of subspecialization in areas of pediatric sports medicine, hand surgery, trauma, and the treatment of adolescent hip pathology. In fact, a recent survey of fellowship graduates showed that 30% of graduating fellows were going to do a second fellowship.3
While technological advances have driven the care of many pediatric orthopedic conditions such as spinal deformity and sports injuries, there also has been a resurgence of interest in the nonoperative treatment of clubfeet using the Ponseti method and of early-onset scoliosis using Mehta casting. Children with clubfeet even a decade ago were being treated with wide comprehensive releases and capsulotomies, leading to stiff painful feet as young adults. Now comprehensive releases are rarely used. Owing to advances in posterior spinal instrumentation as well as studies showing some decline in pulmonary function after thoracotomy and anterior spinal fusion, the treatment of adolescent scoliosis is predominantly done through the posterior approach. Advances in screening have led to a dramatic decrease in the surgical treatment of hip dysplasia. Medical treatment, such as corticosteroids for Duchenne muscular dystrophy, has prolonged length of life and improved quality of life as well as decreased the number of spinal fusions performed. Recombinant factor replacement for hemophilia has almost eliminated the horrible morbidity associated with hemophilic arthropathy and the need for synovectomy, arthrodesis, and arthroplasty, as well as the infectious issues, such as human immunodeficiency virus (HIV) and hepatitis, associated with the use of pooled blood products. The use of growth-friendly spinal implants, such as the Vertical Expandable Prosthetic Titanium Rib (VEPTR; DePuy Synthes), magnetically driven growing rods (MAGEC; Ellipse), and spinal tethers have improved pulmonary outcomes and presumably life expectancy in young patients with early-onset scoliosis who a decade ago may have had an in situ spinal fusion. These are just a few examples, and there are many more.
The articles in this issue highlight some of these changes. Tibial osteotomy and deformity correction, as described in the article by Burton and Hennrikus (pages 16-18), are classic techniques used by pediatric orthopedists over the past decades and will continue to be useful. The article by Hosseinzadeh and Talwalkar (pages 19-22) reviews unique aspects of pediatric compartment syndrome. While the basic concepts of compartment syndrome have not changed, the signs of compartment syndrome, the 5 Ps we all learned a decade ago (pain, paresthesia, paralysis, pallor, and pulselessness) have now been replaced in children with the 3 As (increasing analgesia, anxiety, and agitation). Finally, the article by Sferopoulos (pages 38-41) describing a case of a giant bone island in a child reminds us that we have a lot more to learn as pediatric orthopedists regarding the molecular nature and cause of disease.
The next few years will continue to be an exciting and dynamic time in the field of pediatric orthopedics. Not only is the workforce itself changing and growing, but so are the definitions of what a pediatric orthopedic surgeon is and does. While subspecialization is the trend in most aspects of medicine, it will be important to continue to monitor<hl name="1"/> this trend to ensure that pediatric orthopedics does not become too highly specialized. With the tremendous inflow of new talent, ideas, and technology, the future for pediatric orthopedics has never looked brighter.
References
1. Sawyer JR, Jones KC, Copley LA, Chambers S; POSNA Practice Management Committee. Pediatric orthopaedic workforce in 2014: current workforce and projections for the future [published online ahead of print October 30, 2015]. J Pediatr Orthop.
2. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
3. Glotzbecker MP, Shore BJ, Fletcher ND, Larson AN, Hydorn CR, Sawyer JR; Practice Management Committee of the Pediatric Orthopaedic Society of North America. Early career experience of pediatric orthopaedic fellows: what to expect and need for their services [published online ahead of print March 3, 2015]. J Pediatr Orthop.
In my 16 years of practice, there has been tremendous change in the field of pediatric orthopedics in both demographics and scope of practice. Because of scientific and technological advances, efforts of the Pediatric Orthopaedic Society of North America (POSNA), and a changing workforce, the nature of pediatric orthopedics is changing dramatically and will continue to do so.
In the late 1990s, a “typical” pediatric orthopedic surgeon was treating fractures, developmental dysplasia of the hip, clubfeet, and other congenital deformities. Surgery for adolescent idiopathic scoliosis was moving toward anterior instrumentation and correction of the spine. The concepts of early-onset scoliosis and thoracic insufficiency syndrome were in their infancy. Children with anterior cruciate ligament tears were treated with braces until skeletal maturity, often leading to life-altering meniscal pathology. Essential medical treatments for genetic conditions, including bisphosphonates for osteogenesis imperfecta and corticosteroids for Duchenne muscular dystrophy, were considered experimental.
The field itself also was at a crossroads. In 1993, there were 410 active members in POSNA (vs 653 in 2014), and the vast majority were male.1 In the late 1990s, there were approximately 30 pediatric fellowship spots and 10 fellows being trained per year. Simultaneously, approximately 20 to 30 active POSNA members were retiring annually, leading to a projected shortage of pediatric orthopedic surgeons.1 A 2007 American Orthopaedic Association survey found that 59% of members believed that pediatric orthopedics was the most underserved specialty for a variety of reasons, including perceived lower reimbursement, higher volume of nonoperative treatment, and lifestyle issues (such as on-call burden).2
Owing in part to efforts of POSNA in resident/fellow education and mentorship, the practice of pediatric orthopedics in 2016 is dramatically different from a decade ago. The number of fellowship programs has increased to 44 programs, offering a total of 71 fellowship spots, of which 60 were filled by US applicants in 2014. Interestingly, the current active membership of POSNA is 19% female, and the 2014 fellowship class was 34% female. This is in contrast to the 4.4% of all AAOS members who are female. If current trends continue, POSNA could be 40% female by 2025 as senior, predominantly male members retire.1
Pediatric orthopedic practice in 2016 is also dramatically different owing to the development of subspecialization in areas of pediatric sports medicine, hand surgery, trauma, and the treatment of adolescent hip pathology. In fact, a recent survey of fellowship graduates showed that 30% of graduating fellows were going to do a second fellowship.3
While technological advances have driven the care of many pediatric orthopedic conditions such as spinal deformity and sports injuries, there also has been a resurgence of interest in the nonoperative treatment of clubfeet using the Ponseti method and of early-onset scoliosis using Mehta casting. Children with clubfeet even a decade ago were being treated with wide comprehensive releases and capsulotomies, leading to stiff painful feet as young adults. Now comprehensive releases are rarely used. Owing to advances in posterior spinal instrumentation as well as studies showing some decline in pulmonary function after thoracotomy and anterior spinal fusion, the treatment of adolescent scoliosis is predominantly done through the posterior approach. Advances in screening have led to a dramatic decrease in the surgical treatment of hip dysplasia. Medical treatment, such as corticosteroids for Duchenne muscular dystrophy, has prolonged length of life and improved quality of life as well as decreased the number of spinal fusions performed. Recombinant factor replacement for hemophilia has almost eliminated the horrible morbidity associated with hemophilic arthropathy and the need for synovectomy, arthrodesis, and arthroplasty, as well as the infectious issues, such as human immunodeficiency virus (HIV) and hepatitis, associated with the use of pooled blood products. The use of growth-friendly spinal implants, such as the Vertical Expandable Prosthetic Titanium Rib (VEPTR; DePuy Synthes), magnetically driven growing rods (MAGEC; Ellipse), and spinal tethers have improved pulmonary outcomes and presumably life expectancy in young patients with early-onset scoliosis who a decade ago may have had an in situ spinal fusion. These are just a few examples, and there are many more.
The articles in this issue highlight some of these changes. Tibial osteotomy and deformity correction, as described in the article by Burton and Hennrikus (pages 16-18), are classic techniques used by pediatric orthopedists over the past decades and will continue to be useful. The article by Hosseinzadeh and Talwalkar (pages 19-22) reviews unique aspects of pediatric compartment syndrome. While the basic concepts of compartment syndrome have not changed, the signs of compartment syndrome, the 5 Ps we all learned a decade ago (pain, paresthesia, paralysis, pallor, and pulselessness) have now been replaced in children with the 3 As (increasing analgesia, anxiety, and agitation). Finally, the article by Sferopoulos (pages 38-41) describing a case of a giant bone island in a child reminds us that we have a lot more to learn as pediatric orthopedists regarding the molecular nature and cause of disease.
The next few years will continue to be an exciting and dynamic time in the field of pediatric orthopedics. Not only is the workforce itself changing and growing, but so are the definitions of what a pediatric orthopedic surgeon is and does. While subspecialization is the trend in most aspects of medicine, it will be important to continue to monitor<hl name="1"/> this trend to ensure that pediatric orthopedics does not become too highly specialized. With the tremendous inflow of new talent, ideas, and technology, the future for pediatric orthopedics has never looked brighter.
References
In my 16 years of practice, there has been tremendous change in the field of pediatric orthopedics in both demographics and scope of practice. Because of scientific and technological advances, efforts of the Pediatric Orthopaedic Society of North America (POSNA), and a changing workforce, the nature of pediatric orthopedics is changing dramatically and will continue to do so.
In the late 1990s, a “typical” pediatric orthopedic surgeon was treating fractures, developmental dysplasia of the hip, clubfeet, and other congenital deformities. Surgery for adolescent idiopathic scoliosis was moving toward anterior instrumentation and correction of the spine. The concepts of early-onset scoliosis and thoracic insufficiency syndrome were in their infancy. Children with anterior cruciate ligament tears were treated with braces until skeletal maturity, often leading to life-altering meniscal pathology. Essential medical treatments for genetic conditions, including bisphosphonates for osteogenesis imperfecta and corticosteroids for Duchenne muscular dystrophy, were considered experimental.
The field itself also was at a crossroads. In 1993, there were 410 active members in POSNA (vs 653 in 2014), and the vast majority were male.1 In the late 1990s, there were approximately 30 pediatric fellowship spots and 10 fellows being trained per year. Simultaneously, approximately 20 to 30 active POSNA members were retiring annually, leading to a projected shortage of pediatric orthopedic surgeons.1 A 2007 American Orthopaedic Association survey found that 59% of members believed that pediatric orthopedics was the most underserved specialty for a variety of reasons, including perceived lower reimbursement, higher volume of nonoperative treatment, and lifestyle issues (such as on-call burden).2
Owing in part to efforts of POSNA in resident/fellow education and mentorship, the practice of pediatric orthopedics in 2016 is dramatically different from a decade ago. The number of fellowship programs has increased to 44 programs, offering a total of 71 fellowship spots, of which 60 were filled by US applicants in 2014. Interestingly, the current active membership of POSNA is 19% female, and the 2014 fellowship class was 34% female. This is in contrast to the 4.4% of all AAOS members who are female. If current trends continue, POSNA could be 40% female by 2025 as senior, predominantly male members retire.1
Pediatric orthopedic practice in 2016 is also dramatically different owing to the development of subspecialization in areas of pediatric sports medicine, hand surgery, trauma, and the treatment of adolescent hip pathology. In fact, a recent survey of fellowship graduates showed that 30% of graduating fellows were going to do a second fellowship.3
While technological advances have driven the care of many pediatric orthopedic conditions such as spinal deformity and sports injuries, there also has been a resurgence of interest in the nonoperative treatment of clubfeet using the Ponseti method and of early-onset scoliosis using Mehta casting. Children with clubfeet even a decade ago were being treated with wide comprehensive releases and capsulotomies, leading to stiff painful feet as young adults. Now comprehensive releases are rarely used. Owing to advances in posterior spinal instrumentation as well as studies showing some decline in pulmonary function after thoracotomy and anterior spinal fusion, the treatment of adolescent scoliosis is predominantly done through the posterior approach. Advances in screening have led to a dramatic decrease in the surgical treatment of hip dysplasia. Medical treatment, such as corticosteroids for Duchenne muscular dystrophy, has prolonged length of life and improved quality of life as well as decreased the number of spinal fusions performed. Recombinant factor replacement for hemophilia has almost eliminated the horrible morbidity associated with hemophilic arthropathy and the need for synovectomy, arthrodesis, and arthroplasty, as well as the infectious issues, such as human immunodeficiency virus (HIV) and hepatitis, associated with the use of pooled blood products. The use of growth-friendly spinal implants, such as the Vertical Expandable Prosthetic Titanium Rib (VEPTR; DePuy Synthes), magnetically driven growing rods (MAGEC; Ellipse), and spinal tethers have improved pulmonary outcomes and presumably life expectancy in young patients with early-onset scoliosis who a decade ago may have had an in situ spinal fusion. These are just a few examples, and there are many more.
The articles in this issue highlight some of these changes. Tibial osteotomy and deformity correction, as described in the article by Burton and Hennrikus (pages 16-18), are classic techniques used by pediatric orthopedists over the past decades and will continue to be useful. The article by Hosseinzadeh and Talwalkar (pages 19-22) reviews unique aspects of pediatric compartment syndrome. While the basic concepts of compartment syndrome have not changed, the signs of compartment syndrome, the 5 Ps we all learned a decade ago (pain, paresthesia, paralysis, pallor, and pulselessness) have now been replaced in children with the 3 As (increasing analgesia, anxiety, and agitation). Finally, the article by Sferopoulos (pages 38-41) describing a case of a giant bone island in a child reminds us that we have a lot more to learn as pediatric orthopedists regarding the molecular nature and cause of disease.
The next few years will continue to be an exciting and dynamic time in the field of pediatric orthopedics. Not only is the workforce itself changing and growing, but so are the definitions of what a pediatric orthopedic surgeon is and does. While subspecialization is the trend in most aspects of medicine, it will be important to continue to monitor<hl name="1"/> this trend to ensure that pediatric orthopedics does not become too highly specialized. With the tremendous inflow of new talent, ideas, and technology, the future for pediatric orthopedics has never looked brighter.
References
1. Sawyer JR, Jones KC, Copley LA, Chambers S; POSNA Practice Management Committee. Pediatric orthopaedic workforce in 2014: current workforce and projections for the future [published online ahead of print October 30, 2015]. J Pediatr Orthop.
2. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
3. Glotzbecker MP, Shore BJ, Fletcher ND, Larson AN, Hydorn CR, Sawyer JR; Practice Management Committee of the Pediatric Orthopaedic Society of North America. Early career experience of pediatric orthopaedic fellows: what to expect and need for their services [published online ahead of print March 3, 2015]. J Pediatr Orthop.
1. Sawyer JR, Jones KC, Copley LA, Chambers S; POSNA Practice Management Committee. Pediatric orthopaedic workforce in 2014: current workforce and projections for the future [published online ahead of print October 30, 2015]. J Pediatr Orthop.
2. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
3. Glotzbecker MP, Shore BJ, Fletcher ND, Larson AN, Hydorn CR, Sawyer JR; Practice Management Committee of the Pediatric Orthopaedic Society of North America. Early career experience of pediatric orthopaedic fellows: what to expect and need for their services [published online ahead of print March 3, 2015]. J Pediatr Orthop.
SF-6D best quality of life measure in cervical spine patients
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
AT CSRS 2015
Key clinical point: The Short Form–6D derived from the Neck Disability Index is an effective measure of outcomes in cervical spine patients.
Major finding: The Short Form–6D derived from the Neck Disability Index was a more valid discriminator of meaningful improvement, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D (AUC of .69, .65, and .62, respectively).
Data source: A single-center study that compared three quality of life measures in 420 patients presenting for elective surgical spine procedures.
Disclosures: Dr. Sielatycki reported having no financial disclosures.