User login
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
What is the best treatment for wrist ganglion cysts?
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE-BASED ANSWER:
Open surgical excision of wrist ganglion cysts is associated with a lower recurrence rate than aspiration with or without corticosteroid injection (strength of recommendation [SOR]: B, systematic review of randomized clinical trials [RCTs] and observational trials and RCT).
Even though the recurrence rate with aspiration is about 50%, most patients are satisfied with aspiration and report a decrease in symptoms involving pain, function, and range of motion (SOR: B, individual cohort and case series).
56-year-old woman • worsening pain in left upper arm • influenza vaccination in the arm a few days prior to pain onset • Dx?
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
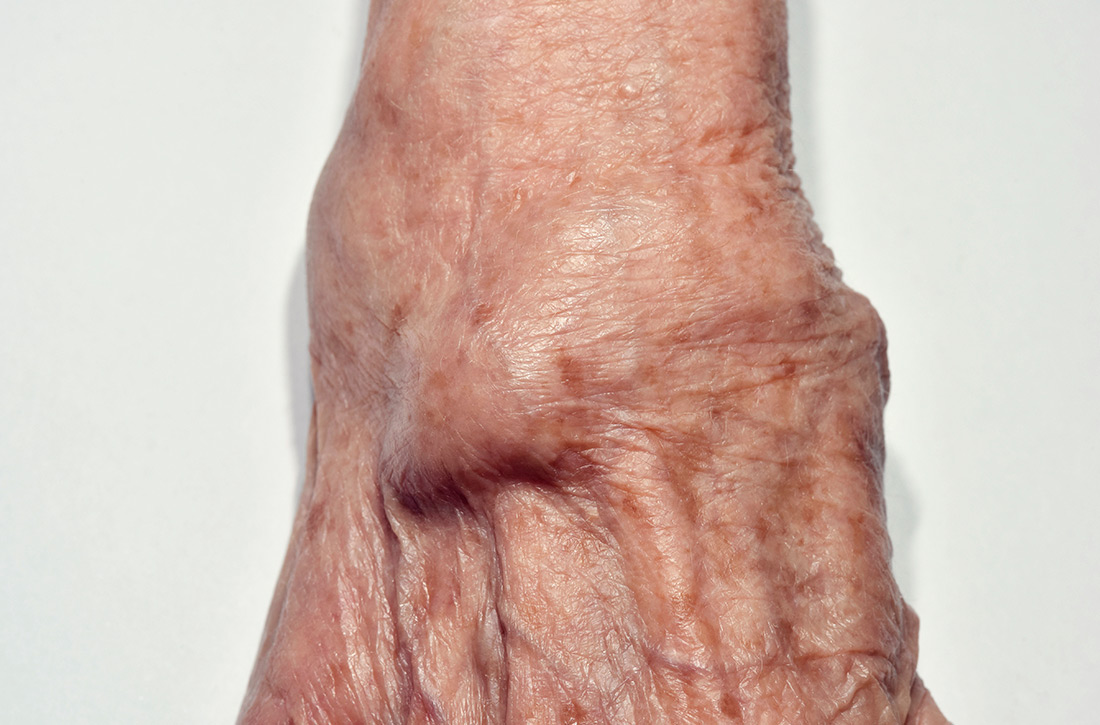
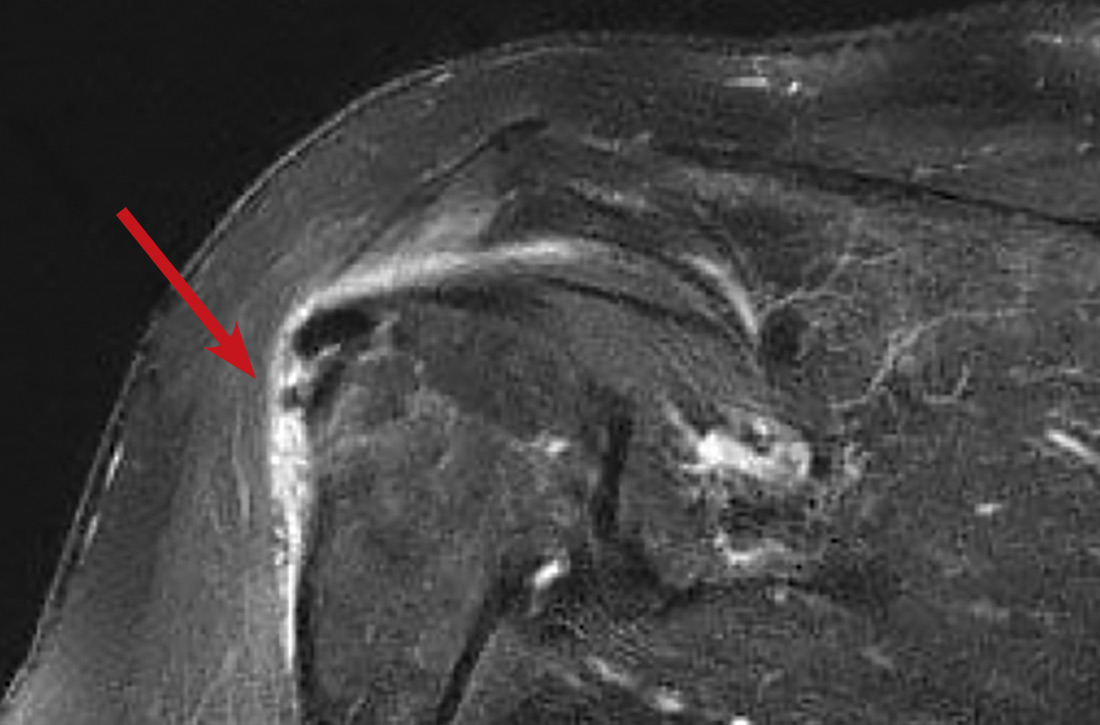
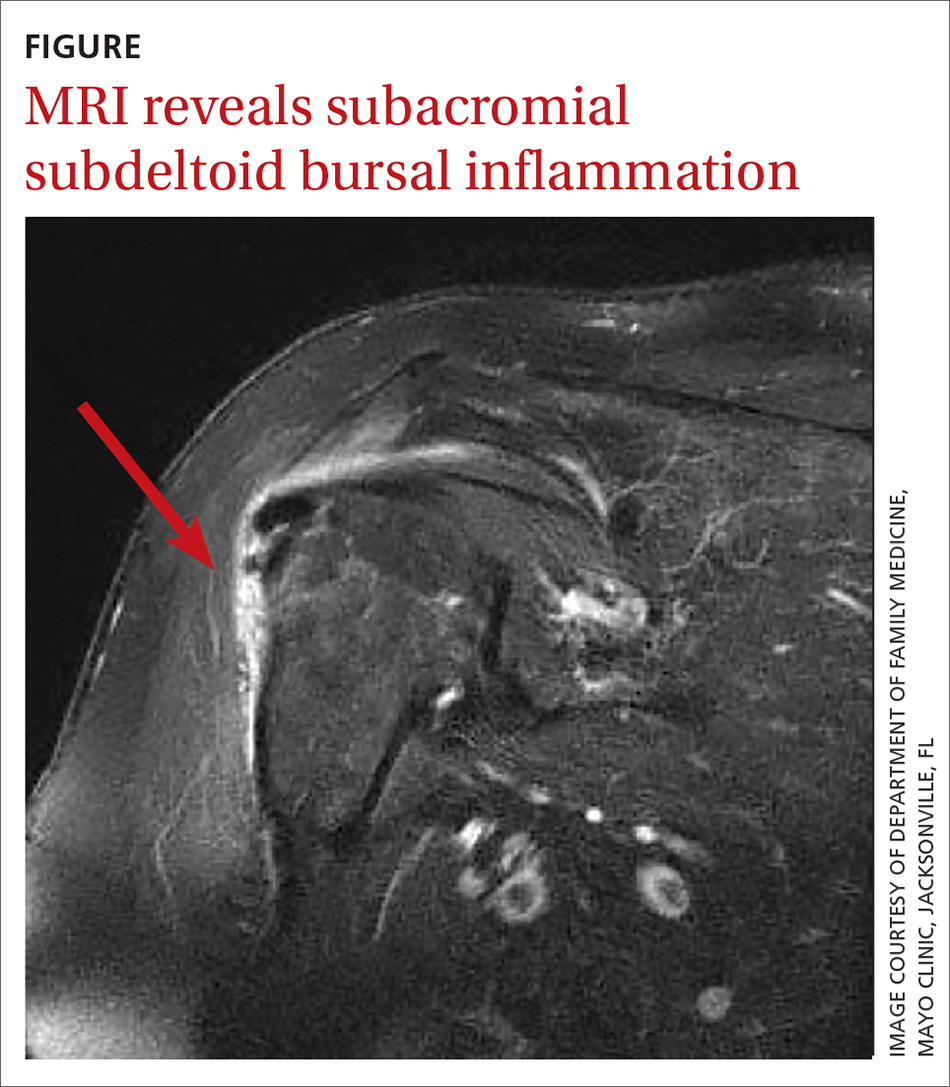
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
Cannabis for sleep: Short-term benefit, long-term disruption?
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM BMJ SUPPORTIVE AND PALLIATIVE CARE
IBD: Inpatient opioids linked with outpatient use
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with inflammatory bowel disease (IBD) who receive opioids while hospitalized are three times as likely to be prescribed opioids after discharge.
Major finding: Patients who were given intravenous opioids while hospitalized were three times as likely to receive a postdischarge opioid prescription, compared with patients who did not receive inpatient intravenous opioids (odds ratio, 3.3).
Study details: A retrospective cohort study involving 862 patients with inflammatory bowel disease.
Disclosures: The investigators disclosed relationships Abbott, Gilead, Romark, and others.
Source: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FDA advisers set high bar for new opioids
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
FDA committee rejects oxycodegol, new opioid designed for less abuse
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
SimLEARN Musculoskeletal Training for VHA Primary Care Providers and Health Professions Educators
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
Incidentally Discovered Ochronosis Explaining Decades of Chronic Pain
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
Antipsychotics, dopamine, and pain
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796