User login
FDA okays ubrogepant for acute migraine treatment
The Food and Drug Administration has approved ubrogepant (Ubrelvy, Allergan) for the acute treatment of migraine with or without aura in adults.
Ubrogepant is the first drug in the class of oral calcitonin gene–related peptide receptor antagonists approved for the acute treatment of migraine. It is approved in two dose strengths (50 mg and 100 mg).
The drug is not indicated, however, for the preventive treatment of migraine.
“Migraine is an often disabling condition that affects an estimated 37 million people in the U.S.,” Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
Ubrogepant represents “an important new option for the acute treatment of migraine in adults, as it is the first drug in its class approved for this indication. The FDA is pleased to approve a novel treatment for patients suffering from migraine and will continue to work with stakeholders to promote the development of new safe and effective migraine therapies,” added Dr. Dunn.
The safety and efficacy of ubrogepant for the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials (ACHIEVE I and ACHIEVE II). In total, 1,439 adults with a history of migraine, with and without aura, received ubrogepant to treat an ongoing migraine.
“Both 50-mg and 100-mg dose strengths demonstrated significantly greater rates of pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours, compared with placebo,” Allergan said in a news release announcing approval.
The most common side effects reported by patients in the clinical trials were nausea, tiredness, and dry mouth. Ubrogepant is contraindicated for coadministration with strong CYP3A4 inhibitors.
The company expects to have ubrogepant available in the first quarter of 2020.
A version of this story originally appeared on Medscape.com.
The Food and Drug Administration has approved ubrogepant (Ubrelvy, Allergan) for the acute treatment of migraine with or without aura in adults.
Ubrogepant is the first drug in the class of oral calcitonin gene–related peptide receptor antagonists approved for the acute treatment of migraine. It is approved in two dose strengths (50 mg and 100 mg).
The drug is not indicated, however, for the preventive treatment of migraine.
“Migraine is an often disabling condition that affects an estimated 37 million people in the U.S.,” Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
Ubrogepant represents “an important new option for the acute treatment of migraine in adults, as it is the first drug in its class approved for this indication. The FDA is pleased to approve a novel treatment for patients suffering from migraine and will continue to work with stakeholders to promote the development of new safe and effective migraine therapies,” added Dr. Dunn.
The safety and efficacy of ubrogepant for the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials (ACHIEVE I and ACHIEVE II). In total, 1,439 adults with a history of migraine, with and without aura, received ubrogepant to treat an ongoing migraine.
“Both 50-mg and 100-mg dose strengths demonstrated significantly greater rates of pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours, compared with placebo,” Allergan said in a news release announcing approval.
The most common side effects reported by patients in the clinical trials were nausea, tiredness, and dry mouth. Ubrogepant is contraindicated for coadministration with strong CYP3A4 inhibitors.
The company expects to have ubrogepant available in the first quarter of 2020.
A version of this story originally appeared on Medscape.com.
The Food and Drug Administration has approved ubrogepant (Ubrelvy, Allergan) for the acute treatment of migraine with or without aura in adults.
Ubrogepant is the first drug in the class of oral calcitonin gene–related peptide receptor antagonists approved for the acute treatment of migraine. It is approved in two dose strengths (50 mg and 100 mg).
The drug is not indicated, however, for the preventive treatment of migraine.
“Migraine is an often disabling condition that affects an estimated 37 million people in the U.S.,” Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
Ubrogepant represents “an important new option for the acute treatment of migraine in adults, as it is the first drug in its class approved for this indication. The FDA is pleased to approve a novel treatment for patients suffering from migraine and will continue to work with stakeholders to promote the development of new safe and effective migraine therapies,” added Dr. Dunn.
The safety and efficacy of ubrogepant for the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials (ACHIEVE I and ACHIEVE II). In total, 1,439 adults with a history of migraine, with and without aura, received ubrogepant to treat an ongoing migraine.
“Both 50-mg and 100-mg dose strengths demonstrated significantly greater rates of pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours, compared with placebo,” Allergan said in a news release announcing approval.
The most common side effects reported by patients in the clinical trials were nausea, tiredness, and dry mouth. Ubrogepant is contraindicated for coadministration with strong CYP3A4 inhibitors.
The company expects to have ubrogepant available in the first quarter of 2020.
A version of this story originally appeared on Medscape.com.
Well-tolerated topical capsaicin formulation reduces knee OA pain
ATLANTA – Use of high-concentration topical capsaicin was associated with reduced pain, a longer duration of clinical response, and was well tolerated in patients with knee osteoarthritis, compared with lower concentrations of capsaicin and placebo, according to recent research presented at the annual meeting of the American College of Rheumatology.
While the ACR has recommended topical capsaicin for the relief of hand and knee OA pain, there are issues with using low-dose capsaicin, including the need for multiple applications and burning, stinging sensations at applications sites. As repeat exposure to capsaicin results in depletion of pain neurotransmitters and a reduction in nerve fibers in a dose-dependent fashion, higher doses of topical capsaicin are a potential topical treatment for OA pain relief, but their tolerability is low, Tim Warneke, vice president of clinical operations at Vizuri Health Sciences in Columbia, Md., said in his presentation.
“[P]oor tolerability has limited the ability to maximize the analgesic effect of capsaicin,” Mr. Warneke said. “While [over-the-counter] preparations of capsaicin provide some pain relief, poor tolerability with higher doses has really left us wondering if we haven’t maximized capsaicin’s ability to provide pain relief.”
Mr. Warneke and colleagues conducted a phase 2, multicenter, double-blind, parallel-group, vehicle-controlled trial where 120 patients with knee OA were randomized in a 1:1:1 ratio to receive 5% capsaicin topical liquid (CGS-200-5), 1% capsaicin topical liquid (CGS-200-1), or vehicle (CGS-200-0) and then followed up to 90 days. “The CGS-200 vehicle was developed to mitigate the burning, stinging pain of capsaicin,” Mr. Warneke said. “It allows the 5% concentration to be well tolerated, which opens the door for increased efficacy, including durability of response.”
Inclusion criteria were radiographically confirmed knee OA using 1986 ACR classification criteria, a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score of 250 mm or greater, and more than 3 months of chronic knee pain. While patients were excluded for use of topical, oral, or injectable corticosteroids in the month prior to enrollment, they were allowed to continue using analgesics such as NSAIDs if they maintained their daily dose throughout the trial. Mr. Warneke noted the study population was typical of an OA population with a mostly female, mostly Caucasian cohort who had a median age of 60 years and a body mass index of 30 kg/m2. Patients had moderate to severe OA and were refractory to previous pain treatments.
The interventions consisted of a single 60-minute application of capsaicin or vehicle to both knees once per day for 4 consecutive days, and patients performed the applications in the clinic. The investigators compared change in WOMAC pain scores between the groups at 31 days, 60 days, and 90 days post dose.
The results at 31 days showed a 46.2% reduction in WOMAC pain scores from baseline for patients using CGS-200-5, compared with a 28.3% reduction in the vehicle group (P = .02). At 60 days, there was a 49.1% reduction in WOMAC pain scores in the CGS-200-5 group, compared with 21.5% in patients using vehicle (P = .0001), and a 42.8% reduction for patients in the CGS-200-5 group at 90 days, compared with 22.8% in the vehicle group (P = .01). The CGS-200-1 group did not reach the primary efficacy WOMAC pain endpoint, compared with vehicle.
A post hoc analysis showed that there was a significantly greater mean reduction in WOMAC total score for patients using CGS-200-5, compared with vehicle at 31 days (P = .02), 60 days (P = .0005), and 90 days post dose (P = .005). “This durability of clinical response for single applications seems to be a promising feature of CGS-200-5,” Mr. Warneke said.
Concerning safety and tolerability, there were no serious adverse events, and one patient discontinued treatment in the CGS-200-5 group. When assessing tolerability at predose, 15-minute, 30-minute, 60-minute, and 90-minute postdose time intervals, the investigators found patients experienced mild or moderate adverse events such as erythema, edema, scaling, and pruritus, with symptoms decreasing by the fourth consecutive day of application.
Mr. Warneke acknowledged the “robust placebo response” in the trial and noted it is not unusual to see in pain studies. “It’s something that is a challenge for all of us who are in this space to overcome, but we still have significant differences here and they are statistically significant as well,” he said. “You have to be pretty good these days to beat the wonder drug placebo, it appears.”
Four authors in addition to Mr. Warneke reported being employees of Vizuri Health Sciences, the company developing CGS-200-5. One author reported being a former consultant for Vizuri. Three authors reported they were current or former employees of CT Clinical Trial & Consulting, a contract research organization employed by Vizuri to execute and manage the study, perform data analysis, and create reports.
SOURCE: Warneke T et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2760.
ATLANTA – Use of high-concentration topical capsaicin was associated with reduced pain, a longer duration of clinical response, and was well tolerated in patients with knee osteoarthritis, compared with lower concentrations of capsaicin and placebo, according to recent research presented at the annual meeting of the American College of Rheumatology.
While the ACR has recommended topical capsaicin for the relief of hand and knee OA pain, there are issues with using low-dose capsaicin, including the need for multiple applications and burning, stinging sensations at applications sites. As repeat exposure to capsaicin results in depletion of pain neurotransmitters and a reduction in nerve fibers in a dose-dependent fashion, higher doses of topical capsaicin are a potential topical treatment for OA pain relief, but their tolerability is low, Tim Warneke, vice president of clinical operations at Vizuri Health Sciences in Columbia, Md., said in his presentation.
“[P]oor tolerability has limited the ability to maximize the analgesic effect of capsaicin,” Mr. Warneke said. “While [over-the-counter] preparations of capsaicin provide some pain relief, poor tolerability with higher doses has really left us wondering if we haven’t maximized capsaicin’s ability to provide pain relief.”
Mr. Warneke and colleagues conducted a phase 2, multicenter, double-blind, parallel-group, vehicle-controlled trial where 120 patients with knee OA were randomized in a 1:1:1 ratio to receive 5% capsaicin topical liquid (CGS-200-5), 1% capsaicin topical liquid (CGS-200-1), or vehicle (CGS-200-0) and then followed up to 90 days. “The CGS-200 vehicle was developed to mitigate the burning, stinging pain of capsaicin,” Mr. Warneke said. “It allows the 5% concentration to be well tolerated, which opens the door for increased efficacy, including durability of response.”
Inclusion criteria were radiographically confirmed knee OA using 1986 ACR classification criteria, a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score of 250 mm or greater, and more than 3 months of chronic knee pain. While patients were excluded for use of topical, oral, or injectable corticosteroids in the month prior to enrollment, they were allowed to continue using analgesics such as NSAIDs if they maintained their daily dose throughout the trial. Mr. Warneke noted the study population was typical of an OA population with a mostly female, mostly Caucasian cohort who had a median age of 60 years and a body mass index of 30 kg/m2. Patients had moderate to severe OA and were refractory to previous pain treatments.
The interventions consisted of a single 60-minute application of capsaicin or vehicle to both knees once per day for 4 consecutive days, and patients performed the applications in the clinic. The investigators compared change in WOMAC pain scores between the groups at 31 days, 60 days, and 90 days post dose.
The results at 31 days showed a 46.2% reduction in WOMAC pain scores from baseline for patients using CGS-200-5, compared with a 28.3% reduction in the vehicle group (P = .02). At 60 days, there was a 49.1% reduction in WOMAC pain scores in the CGS-200-5 group, compared with 21.5% in patients using vehicle (P = .0001), and a 42.8% reduction for patients in the CGS-200-5 group at 90 days, compared with 22.8% in the vehicle group (P = .01). The CGS-200-1 group did not reach the primary efficacy WOMAC pain endpoint, compared with vehicle.
A post hoc analysis showed that there was a significantly greater mean reduction in WOMAC total score for patients using CGS-200-5, compared with vehicle at 31 days (P = .02), 60 days (P = .0005), and 90 days post dose (P = .005). “This durability of clinical response for single applications seems to be a promising feature of CGS-200-5,” Mr. Warneke said.
Concerning safety and tolerability, there were no serious adverse events, and one patient discontinued treatment in the CGS-200-5 group. When assessing tolerability at predose, 15-minute, 30-minute, 60-minute, and 90-minute postdose time intervals, the investigators found patients experienced mild or moderate adverse events such as erythema, edema, scaling, and pruritus, with symptoms decreasing by the fourth consecutive day of application.
Mr. Warneke acknowledged the “robust placebo response” in the trial and noted it is not unusual to see in pain studies. “It’s something that is a challenge for all of us who are in this space to overcome, but we still have significant differences here and they are statistically significant as well,” he said. “You have to be pretty good these days to beat the wonder drug placebo, it appears.”
Four authors in addition to Mr. Warneke reported being employees of Vizuri Health Sciences, the company developing CGS-200-5. One author reported being a former consultant for Vizuri. Three authors reported they were current or former employees of CT Clinical Trial & Consulting, a contract research organization employed by Vizuri to execute and manage the study, perform data analysis, and create reports.
SOURCE: Warneke T et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2760.
ATLANTA – Use of high-concentration topical capsaicin was associated with reduced pain, a longer duration of clinical response, and was well tolerated in patients with knee osteoarthritis, compared with lower concentrations of capsaicin and placebo, according to recent research presented at the annual meeting of the American College of Rheumatology.
While the ACR has recommended topical capsaicin for the relief of hand and knee OA pain, there are issues with using low-dose capsaicin, including the need for multiple applications and burning, stinging sensations at applications sites. As repeat exposure to capsaicin results in depletion of pain neurotransmitters and a reduction in nerve fibers in a dose-dependent fashion, higher doses of topical capsaicin are a potential topical treatment for OA pain relief, but their tolerability is low, Tim Warneke, vice president of clinical operations at Vizuri Health Sciences in Columbia, Md., said in his presentation.
“[P]oor tolerability has limited the ability to maximize the analgesic effect of capsaicin,” Mr. Warneke said. “While [over-the-counter] preparations of capsaicin provide some pain relief, poor tolerability with higher doses has really left us wondering if we haven’t maximized capsaicin’s ability to provide pain relief.”
Mr. Warneke and colleagues conducted a phase 2, multicenter, double-blind, parallel-group, vehicle-controlled trial where 120 patients with knee OA were randomized in a 1:1:1 ratio to receive 5% capsaicin topical liquid (CGS-200-5), 1% capsaicin topical liquid (CGS-200-1), or vehicle (CGS-200-0) and then followed up to 90 days. “The CGS-200 vehicle was developed to mitigate the burning, stinging pain of capsaicin,” Mr. Warneke said. “It allows the 5% concentration to be well tolerated, which opens the door for increased efficacy, including durability of response.”
Inclusion criteria were radiographically confirmed knee OA using 1986 ACR classification criteria, a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score of 250 mm or greater, and more than 3 months of chronic knee pain. While patients were excluded for use of topical, oral, or injectable corticosteroids in the month prior to enrollment, they were allowed to continue using analgesics such as NSAIDs if they maintained their daily dose throughout the trial. Mr. Warneke noted the study population was typical of an OA population with a mostly female, mostly Caucasian cohort who had a median age of 60 years and a body mass index of 30 kg/m2. Patients had moderate to severe OA and were refractory to previous pain treatments.
The interventions consisted of a single 60-minute application of capsaicin or vehicle to both knees once per day for 4 consecutive days, and patients performed the applications in the clinic. The investigators compared change in WOMAC pain scores between the groups at 31 days, 60 days, and 90 days post dose.
The results at 31 days showed a 46.2% reduction in WOMAC pain scores from baseline for patients using CGS-200-5, compared with a 28.3% reduction in the vehicle group (P = .02). At 60 days, there was a 49.1% reduction in WOMAC pain scores in the CGS-200-5 group, compared with 21.5% in patients using vehicle (P = .0001), and a 42.8% reduction for patients in the CGS-200-5 group at 90 days, compared with 22.8% in the vehicle group (P = .01). The CGS-200-1 group did not reach the primary efficacy WOMAC pain endpoint, compared with vehicle.
A post hoc analysis showed that there was a significantly greater mean reduction in WOMAC total score for patients using CGS-200-5, compared with vehicle at 31 days (P = .02), 60 days (P = .0005), and 90 days post dose (P = .005). “This durability of clinical response for single applications seems to be a promising feature of CGS-200-5,” Mr. Warneke said.
Concerning safety and tolerability, there were no serious adverse events, and one patient discontinued treatment in the CGS-200-5 group. When assessing tolerability at predose, 15-minute, 30-minute, 60-minute, and 90-minute postdose time intervals, the investigators found patients experienced mild or moderate adverse events such as erythema, edema, scaling, and pruritus, with symptoms decreasing by the fourth consecutive day of application.
Mr. Warneke acknowledged the “robust placebo response” in the trial and noted it is not unusual to see in pain studies. “It’s something that is a challenge for all of us who are in this space to overcome, but we still have significant differences here and they are statistically significant as well,” he said. “You have to be pretty good these days to beat the wonder drug placebo, it appears.”
Four authors in addition to Mr. Warneke reported being employees of Vizuri Health Sciences, the company developing CGS-200-5. One author reported being a former consultant for Vizuri. Three authors reported they were current or former employees of CT Clinical Trial & Consulting, a contract research organization employed by Vizuri to execute and manage the study, perform data analysis, and create reports.
SOURCE: Warneke T et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2760.
REPORTING FROM ACR 2019
In addiction, abusive partners can wreak havoc
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
Gender-based violence could be driver of opioid epidemic, expert suggests
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
REPORTING FROM AAAP 2019
Addiction specialists: Cannabis policies should go up in smoke
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
REPORTING FROM AAAP 2019
New opioid recommendations: Pain from most dermatologic procedures should be managed with acetaminophen, ibuprofen
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Functional heartburn: An underrecognized cause of PPI-refractory symptoms
A 44-year-old woman presents with an 8-year history of intermittent heartburn, and in the past year she has been experiencing her symptoms daily. She says the heartburn is constant and is worse immediately after eating spicy or acidic foods. She says she has had no dysphagia, weight loss, or vomiting. Her symptoms have persisted despite taking a histamine (H)2-receptor antagonist twice daily plus a proton pump inhibitor (PPI) before breakfast and dinner for more than 3 months.
She has undergone upper endoscopy 3 times in the past 8 years. Each time, the esophagus was normal with a regular Z-line and normal biopsy results from the proximal and distal esophagus.
The patient believes she has severe gastroesophageal reflux disease (GERD) and asks if she is a candidate for fundoplication surgery.
HEARTBURN IS A SYMPTOM; GERD IS A CONDITION
A distinction should be made between heartburn—the symptom of persistent retrosternal burning and discomfort—and gastroesophageal reflux disease—the condition in which reflux of stomach contents causes troublesome symptoms or complications.1 While many clinicians initially diagnose patients who have heartburn as having GERD, there are many other potential causes of their symptoms.
For patients with persistent heartburn, an empiric trial of a once-daily PPI is usually effective, but one-third of patients continue to have heartburn.2,3 The most common cause of this PPI-refractory heartburn is functional heartburn, a functional or hypersensitivity disorder of the esophagus.4
PATHOPHYSIOLOGY IS POORLY UNDERSTOOD
DIAGNOSTIC EVALUATION
Clinicians have several tests available for diagnosing these conditions.
Upper endoscopy
Upper endoscopy is recommended for patients with heartburn that does not respond to a 3-month trial of a PPI.9 Endoscopy is also indicated in any patient who has any of the following “alarm symptoms” that could be due to malignancy or peptic ulcer:
- Dysphagia
- Odynophagia
- Vomiting
- Unexplained weight loss or anemia
- Signs of gastrointestinal bleeding
- Anorexia
- New onset of dyspepsia in a patient over age 60.
During upper endoscopy, the esophagus is evaluated for reflux esophagitis, Barrett esophagus, and other inflammatory disorders such as infectious esophagitis. But even if the esophageal mucosa appears normal, the proximal and distal esophagus should be biopsied to rule out an inflammatory disorder such as eosinophilic or lymphocytic esophagitis.
Esophageal manometry
If endoscopic and esophageal biopsy results are inconclusive, a workup for an esophageal motility disorder is the next step. Dysphagia is the most common symptom of these disorders, although the initial presenting symptom may be heartburn or regurgitation that persists despite PPI therapy.
Manometry is used to test for motility disorders such as achalasia and esophageal spasm.10 After applying a local anesthetic inside the nares, the clinician inserts a flexible catheter (about 4 mm in diameter) with 36 pressure sensors spaced at 1-cm intervals into the nares and passes it through the esophagus and lower esophageal sphincter. The patient then swallows liquid, and the sensors relay the esophageal response, creating a topographic plot that shows esophageal peristalsis and lower esophageal sphincter relaxation.
Achalasia is identified by incomplete lower esophageal sphincter relaxation combined with 100% failed peristalsis in the body of the esophagus. Esophageal spasms are identified by a shortened distal latency, which corresponds to premature contraction of the esophagus during peristalsis.11
Esophageal pH testing
Measuring esophageal pH levels is an important step to quantify gastroesophageal reflux and determine if symptoms occur during reflux events. According to the updated Porto GERD consensus group recommendations,12 a pH test is positive if the acid exposure time is greater than 6% of the testing period. Testing the pH differentiates between GERD (abnormal acid exposure), reflux hypersensitivity (normal acid exposure, strong correlation between symptoms and reflux events), and functional heartburn (normal acid exposure, negative correlation between reflux events and symptoms).5 For this test, a pH probe is placed in the esophagus transnasally or endoscopically. The probe records esophageal pH levels for 24 to 96 hours in an outpatient setting. Antisecretory therapy needs to be withheld for 7 to 10 days before the test.
Transnasal pH probe. For this approach, a thin catheter is inserted through the nares and advanced until the tip is 5 cm proximal to the lower esophageal sphincter. (The placement is guided by the results of esophageal manometry, which is done immediately before pH catheter placement.) The tube is secured with clear tape on the side of the patient’s face, and the end is connected to a portable recorder that compiles the data. The patient pushes a button on the recorder when experiencing heartburn symptoms. (A nurse instructs the patient on proper procedure.) After 24 hours, the patient either removes the catheter or has the clinic remove it. The pH and symptom data are downloaded and analyzed.
Transnasal pH testing can be combined with impedance measurement, which can detect nonacid reflux or weakly acid reflux. However, the clinical significance of this measurement is unclear, as multiple studies have found total acid exposure time to be a better predictor of response to therapy than weakly acid or nonacid reflux.12
Wireless pH probe. This method uses a disposable, catheter-free, capsule device to measure esophageal pH. The capsule, about the size of a gel capsule or pencil eraser, is attached to the patient’s esophageal lining, usually during upper endoscopy. The capsule records pH levels in the lower esophagus for 48 to 96 hours and transmits the data wirelessly to a receiver the patient wears. The patient pushes buttons on the receiver to record symptom-specific data when experiencing heartburn, chest pain, regurgitation, or cough. The capsule detaches from the esophagus spontaneously, generally within 7 days, and is passed out of the body through a bowel movement.
Diagnosing functional heartburn
CASE CONTINUED: NORMAL RESULTS ON TESTING
Based on these results, her condition is diagnosed as functional heartburn, consistent with the Rome IV criteria.5
TREATMENT
Patient education is key
Patient education about the pathogenesis, natural history, and treatment options is the most important aspect of treating any functional gastrointestinal disorder. This includes the “brain-gut connection” and potential mechanisms of dysregulation. Patient education along with assessment of symptoms should be part of every visit, especially before discussing treatment options.
Patients whose condition is diagnosed as functional heartburn need reassurance that the condition is benign and, in particular, that the risk of progression to esophageal adenocarcinoma is minimal in the absence of Barrett esophagus.13 Also important to point out is that the disorder may spontaneously resolve: resolution rates of up to 40% have been reported for other functional gastrointestinal disorders.14
Antisecretory medications may work for some
A PPI or H2-receptor antagonist is the most common first-line treatment for heartburn symptoms. Although most patients with functional heartburn experience no improvement in symptoms with an antisecretory agent, a small number report some relief, which suggests that acid-suppression therapy may have an indirect impact on pain modulation in the esophagus.15 In patients who report symptom relief with an antisecretory agent, we suggest continuing the medication tapered to the lowest effective dose, with repeated reassurance that the medication can be discontinued safely at any time.
Antireflux surgery should be avoided
Antireflux surgery should be avoided in patients with normal pH testing and no objective finding of reflux, as this is associated with worse subjective outcomes than in patients with abnormal pH test results.16
Neuromodulators
It is important to discuss with patients the concept of neuromodulation, including the fact that antidepressants are often used because of their effects on serotonin and norepinephrine, which decrease visceral hypersensitivity.
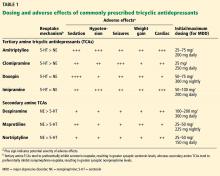
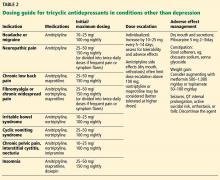
The selective serotonin reuptake inhibitor citalopram has been shown to reduce esophageal hypersensitivity,17 and a tricyclic antidepressant has been shown to improve quality of life.18 These results have led experts to recommend a trial of a low dose of either type of medication.19 The dose of tricyclic antidepressant often needs to be increased sequentially every 2 to 4 weeks.
Interestingly, melatonin 6 mg at bedtime has also shown efficacy for functional heartburn, potentially due to its antinociceptive properties.20
Alternative and complementary therapies
Many esophageal centers use cognitive behavioral therapy and hypnotherapy as first-line treatment for functional esophageal disorders. Here again, it is important for the patient to understand the rationale of therapy for functional gastrointestinal disorders, given the stigma in the general population regarding psychotherapy.
Cognitive behavioral therapy has been used for functional gastrointestinal disorders for many years, as it has been shown to modulate visceral perception.21 Although published studies are limited, research regarding other functional esophageal disorders suggests that patients who commit to long-term behavioral therapy have had a significant improvement in symptoms.22
The goal of esophageal-directed behavioral therapy is to promote focused relaxation using deep breathing techniques, which can help patients manage esophageal hypervigilance, especially if symptoms continue despite neuromodulator therapy. Specifically, hypnotherapy has been shown to modulate functional chest pain through the visceral sensory pathway and also to suppress gastric acid secretion.21,23 A study of a 7-week hypnotherapy program reported significant benefits in heartburn relief and improved quality of life in patients with functional heartburn.24 The data support the use of behavioral therapies as first-line therapy or as adjunctive therapy for patients already taking a neuromodulator.
CASE FOLLOW-UP: IMPROVEMENT WITH TREATMENT
During a follow-up visit, the patient is given several printed resources, including the Rome Foundation article on functional heartburn.5 We again emphasize the benign nature of functional heartburn, noting the minimal risk of progression to esophageal adenocarcinoma, as she had no evidence of Barrett esophagus on endoscopy. And we discuss the natural course of functional heartburn, including the spontaneous resolution rate of about 40%.
For treatment, we present her the rationale for using neuromodulators and reassure her that these medications are for treatment of visceral hypersensitivity, not for anxiety or depression. After the discussion, the patient opts to start amitriptyline therapy at 10 mg every night at bedtime, increasing the dose by 10 mg every 2 weeks until symptoms improve, up to 75–100 mg every day.
After 3 months, the patient reports a 90% improvement in symptoms while on amitriptyline 30 mg every night. She is also able to taper her antisecretory medications once symptoms are controlled. We plan to continue amitriptyline at the current dose for 6 to 12 months, then discuss a slow taper to see if her symptoms spontaneously resolve.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101(8):1900–1920.
- Dean BB, Gano AD Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2(8):656–664. pmid:15290657
- Hachem C, Shaheen NJ. Diagnosis and management of functional heartburn. Am J Gastroenterol 2016; 111(1):53–61. doi:10.1038/ajg.2015.376
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58(2):295–309. doi:10.1136/gut.2007.145581
- Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Esophageal disorders. Gastroenterology 2016; 150(6):1368-1379. doi:10.1053/j.gastro.2016.02.012
- Kondo T, Miwa H. The role of esophageal hypersensitivity in functional heartburn. J Clin Gastroenterol 2017; 51(7):571–578. doi:10.1097/MCG.0000000000000885
- Farmer AD, Ruffle JK, Aziz Q. The role of esophageal hypersensitivity in functional esophageal disorders. J Clin Gastroenterol 2017; 51(2):91–99. doi:10.1097/MCG.0000000000000757
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55(10):1398–1402. doi:10.1136/gut.2005.087668
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108(3):308–328. doi:10.1038/ajg.2012.444
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27(2):160–174. doi:10.1111/nmo.12477
- Kichler AJ, Gabbard S. A man with progressive dysphagia. Cleve Clin J Med 2017; 84(6):443–449. doi:10.3949/ccjm.84a.16055
- Roman S, Gyawali CP, Savarino E, et al; GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29(10):1–15. doi:10.1111/nmo.13067
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007; 133(3):799–807. doi:10.1053/j.gastro.2007.06.010
- Park EY, Choi MG, Baeg M, et al. The value of early wireless esophageal pH monitoring in diagnosing functional heartburn in refractory gastroesophageal reflux disease. Dig Dis Sci 2013; 58(10):2933–2939. doi:10.1007/s10620-013-2728-4
- Khajanchee YS, Hong D, Hansen PD, Swanström LL. Outcomes of antireflux surgery in patients with normal preoperative 24-hour pH test results. Am J Surg 2004; 187(5):599–603. doi:10.1016/j.amjsurg.2004.01.010
- Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2012; 107(11):1662–1667. doi:10.1038/ajg.2011.179
- Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, Leelakusolvong S. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol 2016; 111(2):217–224. doi:10.1038/ajg.2015.413
- Keefer L, Kahrilas PJ. Low-dose tricyclics for esophageal hypersensitivity: is it all placebo effect? Am J Gastroenterol 2016; 111(2):225–227. doi:10.1038/ajg.2016.13
- Basu PP, Hempole H, Krishnaswamy N, Shah NJ, Aloysius, M. The effect of melatonin in functional heartburn: a randomized, placebo-controlled clinical trial. Open J Gastroenterol 2014; 4(2):56–61. doi:10.4236/ojgas.2014.42010
- Watanabe S, Hattori T, Kanazawa M, Kano M, Fukudo S. Role of histaminergic neurons in hypnotic modulation of brain processing of visceral perception. Neurogastroenterol Motil 2007; 19(10):831–838. doi:10.1111/j.1365-2982.2007.00959.x
- Riehl ME, Kinsinger S, Kahrilas PJ, Pandolfino JE, Keefer L. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus 2015; 28(5):428–436. doi:10.1111/dote.12219
- Klein KB, Spiegel D. Modulation of gastric acid secretion by hypnosis. Gastroenterology 1989; 96(6):1383–1387. pmid:2714570
- Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis Esophagus 2016; 29(5):490–496. doi:10.1111/dote.12353
A 44-year-old woman presents with an 8-year history of intermittent heartburn, and in the past year she has been experiencing her symptoms daily. She says the heartburn is constant and is worse immediately after eating spicy or acidic foods. She says she has had no dysphagia, weight loss, or vomiting. Her symptoms have persisted despite taking a histamine (H)2-receptor antagonist twice daily plus a proton pump inhibitor (PPI) before breakfast and dinner for more than 3 months.
She has undergone upper endoscopy 3 times in the past 8 years. Each time, the esophagus was normal with a regular Z-line and normal biopsy results from the proximal and distal esophagus.
The patient believes she has severe gastroesophageal reflux disease (GERD) and asks if she is a candidate for fundoplication surgery.
HEARTBURN IS A SYMPTOM; GERD IS A CONDITION
A distinction should be made between heartburn—the symptom of persistent retrosternal burning and discomfort—and gastroesophageal reflux disease—the condition in which reflux of stomach contents causes troublesome symptoms or complications.1 While many clinicians initially diagnose patients who have heartburn as having GERD, there are many other potential causes of their symptoms.
For patients with persistent heartburn, an empiric trial of a once-daily PPI is usually effective, but one-third of patients continue to have heartburn.2,3 The most common cause of this PPI-refractory heartburn is functional heartburn, a functional or hypersensitivity disorder of the esophagus.4
PATHOPHYSIOLOGY IS POORLY UNDERSTOOD
DIAGNOSTIC EVALUATION
Clinicians have several tests available for diagnosing these conditions.
Upper endoscopy
Upper endoscopy is recommended for patients with heartburn that does not respond to a 3-month trial of a PPI.9 Endoscopy is also indicated in any patient who has any of the following “alarm symptoms” that could be due to malignancy or peptic ulcer:
- Dysphagia
- Odynophagia
- Vomiting
- Unexplained weight loss or anemia
- Signs of gastrointestinal bleeding
- Anorexia
- New onset of dyspepsia in a patient over age 60.
During upper endoscopy, the esophagus is evaluated for reflux esophagitis, Barrett esophagus, and other inflammatory disorders such as infectious esophagitis. But even if the esophageal mucosa appears normal, the proximal and distal esophagus should be biopsied to rule out an inflammatory disorder such as eosinophilic or lymphocytic esophagitis.
Esophageal manometry
If endoscopic and esophageal biopsy results are inconclusive, a workup for an esophageal motility disorder is the next step. Dysphagia is the most common symptom of these disorders, although the initial presenting symptom may be heartburn or regurgitation that persists despite PPI therapy.
Manometry is used to test for motility disorders such as achalasia and esophageal spasm.10 After applying a local anesthetic inside the nares, the clinician inserts a flexible catheter (about 4 mm in diameter) with 36 pressure sensors spaced at 1-cm intervals into the nares and passes it through the esophagus and lower esophageal sphincter. The patient then swallows liquid, and the sensors relay the esophageal response, creating a topographic plot that shows esophageal peristalsis and lower esophageal sphincter relaxation.
Achalasia is identified by incomplete lower esophageal sphincter relaxation combined with 100% failed peristalsis in the body of the esophagus. Esophageal spasms are identified by a shortened distal latency, which corresponds to premature contraction of the esophagus during peristalsis.11
Esophageal pH testing
Measuring esophageal pH levels is an important step to quantify gastroesophageal reflux and determine if symptoms occur during reflux events. According to the updated Porto GERD consensus group recommendations,12 a pH test is positive if the acid exposure time is greater than 6% of the testing period. Testing the pH differentiates between GERD (abnormal acid exposure), reflux hypersensitivity (normal acid exposure, strong correlation between symptoms and reflux events), and functional heartburn (normal acid exposure, negative correlation between reflux events and symptoms).5 For this test, a pH probe is placed in the esophagus transnasally or endoscopically. The probe records esophageal pH levels for 24 to 96 hours in an outpatient setting. Antisecretory therapy needs to be withheld for 7 to 10 days before the test.
Transnasal pH probe. For this approach, a thin catheter is inserted through the nares and advanced until the tip is 5 cm proximal to the lower esophageal sphincter. (The placement is guided by the results of esophageal manometry, which is done immediately before pH catheter placement.) The tube is secured with clear tape on the side of the patient’s face, and the end is connected to a portable recorder that compiles the data. The patient pushes a button on the recorder when experiencing heartburn symptoms. (A nurse instructs the patient on proper procedure.) After 24 hours, the patient either removes the catheter or has the clinic remove it. The pH and symptom data are downloaded and analyzed.
Transnasal pH testing can be combined with impedance measurement, which can detect nonacid reflux or weakly acid reflux. However, the clinical significance of this measurement is unclear, as multiple studies have found total acid exposure time to be a better predictor of response to therapy than weakly acid or nonacid reflux.12
Wireless pH probe. This method uses a disposable, catheter-free, capsule device to measure esophageal pH. The capsule, about the size of a gel capsule or pencil eraser, is attached to the patient’s esophageal lining, usually during upper endoscopy. The capsule records pH levels in the lower esophagus for 48 to 96 hours and transmits the data wirelessly to a receiver the patient wears. The patient pushes buttons on the receiver to record symptom-specific data when experiencing heartburn, chest pain, regurgitation, or cough. The capsule detaches from the esophagus spontaneously, generally within 7 days, and is passed out of the body through a bowel movement.
Diagnosing functional heartburn
CASE CONTINUED: NORMAL RESULTS ON TESTING
Based on these results, her condition is diagnosed as functional heartburn, consistent with the Rome IV criteria.5
TREATMENT
Patient education is key
Patient education about the pathogenesis, natural history, and treatment options is the most important aspect of treating any functional gastrointestinal disorder. This includes the “brain-gut connection” and potential mechanisms of dysregulation. Patient education along with assessment of symptoms should be part of every visit, especially before discussing treatment options.
Patients whose condition is diagnosed as functional heartburn need reassurance that the condition is benign and, in particular, that the risk of progression to esophageal adenocarcinoma is minimal in the absence of Barrett esophagus.13 Also important to point out is that the disorder may spontaneously resolve: resolution rates of up to 40% have been reported for other functional gastrointestinal disorders.14
Antisecretory medications may work for some
A PPI or H2-receptor antagonist is the most common first-line treatment for heartburn symptoms. Although most patients with functional heartburn experience no improvement in symptoms with an antisecretory agent, a small number report some relief, which suggests that acid-suppression therapy may have an indirect impact on pain modulation in the esophagus.15 In patients who report symptom relief with an antisecretory agent, we suggest continuing the medication tapered to the lowest effective dose, with repeated reassurance that the medication can be discontinued safely at any time.
Antireflux surgery should be avoided
Antireflux surgery should be avoided in patients with normal pH testing and no objective finding of reflux, as this is associated with worse subjective outcomes than in patients with abnormal pH test results.16
Neuromodulators
It is important to discuss with patients the concept of neuromodulation, including the fact that antidepressants are often used because of their effects on serotonin and norepinephrine, which decrease visceral hypersensitivity.
The selective serotonin reuptake inhibitor citalopram has been shown to reduce esophageal hypersensitivity,17 and a tricyclic antidepressant has been shown to improve quality of life.18 These results have led experts to recommend a trial of a low dose of either type of medication.19 The dose of tricyclic antidepressant often needs to be increased sequentially every 2 to 4 weeks.
Interestingly, melatonin 6 mg at bedtime has also shown efficacy for functional heartburn, potentially due to its antinociceptive properties.20
Alternative and complementary therapies
Many esophageal centers use cognitive behavioral therapy and hypnotherapy as first-line treatment for functional esophageal disorders. Here again, it is important for the patient to understand the rationale of therapy for functional gastrointestinal disorders, given the stigma in the general population regarding psychotherapy.
Cognitive behavioral therapy has been used for functional gastrointestinal disorders for many years, as it has been shown to modulate visceral perception.21 Although published studies are limited, research regarding other functional esophageal disorders suggests that patients who commit to long-term behavioral therapy have had a significant improvement in symptoms.22
The goal of esophageal-directed behavioral therapy is to promote focused relaxation using deep breathing techniques, which can help patients manage esophageal hypervigilance, especially if symptoms continue despite neuromodulator therapy. Specifically, hypnotherapy has been shown to modulate functional chest pain through the visceral sensory pathway and also to suppress gastric acid secretion.21,23 A study of a 7-week hypnotherapy program reported significant benefits in heartburn relief and improved quality of life in patients with functional heartburn.24 The data support the use of behavioral therapies as first-line therapy or as adjunctive therapy for patients already taking a neuromodulator.
CASE FOLLOW-UP: IMPROVEMENT WITH TREATMENT
During a follow-up visit, the patient is given several printed resources, including the Rome Foundation article on functional heartburn.5 We again emphasize the benign nature of functional heartburn, noting the minimal risk of progression to esophageal adenocarcinoma, as she had no evidence of Barrett esophagus on endoscopy. And we discuss the natural course of functional heartburn, including the spontaneous resolution rate of about 40%.
For treatment, we present her the rationale for using neuromodulators and reassure her that these medications are for treatment of visceral hypersensitivity, not for anxiety or depression. After the discussion, the patient opts to start amitriptyline therapy at 10 mg every night at bedtime, increasing the dose by 10 mg every 2 weeks until symptoms improve, up to 75–100 mg every day.
After 3 months, the patient reports a 90% improvement in symptoms while on amitriptyline 30 mg every night. She is also able to taper her antisecretory medications once symptoms are controlled. We plan to continue amitriptyline at the current dose for 6 to 12 months, then discuss a slow taper to see if her symptoms spontaneously resolve.
A 44-year-old woman presents with an 8-year history of intermittent heartburn, and in the past year she has been experiencing her symptoms daily. She says the heartburn is constant and is worse immediately after eating spicy or acidic foods. She says she has had no dysphagia, weight loss, or vomiting. Her symptoms have persisted despite taking a histamine (H)2-receptor antagonist twice daily plus a proton pump inhibitor (PPI) before breakfast and dinner for more than 3 months.
She has undergone upper endoscopy 3 times in the past 8 years. Each time, the esophagus was normal with a regular Z-line and normal biopsy results from the proximal and distal esophagus.
The patient believes she has severe gastroesophageal reflux disease (GERD) and asks if she is a candidate for fundoplication surgery.
HEARTBURN IS A SYMPTOM; GERD IS A CONDITION
A distinction should be made between heartburn—the symptom of persistent retrosternal burning and discomfort—and gastroesophageal reflux disease—the condition in which reflux of stomach contents causes troublesome symptoms or complications.1 While many clinicians initially diagnose patients who have heartburn as having GERD, there are many other potential causes of their symptoms.
For patients with persistent heartburn, an empiric trial of a once-daily PPI is usually effective, but one-third of patients continue to have heartburn.2,3 The most common cause of this PPI-refractory heartburn is functional heartburn, a functional or hypersensitivity disorder of the esophagus.4
PATHOPHYSIOLOGY IS POORLY UNDERSTOOD
DIAGNOSTIC EVALUATION
Clinicians have several tests available for diagnosing these conditions.
Upper endoscopy
Upper endoscopy is recommended for patients with heartburn that does not respond to a 3-month trial of a PPI.9 Endoscopy is also indicated in any patient who has any of the following “alarm symptoms” that could be due to malignancy or peptic ulcer:
- Dysphagia
- Odynophagia
- Vomiting
- Unexplained weight loss or anemia
- Signs of gastrointestinal bleeding
- Anorexia
- New onset of dyspepsia in a patient over age 60.
During upper endoscopy, the esophagus is evaluated for reflux esophagitis, Barrett esophagus, and other inflammatory disorders such as infectious esophagitis. But even if the esophageal mucosa appears normal, the proximal and distal esophagus should be biopsied to rule out an inflammatory disorder such as eosinophilic or lymphocytic esophagitis.
Esophageal manometry
If endoscopic and esophageal biopsy results are inconclusive, a workup for an esophageal motility disorder is the next step. Dysphagia is the most common symptom of these disorders, although the initial presenting symptom may be heartburn or regurgitation that persists despite PPI therapy.
Manometry is used to test for motility disorders such as achalasia and esophageal spasm.10 After applying a local anesthetic inside the nares, the clinician inserts a flexible catheter (about 4 mm in diameter) with 36 pressure sensors spaced at 1-cm intervals into the nares and passes it through the esophagus and lower esophageal sphincter. The patient then swallows liquid, and the sensors relay the esophageal response, creating a topographic plot that shows esophageal peristalsis and lower esophageal sphincter relaxation.
Achalasia is identified by incomplete lower esophageal sphincter relaxation combined with 100% failed peristalsis in the body of the esophagus. Esophageal spasms are identified by a shortened distal latency, which corresponds to premature contraction of the esophagus during peristalsis.11
Esophageal pH testing
Measuring esophageal pH levels is an important step to quantify gastroesophageal reflux and determine if symptoms occur during reflux events. According to the updated Porto GERD consensus group recommendations,12 a pH test is positive if the acid exposure time is greater than 6% of the testing period. Testing the pH differentiates between GERD (abnormal acid exposure), reflux hypersensitivity (normal acid exposure, strong correlation between symptoms and reflux events), and functional heartburn (normal acid exposure, negative correlation between reflux events and symptoms).5 For this test, a pH probe is placed in the esophagus transnasally or endoscopically. The probe records esophageal pH levels for 24 to 96 hours in an outpatient setting. Antisecretory therapy needs to be withheld for 7 to 10 days before the test.
Transnasal pH probe. For this approach, a thin catheter is inserted through the nares and advanced until the tip is 5 cm proximal to the lower esophageal sphincter. (The placement is guided by the results of esophageal manometry, which is done immediately before pH catheter placement.) The tube is secured with clear tape on the side of the patient’s face, and the end is connected to a portable recorder that compiles the data. The patient pushes a button on the recorder when experiencing heartburn symptoms. (A nurse instructs the patient on proper procedure.) After 24 hours, the patient either removes the catheter or has the clinic remove it. The pH and symptom data are downloaded and analyzed.
Transnasal pH testing can be combined with impedance measurement, which can detect nonacid reflux or weakly acid reflux. However, the clinical significance of this measurement is unclear, as multiple studies have found total acid exposure time to be a better predictor of response to therapy than weakly acid or nonacid reflux.12
Wireless pH probe. This method uses a disposable, catheter-free, capsule device to measure esophageal pH. The capsule, about the size of a gel capsule or pencil eraser, is attached to the patient’s esophageal lining, usually during upper endoscopy. The capsule records pH levels in the lower esophagus for 48 to 96 hours and transmits the data wirelessly to a receiver the patient wears. The patient pushes buttons on the receiver to record symptom-specific data when experiencing heartburn, chest pain, regurgitation, or cough. The capsule detaches from the esophagus spontaneously, generally within 7 days, and is passed out of the body through a bowel movement.
Diagnosing functional heartburn
CASE CONTINUED: NORMAL RESULTS ON TESTING
Based on these results, her condition is diagnosed as functional heartburn, consistent with the Rome IV criteria.5
TREATMENT
Patient education is key
Patient education about the pathogenesis, natural history, and treatment options is the most important aspect of treating any functional gastrointestinal disorder. This includes the “brain-gut connection” and potential mechanisms of dysregulation. Patient education along with assessment of symptoms should be part of every visit, especially before discussing treatment options.
Patients whose condition is diagnosed as functional heartburn need reassurance that the condition is benign and, in particular, that the risk of progression to esophageal adenocarcinoma is minimal in the absence of Barrett esophagus.13 Also important to point out is that the disorder may spontaneously resolve: resolution rates of up to 40% have been reported for other functional gastrointestinal disorders.14
Antisecretory medications may work for some
A PPI or H2-receptor antagonist is the most common first-line treatment for heartburn symptoms. Although most patients with functional heartburn experience no improvement in symptoms with an antisecretory agent, a small number report some relief, which suggests that acid-suppression therapy may have an indirect impact on pain modulation in the esophagus.15 In patients who report symptom relief with an antisecretory agent, we suggest continuing the medication tapered to the lowest effective dose, with repeated reassurance that the medication can be discontinued safely at any time.
Antireflux surgery should be avoided
Antireflux surgery should be avoided in patients with normal pH testing and no objective finding of reflux, as this is associated with worse subjective outcomes than in patients with abnormal pH test results.16
Neuromodulators
It is important to discuss with patients the concept of neuromodulation, including the fact that antidepressants are often used because of their effects on serotonin and norepinephrine, which decrease visceral hypersensitivity.
The selective serotonin reuptake inhibitor citalopram has been shown to reduce esophageal hypersensitivity,17 and a tricyclic antidepressant has been shown to improve quality of life.18 These results have led experts to recommend a trial of a low dose of either type of medication.19 The dose of tricyclic antidepressant often needs to be increased sequentially every 2 to 4 weeks.
Interestingly, melatonin 6 mg at bedtime has also shown efficacy for functional heartburn, potentially due to its antinociceptive properties.20
Alternative and complementary therapies
Many esophageal centers use cognitive behavioral therapy and hypnotherapy as first-line treatment for functional esophageal disorders. Here again, it is important for the patient to understand the rationale of therapy for functional gastrointestinal disorders, given the stigma in the general population regarding psychotherapy.
Cognitive behavioral therapy has been used for functional gastrointestinal disorders for many years, as it has been shown to modulate visceral perception.21 Although published studies are limited, research regarding other functional esophageal disorders suggests that patients who commit to long-term behavioral therapy have had a significant improvement in symptoms.22
The goal of esophageal-directed behavioral therapy is to promote focused relaxation using deep breathing techniques, which can help patients manage esophageal hypervigilance, especially if symptoms continue despite neuromodulator therapy. Specifically, hypnotherapy has been shown to modulate functional chest pain through the visceral sensory pathway and also to suppress gastric acid secretion.21,23 A study of a 7-week hypnotherapy program reported significant benefits in heartburn relief and improved quality of life in patients with functional heartburn.24 The data support the use of behavioral therapies as first-line therapy or as adjunctive therapy for patients already taking a neuromodulator.
CASE FOLLOW-UP: IMPROVEMENT WITH TREATMENT
During a follow-up visit, the patient is given several printed resources, including the Rome Foundation article on functional heartburn.5 We again emphasize the benign nature of functional heartburn, noting the minimal risk of progression to esophageal adenocarcinoma, as she had no evidence of Barrett esophagus on endoscopy. And we discuss the natural course of functional heartburn, including the spontaneous resolution rate of about 40%.
For treatment, we present her the rationale for using neuromodulators and reassure her that these medications are for treatment of visceral hypersensitivity, not for anxiety or depression. After the discussion, the patient opts to start amitriptyline therapy at 10 mg every night at bedtime, increasing the dose by 10 mg every 2 weeks until symptoms improve, up to 75–100 mg every day.
After 3 months, the patient reports a 90% improvement in symptoms while on amitriptyline 30 mg every night. She is also able to taper her antisecretory medications once symptoms are controlled. We plan to continue amitriptyline at the current dose for 6 to 12 months, then discuss a slow taper to see if her symptoms spontaneously resolve.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101(8):1900–1920.
- Dean BB, Gano AD Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2(8):656–664. pmid:15290657
- Hachem C, Shaheen NJ. Diagnosis and management of functional heartburn. Am J Gastroenterol 2016; 111(1):53–61. doi:10.1038/ajg.2015.376
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58(2):295–309. doi:10.1136/gut.2007.145581
- Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Esophageal disorders. Gastroenterology 2016; 150(6):1368-1379. doi:10.1053/j.gastro.2016.02.012
- Kondo T, Miwa H. The role of esophageal hypersensitivity in functional heartburn. J Clin Gastroenterol 2017; 51(7):571–578. doi:10.1097/MCG.0000000000000885
- Farmer AD, Ruffle JK, Aziz Q. The role of esophageal hypersensitivity in functional esophageal disorders. J Clin Gastroenterol 2017; 51(2):91–99. doi:10.1097/MCG.0000000000000757
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55(10):1398–1402. doi:10.1136/gut.2005.087668
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108(3):308–328. doi:10.1038/ajg.2012.444
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27(2):160–174. doi:10.1111/nmo.12477
- Kichler AJ, Gabbard S. A man with progressive dysphagia. Cleve Clin J Med 2017; 84(6):443–449. doi:10.3949/ccjm.84a.16055
- Roman S, Gyawali CP, Savarino E, et al; GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29(10):1–15. doi:10.1111/nmo.13067
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007; 133(3):799–807. doi:10.1053/j.gastro.2007.06.010
- Park EY, Choi MG, Baeg M, et al. The value of early wireless esophageal pH monitoring in diagnosing functional heartburn in refractory gastroesophageal reflux disease. Dig Dis Sci 2013; 58(10):2933–2939. doi:10.1007/s10620-013-2728-4
- Khajanchee YS, Hong D, Hansen PD, Swanström LL. Outcomes of antireflux surgery in patients with normal preoperative 24-hour pH test results. Am J Surg 2004; 187(5):599–603. doi:10.1016/j.amjsurg.2004.01.010
- Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2012; 107(11):1662–1667. doi:10.1038/ajg.2011.179
- Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, Leelakusolvong S. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol 2016; 111(2):217–224. doi:10.1038/ajg.2015.413
- Keefer L, Kahrilas PJ. Low-dose tricyclics for esophageal hypersensitivity: is it all placebo effect? Am J Gastroenterol 2016; 111(2):225–227. doi:10.1038/ajg.2016.13
- Basu PP, Hempole H, Krishnaswamy N, Shah NJ, Aloysius, M. The effect of melatonin in functional heartburn: a randomized, placebo-controlled clinical trial. Open J Gastroenterol 2014; 4(2):56–61. doi:10.4236/ojgas.2014.42010
- Watanabe S, Hattori T, Kanazawa M, Kano M, Fukudo S. Role of histaminergic neurons in hypnotic modulation of brain processing of visceral perception. Neurogastroenterol Motil 2007; 19(10):831–838. doi:10.1111/j.1365-2982.2007.00959.x
- Riehl ME, Kinsinger S, Kahrilas PJ, Pandolfino JE, Keefer L. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus 2015; 28(5):428–436. doi:10.1111/dote.12219
- Klein KB, Spiegel D. Modulation of gastric acid secretion by hypnosis. Gastroenterology 1989; 96(6):1383–1387. pmid:2714570
- Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis Esophagus 2016; 29(5):490–496. doi:10.1111/dote.12353
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101(8):1900–1920.
- Dean BB, Gano AD Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2(8):656–664. pmid:15290657
- Hachem C, Shaheen NJ. Diagnosis and management of functional heartburn. Am J Gastroenterol 2016; 111(1):53–61. doi:10.1038/ajg.2015.376
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58(2):295–309. doi:10.1136/gut.2007.145581
- Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Esophageal disorders. Gastroenterology 2016; 150(6):1368-1379. doi:10.1053/j.gastro.2016.02.012
- Kondo T, Miwa H. The role of esophageal hypersensitivity in functional heartburn. J Clin Gastroenterol 2017; 51(7):571–578. doi:10.1097/MCG.0000000000000885
- Farmer AD, Ruffle JK, Aziz Q. The role of esophageal hypersensitivity in functional esophageal disorders. J Clin Gastroenterol 2017; 51(2):91–99. doi:10.1097/MCG.0000000000000757
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55(10):1398–1402. doi:10.1136/gut.2005.087668
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108(3):308–328. doi:10.1038/ajg.2012.444
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27(2):160–174. doi:10.1111/nmo.12477
- Kichler AJ, Gabbard S. A man with progressive dysphagia. Cleve Clin J Med 2017; 84(6):443–449. doi:10.3949/ccjm.84a.16055
- Roman S, Gyawali CP, Savarino E, et al; GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29(10):1–15. doi:10.1111/nmo.13067
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007; 133(3):799–807. doi:10.1053/j.gastro.2007.06.010
- Park EY, Choi MG, Baeg M, et al. The value of early wireless esophageal pH monitoring in diagnosing functional heartburn in refractory gastroesophageal reflux disease. Dig Dis Sci 2013; 58(10):2933–2939. doi:10.1007/s10620-013-2728-4
- Khajanchee YS, Hong D, Hansen PD, Swanström LL. Outcomes of antireflux surgery in patients with normal preoperative 24-hour pH test results. Am J Surg 2004; 187(5):599–603. doi:10.1016/j.amjsurg.2004.01.010
- Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2012; 107(11):1662–1667. doi:10.1038/ajg.2011.179
- Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, Leelakusolvong S. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol 2016; 111(2):217–224. doi:10.1038/ajg.2015.413
- Keefer L, Kahrilas PJ. Low-dose tricyclics for esophageal hypersensitivity: is it all placebo effect? Am J Gastroenterol 2016; 111(2):225–227. doi:10.1038/ajg.2016.13
- Basu PP, Hempole H, Krishnaswamy N, Shah NJ, Aloysius, M. The effect of melatonin in functional heartburn: a randomized, placebo-controlled clinical trial. Open J Gastroenterol 2014; 4(2):56–61. doi:10.4236/ojgas.2014.42010
- Watanabe S, Hattori T, Kanazawa M, Kano M, Fukudo S. Role of histaminergic neurons in hypnotic modulation of brain processing of visceral perception. Neurogastroenterol Motil 2007; 19(10):831–838. doi:10.1111/j.1365-2982.2007.00959.x
- Riehl ME, Kinsinger S, Kahrilas PJ, Pandolfino JE, Keefer L. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus 2015; 28(5):428–436. doi:10.1111/dote.12219
- Klein KB, Spiegel D. Modulation of gastric acid secretion by hypnosis. Gastroenterology 1989; 96(6):1383–1387. pmid:2714570
- Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis Esophagus 2016; 29(5):490–496. doi:10.1111/dote.12353
KEY POINTS
- Functional heartburn accounts for more than half of all referrals for PPI-refractory GERD.
- Diagnostic criteria require at least 3 months of symptoms in the 6 months before presentation.
- Results of upper endoscopy with biopsy, esophageal manometry, and esophageal pH monitoring must be normal.
- Patient education is key, with reassurance that the risk of progression to malignancy is low in the absence of Barrett esophagus, and that the condition remits spontaneously in up to 40% of cases.
- Neuromodulators to reduce pain perception are the mainstay of treatment for functional gastrointestinal disorders such as functional heartburn. Cognitive behavioral therapy and hypnotherapy are also used as first-line treatment.
Beyond depression: Other uses for tricyclic antidepressants
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
KEY POINTS
- Amitriptyline is the most useful TCA for many painful conditions.
- TCAs can be especially helpful for patients with a pain syndrome or insomnia with comorbid depression, although their benefits appear to be independent of antidepressant effects.
- TCAs have long half-lives and so can be taken once a day.
- Effective dosages for symptom control in many conditions are lower than for severe depression; dosage should start low and be gradually increased while monitoring efficacy and adverse effects.
- TCAs should not be used concurrently with a monoamine oxidase inhibitor and by certain patient groups: the elderly, pregnant women, and patients with certain cardiac conduction abnormalities, epilepsy, or risk of suicide.
Off-label and oft-prescribed
In no way do I minimize the value of the imprimatur of FDA approval stating a drug, after appropriate preclinical and clinical studies, is deemed safe and effective. Whatever the agency’s shortcomings, the story of thalidomide (a drug never approved by the FDA) gives credence to the value of having a robust approval process. Arguments will likely continue forever as to whether the agency errs on the side of being too permissive or too restrictive in its approval process.
Nonetheless, I believe there are valid clinical reasons why we should continue to prescribe FDA-approved medications for nonapproved indications. In my practice, I treat some conditions that are sufficiently uncommon or heterogeneous in expression that large-scale clinical trials are logistically hard to carry out or deemed financially unviable by the corporate sponsor, even though clinical experience has informed us of a reasonable likelihood of efficacy. Sometimes drugs have “failed” in clinical trials, but experience and post hoc subset analysis of data have indicated a likely positive response in certain patients.
Although a drug that has been FDA approved has passed significant safety testing, the patients exposed to the drug when it was evaluated for treating a certain disease may be strikingly different from patients who have a different disease—the age, sex, comorbidities, and coprescribed medications may all differ significantly in the population of patients with the “off-label” disorder. Hence, appropriate caution is warranted, and if relevant, this should be explained to patients before giving them the medication.
In this issue of the Journal, 2 papers address the use of medications in an “off-label” manner. Schneider and colleagues discuss several frequent clinical uses of tricyclic antidepressants for reasons other than depression, and Modesto-Lowe and colleagues review the more controversial use of gabapentin in patients with alcohol use disorder. The hoped-for benefits in both circumstances are symptomatic, and both benefits and side effects are dose-related in ways not necessarily coinciding with those in the FDA-labeled indications.
My experience in using tricyclics as adjunctive treatment for fibromyalgia is that patients are quite sensitive to some of the side effects of the drugs (eg, oral dryness and fatigue), even in low doses. Moreover, we should expect only modest benefits, which should be explicitly described to the patient: improved quality of sleep with resultant decreased fatigue (while we watch closely for worsened fatigue from too-high dosing) and a modest reduction in pain over time as part of a multimodality treatment plan. I often find that practitioners who are less familiar with the use of these medications in this setting tend to start at lowish (but higher than often tolerated) doses, have patients take the medication too close to bedtime (resulting in some morning hangover sensation), fail to discuss the timing and degree of expected pain relief, don’t titrate the dose over time, and are not aware of the different responses that patients may experience with different medications within the same class. As with all prescribed medications, the benefits and ill effects must be frequently assessed, and particularly with these medications, one must be willing to discontinue them if appropriate outcomes are not achieved.
“Off-label” should not imply off the table as a therapeutic option. But it is incumbent on us to devote sufficient time to explain to each patient the anticipated side effects and hoped-for benefits, particularly since in most cases, we and our patients cannot refer to the results of definitive phase 3 clinical trials or patient online information sites that are totally relevant, reliable, data supported, and FDA reviewed.
In no way do I minimize the value of the imprimatur of FDA approval stating a drug, after appropriate preclinical and clinical studies, is deemed safe and effective. Whatever the agency’s shortcomings, the story of thalidomide (a drug never approved by the FDA) gives credence to the value of having a robust approval process. Arguments will likely continue forever as to whether the agency errs on the side of being too permissive or too restrictive in its approval process.
Nonetheless, I believe there are valid clinical reasons why we should continue to prescribe FDA-approved medications for nonapproved indications. In my practice, I treat some conditions that are sufficiently uncommon or heterogeneous in expression that large-scale clinical trials are logistically hard to carry out or deemed financially unviable by the corporate sponsor, even though clinical experience has informed us of a reasonable likelihood of efficacy. Sometimes drugs have “failed” in clinical trials, but experience and post hoc subset analysis of data have indicated a likely positive response in certain patients.
Although a drug that has been FDA approved has passed significant safety testing, the patients exposed to the drug when it was evaluated for treating a certain disease may be strikingly different from patients who have a different disease—the age, sex, comorbidities, and coprescribed medications may all differ significantly in the population of patients with the “off-label” disorder. Hence, appropriate caution is warranted, and if relevant, this should be explained to patients before giving them the medication.
In this issue of the Journal, 2 papers address the use of medications in an “off-label” manner. Schneider and colleagues discuss several frequent clinical uses of tricyclic antidepressants for reasons other than depression, and Modesto-Lowe and colleagues review the more controversial use of gabapentin in patients with alcohol use disorder. The hoped-for benefits in both circumstances are symptomatic, and both benefits and side effects are dose-related in ways not necessarily coinciding with those in the FDA-labeled indications.
My experience in using tricyclics as adjunctive treatment for fibromyalgia is that patients are quite sensitive to some of the side effects of the drugs (eg, oral dryness and fatigue), even in low doses. Moreover, we should expect only modest benefits, which should be explicitly described to the patient: improved quality of sleep with resultant decreased fatigue (while we watch closely for worsened fatigue from too-high dosing) and a modest reduction in pain over time as part of a multimodality treatment plan. I often find that practitioners who are less familiar with the use of these medications in this setting tend to start at lowish (but higher than often tolerated) doses, have patients take the medication too close to bedtime (resulting in some morning hangover sensation), fail to discuss the timing and degree of expected pain relief, don’t titrate the dose over time, and are not aware of the different responses that patients may experience with different medications within the same class. As with all prescribed medications, the benefits and ill effects must be frequently assessed, and particularly with these medications, one must be willing to discontinue them if appropriate outcomes are not achieved.
“Off-label” should not imply off the table as a therapeutic option. But it is incumbent on us to devote sufficient time to explain to each patient the anticipated side effects and hoped-for benefits, particularly since in most cases, we and our patients cannot refer to the results of definitive phase 3 clinical trials or patient online information sites that are totally relevant, reliable, data supported, and FDA reviewed.
In no way do I minimize the value of the imprimatur of FDA approval stating a drug, after appropriate preclinical and clinical studies, is deemed safe and effective. Whatever the agency’s shortcomings, the story of thalidomide (a drug never approved by the FDA) gives credence to the value of having a robust approval process. Arguments will likely continue forever as to whether the agency errs on the side of being too permissive or too restrictive in its approval process.
Nonetheless, I believe there are valid clinical reasons why we should continue to prescribe FDA-approved medications for nonapproved indications. In my practice, I treat some conditions that are sufficiently uncommon or heterogeneous in expression that large-scale clinical trials are logistically hard to carry out or deemed financially unviable by the corporate sponsor, even though clinical experience has informed us of a reasonable likelihood of efficacy. Sometimes drugs have “failed” in clinical trials, but experience and post hoc subset analysis of data have indicated a likely positive response in certain patients.
Although a drug that has been FDA approved has passed significant safety testing, the patients exposed to the drug when it was evaluated for treating a certain disease may be strikingly different from patients who have a different disease—the age, sex, comorbidities, and coprescribed medications may all differ significantly in the population of patients with the “off-label” disorder. Hence, appropriate caution is warranted, and if relevant, this should be explained to patients before giving them the medication.
In this issue of the Journal, 2 papers address the use of medications in an “off-label” manner. Schneider and colleagues discuss several frequent clinical uses of tricyclic antidepressants for reasons other than depression, and Modesto-Lowe and colleagues review the more controversial use of gabapentin in patients with alcohol use disorder. The hoped-for benefits in both circumstances are symptomatic, and both benefits and side effects are dose-related in ways not necessarily coinciding with those in the FDA-labeled indications.
My experience in using tricyclics as adjunctive treatment for fibromyalgia is that patients are quite sensitive to some of the side effects of the drugs (eg, oral dryness and fatigue), even in low doses. Moreover, we should expect only modest benefits, which should be explicitly described to the patient: improved quality of sleep with resultant decreased fatigue (while we watch closely for worsened fatigue from too-high dosing) and a modest reduction in pain over time as part of a multimodality treatment plan. I often find that practitioners who are less familiar with the use of these medications in this setting tend to start at lowish (but higher than often tolerated) doses, have patients take the medication too close to bedtime (resulting in some morning hangover sensation), fail to discuss the timing and degree of expected pain relief, don’t titrate the dose over time, and are not aware of the different responses that patients may experience with different medications within the same class. As with all prescribed medications, the benefits and ill effects must be frequently assessed, and particularly with these medications, one must be willing to discontinue them if appropriate outcomes are not achieved.
“Off-label” should not imply off the table as a therapeutic option. But it is incumbent on us to devote sufficient time to explain to each patient the anticipated side effects and hoped-for benefits, particularly since in most cases, we and our patients cannot refer to the results of definitive phase 3 clinical trials or patient online information sites that are totally relevant, reliable, data supported, and FDA reviewed.
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
OA management guidelines forgo treatment hierarchy or order but emphasize severity, patient risk factors
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
REPORTING FROM ACR 2019