User login
FDA approves first IV migraine prevention drug
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
FROM MEDSCAPE.COM
Prescription osteoarthritis relief gets OTC approval
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
The Food and Drug Administration has approved formerly prescription-only Voltaren Arthritis Pain (diclofenac sodium topical gel, 1%) for nonprescription use via a process known as a prescription to over-the-counter (Rx-to-OTC) switch, according to a news release from the agency.
“As a result of the Rx-to-OTC switch process, many products sold over the counter today use ingredients or dosage strengths that were available only by prescription 30 years ago,” Karen Mahoney, MD, acting deputy director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the release.
This switch to nonprescription status is usually initiated by the manufacturer, who must provide data that demonstrates the drug in question is both safe and effective as self-medication in accordance with the proposed labeling and that consumers can use it safely and effectively without the supervision of a health care professional.
This particular therapy is a topical NSAID gel and was first approved by the FDA in 2007 with the indication for relief of osteoarthritis pain. It can take 7 days to have an effect, but if patients find it takes longer than that or they need to use it for more than 21 days, they should seek medical attention. The gel can cause severe allergic reactions, especially in people allergic to aspirin; patients who experience such reactions are advised to stop use and seek immediate medical care. Other concerns include potential for liver damage with extended use; the possibility of severe stomach bleeds; and risk of heart attack, heart failure, and stroke.
The gel will no longer be available in prescription form.
Full prescribing information can be found on the FDA website, as can the full news release regarding this approval.
Tramadol use for noncancer pain linked with increased hip fracture risk
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
Pharmacologic prophylaxis fails in pediatric migraine
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
FROM JAMA PEDIATRICS
Shift in approach is encouraged in assessing chronic pain
In many cases, dietary interventions can lead to less inflammation
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
In many cases, dietary interventions can lead to less inflammation
In many cases, dietary interventions can lead to less inflammation
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
REPORTING FROM A NATURAL SUPPLEMENTS UPDATE
Lidocaine-prilocaine cream tops lidocaine injections for vulvar biopsy pain
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
FROM OBSTETRICS AND GYNECOLOGY
Patients remain satisfied despite reduced use of opioids post partum
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
REPORTING FROM THE PREGNANCY MEETING
IBD quality initiative slashes ED utilization
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.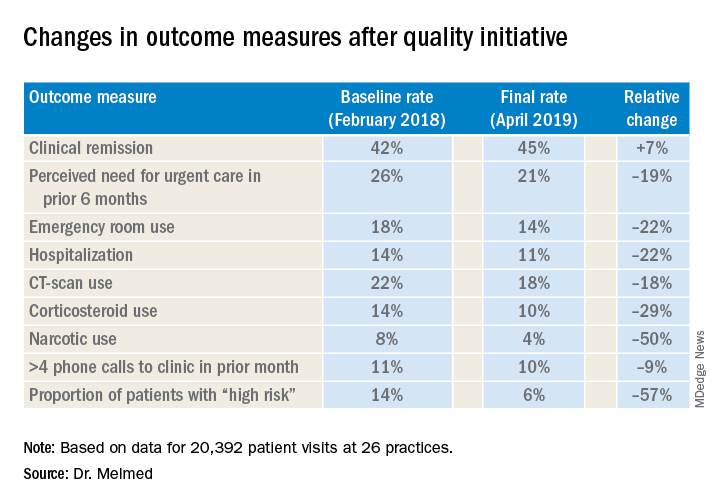
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
REPORTING FROM CROHN’S & COLITIS CONGRESS
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.

NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Dependent trait in chronic migraine may predict nonresponse to onabotulinumtoxin A
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
FROM HEADACHE