User login
Antifungals during pregnancy and breastfeeding
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Study: Spanking may change children’s brains
Rare is the parent who has never so much as thought about spanking an unruly child. But a new study provides another reason to avoid corporal punishment: Spanking may cause changes in the same areas of a child’s brain that are affected by more severe physical and sexual abuse.
Previous research has consistently found links between spanking and behavioral problems, aggression, depression, and anxiety, says Jorge Cuartas, a doctoral candidate at the Harvard Graduate School of Education and first author of the study. “We wanted to look at one potential mechanism, brain development, that might explain how corporal punishment can impact children’s behavior and cognitive development.”
The study, published in Child Development, used functional MRIs to map brain changes in 147 tweens who’d never experienced physical or sexual abuse. Researchers tracked which parts of the children’s brains activated in response to neutral or fearful facial expressions. When shown pictures of someone looking fearful, kids who reported having been spanked had a larger response in certain parts of the brain than kids who hadn’t been. Those areas drive the response to environmental cues, recognizing threats and reacting to them. If a child’s brain overreacts, behavioral challenges can result.
“We saw those changes in the same areas as more severe forms of abuse or domestic violence. It suggests the difference is of degree rather than type,” Mr. Cuartas says. As far as a child’s brain is concerned, “It’s all violence.”
It’s a significant finding because many parents don’t think of spanking as being violent, says Vincent J. Palusci, MD, a pediatrician and editor-in-chief of the journal Child Maltreatment. “We want to raise kids who are happy and healthy, and many parents who use spanking are doing it with that goal.”
Spanking in the U.S.
Around the world, 62 states and countries have outlawed corporal punishment. While the U.S. has no such protections, both the American Academy of Pediatrics and the American Psychological Association have condemned the practice. Acceptance of spanking seems to be shrinking: The percentage of parents in this country who say they spank their children is trending downward. In 1993, 50% of parents surveyed said they did, but by 2017 that number had fallen to 35%. Still far too many, Mr. Cuartas and Dr. Palusci say, but a promising trend.
“While we wouldn’t as parents want to hurt our kids,” Dr. Palusci says, “we need to understand that spanking can be just as bad as things we’d never do.”
Discipline vs. punishment
For some parents, it may require a shift in thinking, differentiating between discipline and punishment. “Discipline changes behavior – it teaches positive behavior, empathy, essential social skills. But that’s different from punishment,” Mr. Cuartas says. “That makes somebody feel pain or shame. We have to start thinking about spanking as punishment.”
That can be difficult, especially for adults who’ve been spanked themselves. They may believe that since they turned out fine, spanking must be fine, too. But the study doesn’t suggest that every child who’s spanked will have these difficulties – it just shows they happen, Mr. Cuartas says. “Compare this to smoking. We all know someone who smokes who’s healthy, but that doesn’t mean smoking is good,” he says. “Individual cases aren’t enough to understand whether certain experiences are good or bad.”
Dr. Palusci draws parallels to the advice pregnant women receive about taking medications: If it hasn’t been tested in pregnancy specifically, no amount can be considered safe. “We don’t have the studies to say how much spanking is dangerous, so we have to think that any amount has this potential.”
A version of this article first appeared on Medscape.com.
Rare is the parent who has never so much as thought about spanking an unruly child. But a new study provides another reason to avoid corporal punishment: Spanking may cause changes in the same areas of a child’s brain that are affected by more severe physical and sexual abuse.
Previous research has consistently found links between spanking and behavioral problems, aggression, depression, and anxiety, says Jorge Cuartas, a doctoral candidate at the Harvard Graduate School of Education and first author of the study. “We wanted to look at one potential mechanism, brain development, that might explain how corporal punishment can impact children’s behavior and cognitive development.”
The study, published in Child Development, used functional MRIs to map brain changes in 147 tweens who’d never experienced physical or sexual abuse. Researchers tracked which parts of the children’s brains activated in response to neutral or fearful facial expressions. When shown pictures of someone looking fearful, kids who reported having been spanked had a larger response in certain parts of the brain than kids who hadn’t been. Those areas drive the response to environmental cues, recognizing threats and reacting to them. If a child’s brain overreacts, behavioral challenges can result.
“We saw those changes in the same areas as more severe forms of abuse or domestic violence. It suggests the difference is of degree rather than type,” Mr. Cuartas says. As far as a child’s brain is concerned, “It’s all violence.”
It’s a significant finding because many parents don’t think of spanking as being violent, says Vincent J. Palusci, MD, a pediatrician and editor-in-chief of the journal Child Maltreatment. “We want to raise kids who are happy and healthy, and many parents who use spanking are doing it with that goal.”
Spanking in the U.S.
Around the world, 62 states and countries have outlawed corporal punishment. While the U.S. has no such protections, both the American Academy of Pediatrics and the American Psychological Association have condemned the practice. Acceptance of spanking seems to be shrinking: The percentage of parents in this country who say they spank their children is trending downward. In 1993, 50% of parents surveyed said they did, but by 2017 that number had fallen to 35%. Still far too many, Mr. Cuartas and Dr. Palusci say, but a promising trend.
“While we wouldn’t as parents want to hurt our kids,” Dr. Palusci says, “we need to understand that spanking can be just as bad as things we’d never do.”
Discipline vs. punishment
For some parents, it may require a shift in thinking, differentiating between discipline and punishment. “Discipline changes behavior – it teaches positive behavior, empathy, essential social skills. But that’s different from punishment,” Mr. Cuartas says. “That makes somebody feel pain or shame. We have to start thinking about spanking as punishment.”
That can be difficult, especially for adults who’ve been spanked themselves. They may believe that since they turned out fine, spanking must be fine, too. But the study doesn’t suggest that every child who’s spanked will have these difficulties – it just shows they happen, Mr. Cuartas says. “Compare this to smoking. We all know someone who smokes who’s healthy, but that doesn’t mean smoking is good,” he says. “Individual cases aren’t enough to understand whether certain experiences are good or bad.”
Dr. Palusci draws parallels to the advice pregnant women receive about taking medications: If it hasn’t been tested in pregnancy specifically, no amount can be considered safe. “We don’t have the studies to say how much spanking is dangerous, so we have to think that any amount has this potential.”
A version of this article first appeared on Medscape.com.
Rare is the parent who has never so much as thought about spanking an unruly child. But a new study provides another reason to avoid corporal punishment: Spanking may cause changes in the same areas of a child’s brain that are affected by more severe physical and sexual abuse.
Previous research has consistently found links between spanking and behavioral problems, aggression, depression, and anxiety, says Jorge Cuartas, a doctoral candidate at the Harvard Graduate School of Education and first author of the study. “We wanted to look at one potential mechanism, brain development, that might explain how corporal punishment can impact children’s behavior and cognitive development.”
The study, published in Child Development, used functional MRIs to map brain changes in 147 tweens who’d never experienced physical or sexual abuse. Researchers tracked which parts of the children’s brains activated in response to neutral or fearful facial expressions. When shown pictures of someone looking fearful, kids who reported having been spanked had a larger response in certain parts of the brain than kids who hadn’t been. Those areas drive the response to environmental cues, recognizing threats and reacting to them. If a child’s brain overreacts, behavioral challenges can result.
“We saw those changes in the same areas as more severe forms of abuse or domestic violence. It suggests the difference is of degree rather than type,” Mr. Cuartas says. As far as a child’s brain is concerned, “It’s all violence.”
It’s a significant finding because many parents don’t think of spanking as being violent, says Vincent J. Palusci, MD, a pediatrician and editor-in-chief of the journal Child Maltreatment. “We want to raise kids who are happy and healthy, and many parents who use spanking are doing it with that goal.”
Spanking in the U.S.
Around the world, 62 states and countries have outlawed corporal punishment. While the U.S. has no such protections, both the American Academy of Pediatrics and the American Psychological Association have condemned the practice. Acceptance of spanking seems to be shrinking: The percentage of parents in this country who say they spank their children is trending downward. In 1993, 50% of parents surveyed said they did, but by 2017 that number had fallen to 35%. Still far too many, Mr. Cuartas and Dr. Palusci say, but a promising trend.
“While we wouldn’t as parents want to hurt our kids,” Dr. Palusci says, “we need to understand that spanking can be just as bad as things we’d never do.”
Discipline vs. punishment
For some parents, it may require a shift in thinking, differentiating between discipline and punishment. “Discipline changes behavior – it teaches positive behavior, empathy, essential social skills. But that’s different from punishment,” Mr. Cuartas says. “That makes somebody feel pain or shame. We have to start thinking about spanking as punishment.”
That can be difficult, especially for adults who’ve been spanked themselves. They may believe that since they turned out fine, spanking must be fine, too. But the study doesn’t suggest that every child who’s spanked will have these difficulties – it just shows they happen, Mr. Cuartas says. “Compare this to smoking. We all know someone who smokes who’s healthy, but that doesn’t mean smoking is good,” he says. “Individual cases aren’t enough to understand whether certain experiences are good or bad.”
Dr. Palusci draws parallels to the advice pregnant women receive about taking medications: If it hasn’t been tested in pregnancy specifically, no amount can be considered safe. “We don’t have the studies to say how much spanking is dangerous, so we have to think that any amount has this potential.”
A version of this article first appeared on Medscape.com.
Children’s share of COVID-19 burden has never been higher
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
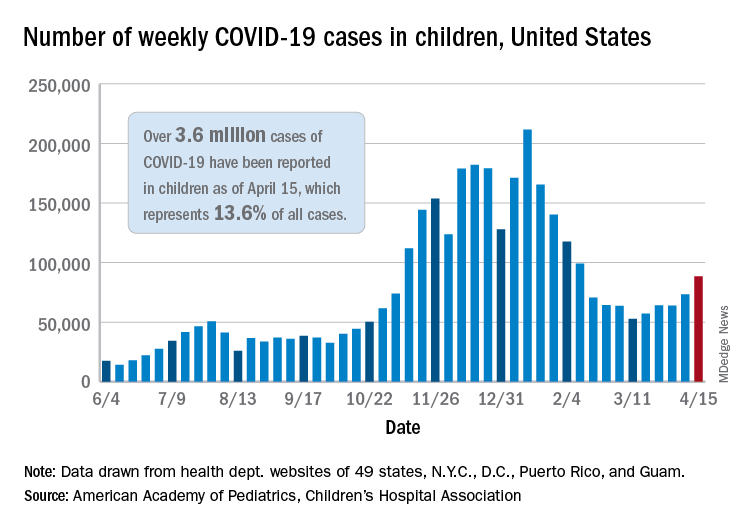
That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
Teen tanning bed ban would prevent more than 15,000 melanoma cases
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.
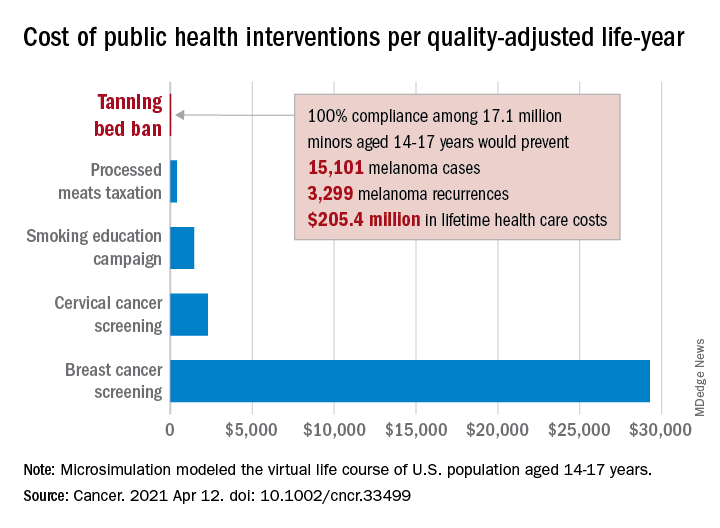
“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
FROM CANCER
Epidural use shows no association with autism spectrum disorder in children
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
FROM JAMA PEDIATRICS
Risk of hypogammaglobulinemia, infections with rituximab increased in pediatric patients
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
FROM CARRA 2021
COVID-19 vaccine response lower in kidney dialysis patients
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A 12-year-old male has persistent purple toes and new red lesions on his hands
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
He denied any hair loss, mouth sores, sun sensitivity, headaches, gastrointestinal complaints, joint pain, or muscle weakness.
He is not taking any medications.
He has been at home doing virtual school and has not traveled. He likes to play the piano. There is no family history of similar lesions, connective tissue disorder, or autoimmunity.
On physical exam he has purple discoloration on the toes with some violaceous and pink papules. On the fingers he has pink to violaceous papules and macules.
There is no joint edema or pain.
Stethoscope and Doppler may outperform newer intrapartum fetal monitoring techniques
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Tick talk for families and pediatricians
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.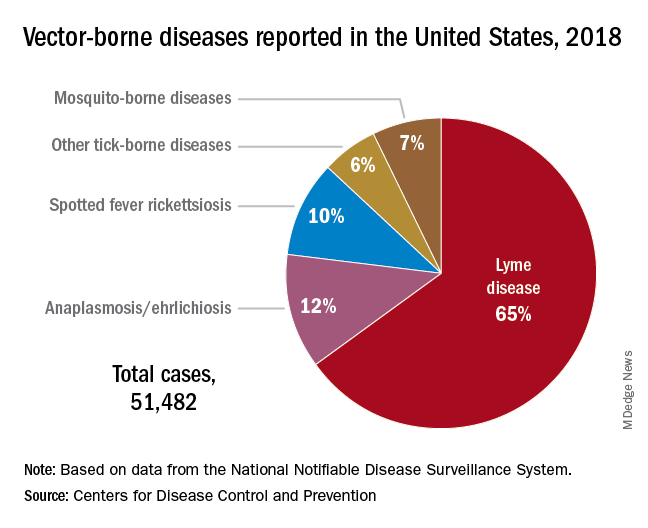
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.