User login
Flu activity dropped in early December
according to the Centers for Disease Control and Prevention.
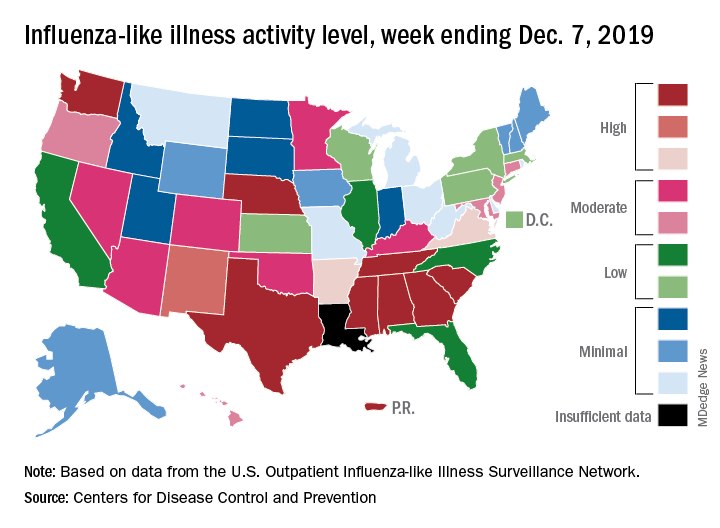
Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
Could liraglutide stall the onset of type 2 diabetes in children?
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
EXPERT ANALYSIS FROM THE WCIRDC 2019
Asthma exacerbation in pregnancy impacts mothers, infants
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
Poor sleep due to ADHD or ADHD due to poor sleep?
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
ADA2 is a potent new biomarker for macrophage activation syndrome
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
REPORTING FROM ACR 2019
Tofacitinib improves disease activity in patients with polyarticular-course JIA
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
REPORTING FROM ACR 2019
Bringing the HPV vaccination rate into line with other adolescent immunizations
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
From The Journal of Family Practice | 2019;68(10):E1-E7.
PRACTICE RECOMMENDATIONS
› Review vaccination status at every adolescent health care visit. C
› Give a clear, unambiguous, strong recommendation to vaccinate with human papillomavirus (HPV) to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts. A
› Schedule follow-up appointments at checkout following initiation of HPV vaccination to help ensure completion of the series. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
High infantile spasm risk should contraindicate sodium channel blocker antiepileptics
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
REPORTING FROM AES 2019
Think twice: Choosing Wisely recommendations on testing to avoid in pediatric hematology
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
REPORTING FROM ASH 2019
E-cigarette use, interest in flavors remains high among youth
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
FROM THE MMWR