User login
Adolescent substance use and the COVID-19 pandemic
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
I sent my suicidal teen patient to the ED: Whew?
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
Hospitalization not rare for children with COVID, study says
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
FDA to recommend limits on heavy metals in baby foods
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
Low-calorie diet linked to improved chemo response in leukemia
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
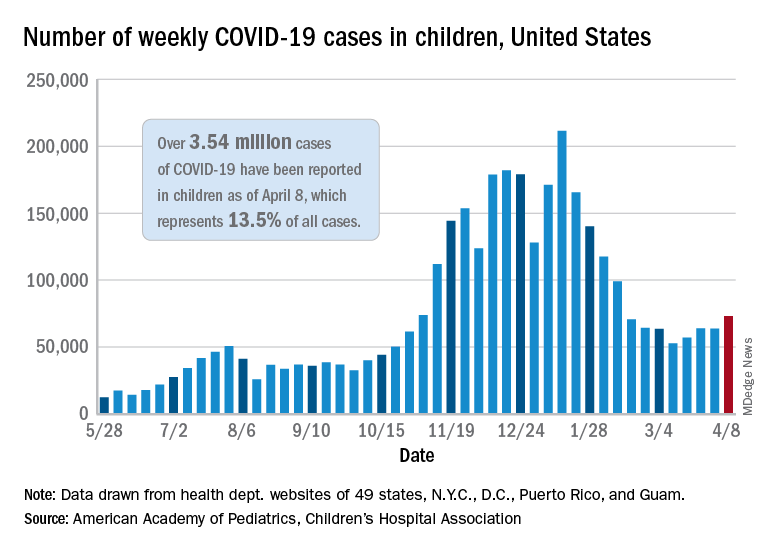
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The obesity risk everyone forgets
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Family psychoeducation is critical in care of children with disabilities
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.
Survey shines light on pediatric dermatologists’ earnings
For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.

For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.

For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.









