User login
Adolescents should know risks of tattoos and piercings
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
EXPERT ANALYSIS FROM AAP 19
Make the Diagnosis
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
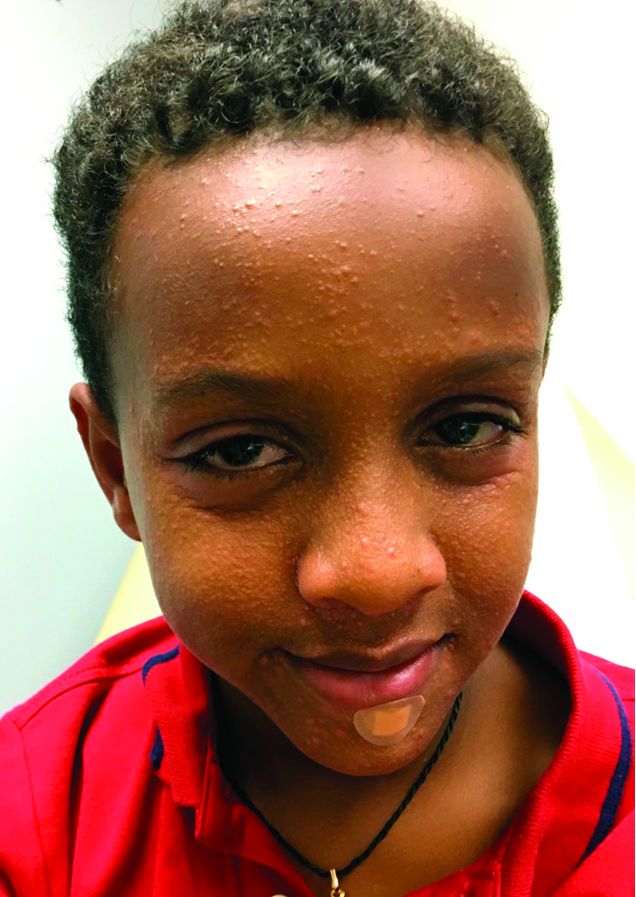
An 8-year-old African American male presented to our pediatric dermatology clinic for evaluation of a 3-month history of flesh-colored bumps on the face. According to the patient's mother, the lesions started with small pimple-like lesions around the nose and then spread to the whole face. Some lesions were crusty and somewhat itchy. He was treated with cephalexin and pimecrolimus with no improvement. The mother was very concerned because the lesions were close to the eyes and spreading.
He had no fevers, arthritis, or upper respiratory or gastrointestinal symptoms. He recently came back from a trip to Africa to visit his family. No other family members were affected. He used some new soaps, sunscreens, and moisturizers while he was in Africa.
On physical examination, the boy was in no acute distress. He had multiple flesh-colored papules on the face, especially around the eyes, nose, and mouth, where some lesions appeared crusted. There were no other skin lesions elsewhere on his body. There was no lymphadenopathy or hepatosplenomegaly.
The vaping problem
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Breakfast or not?
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
FDA approves Vyondys 53 for Duchenne muscular dystrophy subtype
The Food and Drug Administration has granted accelerated approval to Vyondys 53 (golodirsen) to treat patients with Duchenne muscular dystrophy (DMD) who have a mutation of the dystrophin gene that is amenable to exon 53 skipping. About 8% of patients with DMD have this type of mutation. Further research is required to establish whether the antisense oligonucleotide provides clinical benefit, the agency said.
Separately, the agency approved the first newborn screening test for DMD.
DMD is a “rare and devastating disease,” said Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research.
“Patients ... who have a confirmed mutation of the dystrophin gene amenable to exon 53 skipping will now have available the first treatment targeted specifically for this disease subtype,” Dr. Dunn said in a news release. “Use of the accelerated approval pathway will make Vyondys 53 available to patients based on initial data, and we look forward to learning more about the drug’s clinical benefit from the ongoing confirmatory clinical trial.”
A surrogate endpoint
The FDA approved Vyondys 53 based on the surrogate endpoint of increased dystrophin production in the skeletal muscle in some patients treated with the drug. Sarepta Therapeutics, the developer of Vyondys 53, evaluated the treatment in a two-part clinical study. In the first part, eight patients with DMD received Vyondys 53, and four received placebo. In the second part, 25 patients, including the 12 patients from the first part, received open-label treatment. Dystrophin levels increased from 0.10% of normal at baseline to 1.02% of normal after at least 48 weeks of treatment.
A placebo-controlled, confirmatory trial is expected to conclude by 2024, the company said. If the trial does not confirm clinical benefit, the FDA could withdraw approval of the drug.
The most common side effects in patients who received Vyondys 53 include headache, fever, fall, cough, vomiting, abdominal pain, cold symptoms, and nausea. Some patients had hypersensitivity reactions. Renal toxicity occurred in animal studies of golodirsen, but not in the clinical studies. Renal toxicity, however, has occurred after treatment with other antisense oligonucleotides, the FDA noted.
Sarepta said Vyondys 53, an injection, would be available immediately. The drug is the company’s second RNA exon-skipping treatment for DMD. The FDA approved the first treatment, Exondys 51 (eteplirsen), in 2016. Together, the two drugs can treat about 20% of patients with DMD, the company said.
Newborn screening
On the same day, Dec. 12, 2019, the FDA authorized marketing of the first test to aid in newborn screening for DMD. Although authorization for the GSP Neonatal Creatine Kinase–MM kit enables laboratories to add this test to their newborn screening panel, it “does not signal a recommendation for DMD to be added ... as a condition for which newborn screening is recommended,” the agency said. In addition, the FDA noted that the kit is not meant to diagnose DMD or to screen for other muscular dystrophies.
The GSP Neonatal Creatine Kinase–MM kit measures the concentration of CK-MM, a type of protein that increases when there is muscle damage. The test measures CK-MM in dried blood samples collected from a newborn’s heel 24-48 hours after birth. Elevated levels may indicate DMD, but physicians must confirm the diagnosis using other methods, such as muscle biopsies, genetic testing, and other laboratory tests.
DMD primarily affects boys, and patients often do not have a family history of the condition. About 1 in 3,600 male live-born infants worldwide have DMD. Symptom onset usually occurs between the ages of 3 and 5 years.
The FDA reviewed the kit through the de novo premarket review pathway for low to moderate risk devices. In a clinical study of 3,041 newborns, the kit identified the four screened newborns who had DMD-causing genetic mutations. In addition, the test correctly identified 30 samples from newborns with clinically confirmed cases of DMD.
PerkinElmer developed the GSP Neonatal Creatine Kinase–MM kit.
The Food and Drug Administration has granted accelerated approval to Vyondys 53 (golodirsen) to treat patients with Duchenne muscular dystrophy (DMD) who have a mutation of the dystrophin gene that is amenable to exon 53 skipping. About 8% of patients with DMD have this type of mutation. Further research is required to establish whether the antisense oligonucleotide provides clinical benefit, the agency said.
Separately, the agency approved the first newborn screening test for DMD.
DMD is a “rare and devastating disease,” said Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research.
“Patients ... who have a confirmed mutation of the dystrophin gene amenable to exon 53 skipping will now have available the first treatment targeted specifically for this disease subtype,” Dr. Dunn said in a news release. “Use of the accelerated approval pathway will make Vyondys 53 available to patients based on initial data, and we look forward to learning more about the drug’s clinical benefit from the ongoing confirmatory clinical trial.”
A surrogate endpoint
The FDA approved Vyondys 53 based on the surrogate endpoint of increased dystrophin production in the skeletal muscle in some patients treated with the drug. Sarepta Therapeutics, the developer of Vyondys 53, evaluated the treatment in a two-part clinical study. In the first part, eight patients with DMD received Vyondys 53, and four received placebo. In the second part, 25 patients, including the 12 patients from the first part, received open-label treatment. Dystrophin levels increased from 0.10% of normal at baseline to 1.02% of normal after at least 48 weeks of treatment.
A placebo-controlled, confirmatory trial is expected to conclude by 2024, the company said. If the trial does not confirm clinical benefit, the FDA could withdraw approval of the drug.
The most common side effects in patients who received Vyondys 53 include headache, fever, fall, cough, vomiting, abdominal pain, cold symptoms, and nausea. Some patients had hypersensitivity reactions. Renal toxicity occurred in animal studies of golodirsen, but not in the clinical studies. Renal toxicity, however, has occurred after treatment with other antisense oligonucleotides, the FDA noted.
Sarepta said Vyondys 53, an injection, would be available immediately. The drug is the company’s second RNA exon-skipping treatment for DMD. The FDA approved the first treatment, Exondys 51 (eteplirsen), in 2016. Together, the two drugs can treat about 20% of patients with DMD, the company said.
Newborn screening
On the same day, Dec. 12, 2019, the FDA authorized marketing of the first test to aid in newborn screening for DMD. Although authorization for the GSP Neonatal Creatine Kinase–MM kit enables laboratories to add this test to their newborn screening panel, it “does not signal a recommendation for DMD to be added ... as a condition for which newborn screening is recommended,” the agency said. In addition, the FDA noted that the kit is not meant to diagnose DMD or to screen for other muscular dystrophies.
The GSP Neonatal Creatine Kinase–MM kit measures the concentration of CK-MM, a type of protein that increases when there is muscle damage. The test measures CK-MM in dried blood samples collected from a newborn’s heel 24-48 hours after birth. Elevated levels may indicate DMD, but physicians must confirm the diagnosis using other methods, such as muscle biopsies, genetic testing, and other laboratory tests.
DMD primarily affects boys, and patients often do not have a family history of the condition. About 1 in 3,600 male live-born infants worldwide have DMD. Symptom onset usually occurs between the ages of 3 and 5 years.
The FDA reviewed the kit through the de novo premarket review pathway for low to moderate risk devices. In a clinical study of 3,041 newborns, the kit identified the four screened newborns who had DMD-causing genetic mutations. In addition, the test correctly identified 30 samples from newborns with clinically confirmed cases of DMD.
PerkinElmer developed the GSP Neonatal Creatine Kinase–MM kit.
The Food and Drug Administration has granted accelerated approval to Vyondys 53 (golodirsen) to treat patients with Duchenne muscular dystrophy (DMD) who have a mutation of the dystrophin gene that is amenable to exon 53 skipping. About 8% of patients with DMD have this type of mutation. Further research is required to establish whether the antisense oligonucleotide provides clinical benefit, the agency said.
Separately, the agency approved the first newborn screening test for DMD.
DMD is a “rare and devastating disease,” said Billy Dunn, MD, acting director of the office of neuroscience in the FDA’s Center for Drug Evaluation and Research.
“Patients ... who have a confirmed mutation of the dystrophin gene amenable to exon 53 skipping will now have available the first treatment targeted specifically for this disease subtype,” Dr. Dunn said in a news release. “Use of the accelerated approval pathway will make Vyondys 53 available to patients based on initial data, and we look forward to learning more about the drug’s clinical benefit from the ongoing confirmatory clinical trial.”
A surrogate endpoint
The FDA approved Vyondys 53 based on the surrogate endpoint of increased dystrophin production in the skeletal muscle in some patients treated with the drug. Sarepta Therapeutics, the developer of Vyondys 53, evaluated the treatment in a two-part clinical study. In the first part, eight patients with DMD received Vyondys 53, and four received placebo. In the second part, 25 patients, including the 12 patients from the first part, received open-label treatment. Dystrophin levels increased from 0.10% of normal at baseline to 1.02% of normal after at least 48 weeks of treatment.
A placebo-controlled, confirmatory trial is expected to conclude by 2024, the company said. If the trial does not confirm clinical benefit, the FDA could withdraw approval of the drug.
The most common side effects in patients who received Vyondys 53 include headache, fever, fall, cough, vomiting, abdominal pain, cold symptoms, and nausea. Some patients had hypersensitivity reactions. Renal toxicity occurred in animal studies of golodirsen, but not in the clinical studies. Renal toxicity, however, has occurred after treatment with other antisense oligonucleotides, the FDA noted.
Sarepta said Vyondys 53, an injection, would be available immediately. The drug is the company’s second RNA exon-skipping treatment for DMD. The FDA approved the first treatment, Exondys 51 (eteplirsen), in 2016. Together, the two drugs can treat about 20% of patients with DMD, the company said.
Newborn screening
On the same day, Dec. 12, 2019, the FDA authorized marketing of the first test to aid in newborn screening for DMD. Although authorization for the GSP Neonatal Creatine Kinase–MM kit enables laboratories to add this test to their newborn screening panel, it “does not signal a recommendation for DMD to be added ... as a condition for which newborn screening is recommended,” the agency said. In addition, the FDA noted that the kit is not meant to diagnose DMD or to screen for other muscular dystrophies.
The GSP Neonatal Creatine Kinase–MM kit measures the concentration of CK-MM, a type of protein that increases when there is muscle damage. The test measures CK-MM in dried blood samples collected from a newborn’s heel 24-48 hours after birth. Elevated levels may indicate DMD, but physicians must confirm the diagnosis using other methods, such as muscle biopsies, genetic testing, and other laboratory tests.
DMD primarily affects boys, and patients often do not have a family history of the condition. About 1 in 3,600 male live-born infants worldwide have DMD. Symptom onset usually occurs between the ages of 3 and 5 years.
The FDA reviewed the kit through the de novo premarket review pathway for low to moderate risk devices. In a clinical study of 3,041 newborns, the kit identified the four screened newborns who had DMD-causing genetic mutations. In addition, the test correctly identified 30 samples from newborns with clinically confirmed cases of DMD.
PerkinElmer developed the GSP Neonatal Creatine Kinase–MM kit.
Fast, aggressive eczema treatment linked to fewer food allergies by age 2
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Atopic dermatitis in egg-, milk-allergic kids may up anaphylaxis risk
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Being whole
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Frequent soaks ease pediatric atopic dermatitis
A regimen of twice-daily baths followed by occlusive moisturizer improved atopic dermatitis in children with moderate to severe disease more effectively than did a twice-weekly protocol, based on data from 42 children.
Guidelines for bathing frequency for children with atopic dermatitis are inconsistent and often confusing for parents, according to Ivan D. Cardona, MD, of Maine Medical Research Institute, Portland, and colleagues.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers randomized 42 children aged 6 months to 11 years with moderate to severe atopic dermatitis to a routine of twice-weekly “soak and seal” (SS) procedures consisting of soaking baths for 10 minutes or less, followed by an occlusive emollient, or to twice-daily SS with baths of 15-20 minutes followed by emollient. The groups were treated for 2 weeks, then switched protocols. The study included a total of four clinic visits over 5 weeks. All patients also received standard of care low-potency topical corticosteroids and moisturizer.
Overall, the frequent bathing (“wet method”) led to a decrease of 21.2 on the SCORing Atopic Dermatitis Index (SCORAD) compared with the less frequent bathing (“dry method”). Improvements in SCORAD (the primary outcome) correlated with a secondary outcome of improved scores on the parent-rated Atopic Dermatitis Quickscore.
The findings were limited by several factors including the small sample size, large rate of attrition prior to randomization among initially screened children, lack of data on environmental factors such as water temperature and quality, and the lack of a washout period between the treatment protocols, the researchers noted. They acknowledged that “twice-daily SS bathing in the real world can be time consuming, making adherence difficult for families.”
However, the results suggest that the frequent bathing protocol was safe and effective at improving symptoms of atopic dermatitis, and may reduce steroid use, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Cardona ID et al. J Allergy Clin Immunol Pract. 2019 Nov 13. doi: 10.1016/j.jaip.2019.10.042.
A regimen of twice-daily baths followed by occlusive moisturizer improved atopic dermatitis in children with moderate to severe disease more effectively than did a twice-weekly protocol, based on data from 42 children.
Guidelines for bathing frequency for children with atopic dermatitis are inconsistent and often confusing for parents, according to Ivan D. Cardona, MD, of Maine Medical Research Institute, Portland, and colleagues.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers randomized 42 children aged 6 months to 11 years with moderate to severe atopic dermatitis to a routine of twice-weekly “soak and seal” (SS) procedures consisting of soaking baths for 10 minutes or less, followed by an occlusive emollient, or to twice-daily SS with baths of 15-20 minutes followed by emollient. The groups were treated for 2 weeks, then switched protocols. The study included a total of four clinic visits over 5 weeks. All patients also received standard of care low-potency topical corticosteroids and moisturizer.
Overall, the frequent bathing (“wet method”) led to a decrease of 21.2 on the SCORing Atopic Dermatitis Index (SCORAD) compared with the less frequent bathing (“dry method”). Improvements in SCORAD (the primary outcome) correlated with a secondary outcome of improved scores on the parent-rated Atopic Dermatitis Quickscore.
The findings were limited by several factors including the small sample size, large rate of attrition prior to randomization among initially screened children, lack of data on environmental factors such as water temperature and quality, and the lack of a washout period between the treatment protocols, the researchers noted. They acknowledged that “twice-daily SS bathing in the real world can be time consuming, making adherence difficult for families.”
However, the results suggest that the frequent bathing protocol was safe and effective at improving symptoms of atopic dermatitis, and may reduce steroid use, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Cardona ID et al. J Allergy Clin Immunol Pract. 2019 Nov 13. doi: 10.1016/j.jaip.2019.10.042.
A regimen of twice-daily baths followed by occlusive moisturizer improved atopic dermatitis in children with moderate to severe disease more effectively than did a twice-weekly protocol, based on data from 42 children.
Guidelines for bathing frequency for children with atopic dermatitis are inconsistent and often confusing for parents, according to Ivan D. Cardona, MD, of Maine Medical Research Institute, Portland, and colleagues.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers randomized 42 children aged 6 months to 11 years with moderate to severe atopic dermatitis to a routine of twice-weekly “soak and seal” (SS) procedures consisting of soaking baths for 10 minutes or less, followed by an occlusive emollient, or to twice-daily SS with baths of 15-20 minutes followed by emollient. The groups were treated for 2 weeks, then switched protocols. The study included a total of four clinic visits over 5 weeks. All patients also received standard of care low-potency topical corticosteroids and moisturizer.
Overall, the frequent bathing (“wet method”) led to a decrease of 21.2 on the SCORing Atopic Dermatitis Index (SCORAD) compared with the less frequent bathing (“dry method”). Improvements in SCORAD (the primary outcome) correlated with a secondary outcome of improved scores on the parent-rated Atopic Dermatitis Quickscore.
The findings were limited by several factors including the small sample size, large rate of attrition prior to randomization among initially screened children, lack of data on environmental factors such as water temperature and quality, and the lack of a washout period between the treatment protocols, the researchers noted. They acknowledged that “twice-daily SS bathing in the real world can be time consuming, making adherence difficult for families.”
However, the results suggest that the frequent bathing protocol was safe and effective at improving symptoms of atopic dermatitis, and may reduce steroid use, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Cardona ID et al. J Allergy Clin Immunol Pract. 2019 Nov 13. doi: 10.1016/j.jaip.2019.10.042.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Clinic goes to bat for bullied kids
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 2019