User login
ACR looks for continued growth in awareness of rheumatic diseases with latest annual campaign
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
Antisuicide program promotes resilience, peer support
As youth suicides continue to climb nationwide, a growing body of research shows that the deaths are happening at higher rates in rural communities.
In 2017, suicides reached their highest point since 2000, a trend driven by a sharp rise in male suicides and in youth aged 15-19 years, according to an analysis published recently in JAMA (2019 Jun 18. doi: 10.1001/jama.2019.5054). Among youth aged 15-19 years, the suicide rate was 12 per 100,000 in 2017 (18 per 100,000 in males and 5 per 100,000 in females), compared with 8 per 100,000 in 2000, the study found. Across all age groups, the highest suicide rates and greatest rate increases are in rural counties, according to data from the Centers for Disease Control and Prevention (CDC).
Now, a unique initiative in New Mexico is working to combat those alarming trends through an alliance of community leaders that strives to strengthen resilience and build peer support for at-risk youth.
The Alliance-Building for Suicide Prevention & Youth Resilience (ASPYR) program, created by the University of New Mexico (UNM), Albuquerque, focuses on training professionals and advocates within New Mexico communities in a strength-based, youth-directed, collaborative approach for the assessment and treatment of suicidality. A diversity of community members undergo the training, including health and behavioral health care providers, peer support and community support workers, youth and community advocates, educators, and first responders. The initiative also supports and facilitates the development of a communitywide crisis intervention plan that promotes youth safety and resilience.
“ASPYR is unique, in that we actively involve youth to guide our program, versus an adult-only led program,” says Laura Rombach, program manager for ASPYR and a senior program therapist in the department of psychiatry and behavioral sciences at UNM. “Youth offer feedback about our training and ideas about how to best prevent suicide in their schools and communities. New Mexico is underresourced, and individuals living in rural/frontier areas do not always have access to licensed behavioral health providers, so our training is developed for licensed providers as well as peers and paraprofessionals to increase the knowledge of care for individuals experiencing a suicidal crisis.”
Rural populations present challenges
The many rural pockets of New Mexico pose numerous obstacles for antisuicide advocates.
Of the 33 counties in New Mexico, six are identified by the Census Bureau as completely “rural,” and an additional six are defined as mostly rural, according to the University of New Mexico Bureau of Business & Economic Research. Even among counties considered “urban” however, a considerable amount of the population lives in rural areas, according to the bureau. San Juan County, for example, which is considered urban by the Census Bureau, had an estimated 34% of residents living in rural areas in 2010.
Poverty adds to the difficulty. In 2017, nearly one in five New Mexicans (20%) lived below the poverty line, and the state had the second-highest rate of children under 18 years living in poverty in the country, according to a report by the New Mexico Department of Workforce Solutions.
“New Mexico is an impoverished state with limited capacity, especially in regards to behavioral health services,” said Avi Kriechman, MD, principal investigator for ASPYR at UNM and a child, adolescent, and family psychiatrist at the university. “It is also challenging to create a truly statewide effort where there is limited public transportation, problematic Internet connection, and other barriers to involving those who live and work in rural and frontier New Mexico.”
Addressing suicide among the many native and Indigenous people in rural New Mexico presents another unique set of challenges, said Mary Roessel, MD, a Santa Fe, N.M.–based psychiatrist who specializes in cultural psychiatry. Native and Indigenous residents often have a general mistrust of outsiders and a stigma against mental illnesses, Dr. Roessel said in an interview.
“One of the problems is being able to identify when a person has attempted suicide in some of these small, private, Pueblo communities because they are very closed,” she said. “At times, we don’t get the information to go in and help them. They’re trying to address or deal with the problem themselves.”
To address the many barriers of rural New Mexico, ASPYR works hard to recognize, identify, and support preexisting community resources that are often neglected in needs assessment and stakeholder identification, Dr. Kriechman said. This can include food banks, church care committees, youth advocacy groups, local caregiving, and spiritual traditions, among others. Frequently, many community caregivers and agencies have not connected or communicated with one another and often are unaware of all they have to offer, he said.
“We try to build capacity through community trainings, which include a widely diverse group of providers, advocates, and supports,” he continued. “Our trainings involve highlighting and building upon local and cultural practices and traditions of healing, caregiving, and support. A significant part of our onsite training involves assembling a representative group of local providers in health care, behavioral health care, peer & community support and advocacy, education, first responders to community crises, and government and nonprofit agencies, then facilitating a community conversation between the panel and training attendees about how best to move forward in a synergistic and systemic manner to support youth safety and resilience.”
Peers support peers
While ASPYR encompasses elements of other suicide prevention models, two unique cornerstones of the program are its emphasis on resilience and promotion of peer support. The strength-based, youth-directed approach includes creating a youth-directed safety plan, enlisting peers as support and reducing access to lethal means.
Regarding the youth safety plan, Dr. Kriechman explained that, rather than being prescribed and instructed in expert-selected and expert-driven coping skills, youth are offered a menu of options that most speak to their strengths, values, experience, and preferences. Young people also select a peer who, if they wish, accompanies them to sessions, and supports and coaches them at home.
“Peers are often more influential than parents, siblings, family members, and adults regarding youth behavior,” Dr. Kriechman said. “Most often, it is a peer that a youth-at-risk turns to for support, counsel, role models, and understanding. Youth who wish to offer their peers support can quickly be trained to provide early identification of youth at risk, motivational support to seek help, and a ‘warm hand-off’ to community resources.”
In addition, a Youth Advisory Council established as part of the program draws from young people across New Mexico to participate in state and national conferences, and conduct outreach efforts to peers.
ASPYR Youth council member Serenity Gomez, a senior at the Public Academy for Performing Arts in Albuquerque, became interested in ASPYR after volunteering for the American Foundation for Suicide Prevention in 2016. As a youth council member, Ms. Gomez said she helps create projects to raise suicide awareness, whether through posters, stickers, social media, poetry, or songs.
“My experience as a youth council member has really opened my eyes and has made me more motivated to help others,” she said in an interview. “It has also showed me that talking about suicide doesn’t always have to be a slideshow of facts. You can reach people through music, poetry, storytelling, and so much more. Many people are afraid to talk about suicide because it’s such a scary idea, but if we all talk about it and bring more awareness, then we can find the support everyone needs. In ASPYR, specifically, I hope to reach youth and help all youth learn to support each other.”
Since ASPYR launched in 2017, the program has provided both onsite and online trainings to hundreds of New Mexicans, and has helped rural and frontier communities start working on collaborative approaches to promoting youth safety and resilience, Dr. Kriechman said. Following community consultations, numerous rural communities have since formed systems of care to identify, support, and treat youth at risk. In addition to the youth council, an Advisory Community Council has also been established that welcomes any New Mexico resident interested in working on the mission of preventing youth suicide.
For example, the program shifts from “no-suicide contracts” to safety planning, focusing on reasons for living rather than reasons for dying, and shifting from prescribing coping skills to strengthening preexisting coping skills in young people.
“An ultimate hope for ASPYR is emphasizing that recovery from any of life’s challenges is far more than symptom reduction or agency collaboration,” Dr. Kriechman said. “It is the understanding that a life of value and meaning, the instillation of hope and support for the unique strengths, competencies, skills, and understandings of each individual, is honored, respected, and supported.”
As youth suicides continue to climb nationwide, a growing body of research shows that the deaths are happening at higher rates in rural communities.
In 2017, suicides reached their highest point since 2000, a trend driven by a sharp rise in male suicides and in youth aged 15-19 years, according to an analysis published recently in JAMA (2019 Jun 18. doi: 10.1001/jama.2019.5054). Among youth aged 15-19 years, the suicide rate was 12 per 100,000 in 2017 (18 per 100,000 in males and 5 per 100,000 in females), compared with 8 per 100,000 in 2000, the study found. Across all age groups, the highest suicide rates and greatest rate increases are in rural counties, according to data from the Centers for Disease Control and Prevention (CDC).
Now, a unique initiative in New Mexico is working to combat those alarming trends through an alliance of community leaders that strives to strengthen resilience and build peer support for at-risk youth.
The Alliance-Building for Suicide Prevention & Youth Resilience (ASPYR) program, created by the University of New Mexico (UNM), Albuquerque, focuses on training professionals and advocates within New Mexico communities in a strength-based, youth-directed, collaborative approach for the assessment and treatment of suicidality. A diversity of community members undergo the training, including health and behavioral health care providers, peer support and community support workers, youth and community advocates, educators, and first responders. The initiative also supports and facilitates the development of a communitywide crisis intervention plan that promotes youth safety and resilience.
“ASPYR is unique, in that we actively involve youth to guide our program, versus an adult-only led program,” says Laura Rombach, program manager for ASPYR and a senior program therapist in the department of psychiatry and behavioral sciences at UNM. “Youth offer feedback about our training and ideas about how to best prevent suicide in their schools and communities. New Mexico is underresourced, and individuals living in rural/frontier areas do not always have access to licensed behavioral health providers, so our training is developed for licensed providers as well as peers and paraprofessionals to increase the knowledge of care for individuals experiencing a suicidal crisis.”
Rural populations present challenges
The many rural pockets of New Mexico pose numerous obstacles for antisuicide advocates.
Of the 33 counties in New Mexico, six are identified by the Census Bureau as completely “rural,” and an additional six are defined as mostly rural, according to the University of New Mexico Bureau of Business & Economic Research. Even among counties considered “urban” however, a considerable amount of the population lives in rural areas, according to the bureau. San Juan County, for example, which is considered urban by the Census Bureau, had an estimated 34% of residents living in rural areas in 2010.
Poverty adds to the difficulty. In 2017, nearly one in five New Mexicans (20%) lived below the poverty line, and the state had the second-highest rate of children under 18 years living in poverty in the country, according to a report by the New Mexico Department of Workforce Solutions.
“New Mexico is an impoverished state with limited capacity, especially in regards to behavioral health services,” said Avi Kriechman, MD, principal investigator for ASPYR at UNM and a child, adolescent, and family psychiatrist at the university. “It is also challenging to create a truly statewide effort where there is limited public transportation, problematic Internet connection, and other barriers to involving those who live and work in rural and frontier New Mexico.”
Addressing suicide among the many native and Indigenous people in rural New Mexico presents another unique set of challenges, said Mary Roessel, MD, a Santa Fe, N.M.–based psychiatrist who specializes in cultural psychiatry. Native and Indigenous residents often have a general mistrust of outsiders and a stigma against mental illnesses, Dr. Roessel said in an interview.
“One of the problems is being able to identify when a person has attempted suicide in some of these small, private, Pueblo communities because they are very closed,” she said. “At times, we don’t get the information to go in and help them. They’re trying to address or deal with the problem themselves.”
To address the many barriers of rural New Mexico, ASPYR works hard to recognize, identify, and support preexisting community resources that are often neglected in needs assessment and stakeholder identification, Dr. Kriechman said. This can include food banks, church care committees, youth advocacy groups, local caregiving, and spiritual traditions, among others. Frequently, many community caregivers and agencies have not connected or communicated with one another and often are unaware of all they have to offer, he said.
“We try to build capacity through community trainings, which include a widely diverse group of providers, advocates, and supports,” he continued. “Our trainings involve highlighting and building upon local and cultural practices and traditions of healing, caregiving, and support. A significant part of our onsite training involves assembling a representative group of local providers in health care, behavioral health care, peer & community support and advocacy, education, first responders to community crises, and government and nonprofit agencies, then facilitating a community conversation between the panel and training attendees about how best to move forward in a synergistic and systemic manner to support youth safety and resilience.”
Peers support peers
While ASPYR encompasses elements of other suicide prevention models, two unique cornerstones of the program are its emphasis on resilience and promotion of peer support. The strength-based, youth-directed approach includes creating a youth-directed safety plan, enlisting peers as support and reducing access to lethal means.
Regarding the youth safety plan, Dr. Kriechman explained that, rather than being prescribed and instructed in expert-selected and expert-driven coping skills, youth are offered a menu of options that most speak to their strengths, values, experience, and preferences. Young people also select a peer who, if they wish, accompanies them to sessions, and supports and coaches them at home.
“Peers are often more influential than parents, siblings, family members, and adults regarding youth behavior,” Dr. Kriechman said. “Most often, it is a peer that a youth-at-risk turns to for support, counsel, role models, and understanding. Youth who wish to offer their peers support can quickly be trained to provide early identification of youth at risk, motivational support to seek help, and a ‘warm hand-off’ to community resources.”
In addition, a Youth Advisory Council established as part of the program draws from young people across New Mexico to participate in state and national conferences, and conduct outreach efforts to peers.
ASPYR Youth council member Serenity Gomez, a senior at the Public Academy for Performing Arts in Albuquerque, became interested in ASPYR after volunteering for the American Foundation for Suicide Prevention in 2016. As a youth council member, Ms. Gomez said she helps create projects to raise suicide awareness, whether through posters, stickers, social media, poetry, or songs.
“My experience as a youth council member has really opened my eyes and has made me more motivated to help others,” she said in an interview. “It has also showed me that talking about suicide doesn’t always have to be a slideshow of facts. You can reach people through music, poetry, storytelling, and so much more. Many people are afraid to talk about suicide because it’s such a scary idea, but if we all talk about it and bring more awareness, then we can find the support everyone needs. In ASPYR, specifically, I hope to reach youth and help all youth learn to support each other.”
Since ASPYR launched in 2017, the program has provided both onsite and online trainings to hundreds of New Mexicans, and has helped rural and frontier communities start working on collaborative approaches to promoting youth safety and resilience, Dr. Kriechman said. Following community consultations, numerous rural communities have since formed systems of care to identify, support, and treat youth at risk. In addition to the youth council, an Advisory Community Council has also been established that welcomes any New Mexico resident interested in working on the mission of preventing youth suicide.
For example, the program shifts from “no-suicide contracts” to safety planning, focusing on reasons for living rather than reasons for dying, and shifting from prescribing coping skills to strengthening preexisting coping skills in young people.
“An ultimate hope for ASPYR is emphasizing that recovery from any of life’s challenges is far more than symptom reduction or agency collaboration,” Dr. Kriechman said. “It is the understanding that a life of value and meaning, the instillation of hope and support for the unique strengths, competencies, skills, and understandings of each individual, is honored, respected, and supported.”
As youth suicides continue to climb nationwide, a growing body of research shows that the deaths are happening at higher rates in rural communities.
In 2017, suicides reached their highest point since 2000, a trend driven by a sharp rise in male suicides and in youth aged 15-19 years, according to an analysis published recently in JAMA (2019 Jun 18. doi: 10.1001/jama.2019.5054). Among youth aged 15-19 years, the suicide rate was 12 per 100,000 in 2017 (18 per 100,000 in males and 5 per 100,000 in females), compared with 8 per 100,000 in 2000, the study found. Across all age groups, the highest suicide rates and greatest rate increases are in rural counties, according to data from the Centers for Disease Control and Prevention (CDC).
Now, a unique initiative in New Mexico is working to combat those alarming trends through an alliance of community leaders that strives to strengthen resilience and build peer support for at-risk youth.
The Alliance-Building for Suicide Prevention & Youth Resilience (ASPYR) program, created by the University of New Mexico (UNM), Albuquerque, focuses on training professionals and advocates within New Mexico communities in a strength-based, youth-directed, collaborative approach for the assessment and treatment of suicidality. A diversity of community members undergo the training, including health and behavioral health care providers, peer support and community support workers, youth and community advocates, educators, and first responders. The initiative also supports and facilitates the development of a communitywide crisis intervention plan that promotes youth safety and resilience.
“ASPYR is unique, in that we actively involve youth to guide our program, versus an adult-only led program,” says Laura Rombach, program manager for ASPYR and a senior program therapist in the department of psychiatry and behavioral sciences at UNM. “Youth offer feedback about our training and ideas about how to best prevent suicide in their schools and communities. New Mexico is underresourced, and individuals living in rural/frontier areas do not always have access to licensed behavioral health providers, so our training is developed for licensed providers as well as peers and paraprofessionals to increase the knowledge of care for individuals experiencing a suicidal crisis.”
Rural populations present challenges
The many rural pockets of New Mexico pose numerous obstacles for antisuicide advocates.
Of the 33 counties in New Mexico, six are identified by the Census Bureau as completely “rural,” and an additional six are defined as mostly rural, according to the University of New Mexico Bureau of Business & Economic Research. Even among counties considered “urban” however, a considerable amount of the population lives in rural areas, according to the bureau. San Juan County, for example, which is considered urban by the Census Bureau, had an estimated 34% of residents living in rural areas in 2010.
Poverty adds to the difficulty. In 2017, nearly one in five New Mexicans (20%) lived below the poverty line, and the state had the second-highest rate of children under 18 years living in poverty in the country, according to a report by the New Mexico Department of Workforce Solutions.
“New Mexico is an impoverished state with limited capacity, especially in regards to behavioral health services,” said Avi Kriechman, MD, principal investigator for ASPYR at UNM and a child, adolescent, and family psychiatrist at the university. “It is also challenging to create a truly statewide effort where there is limited public transportation, problematic Internet connection, and other barriers to involving those who live and work in rural and frontier New Mexico.”
Addressing suicide among the many native and Indigenous people in rural New Mexico presents another unique set of challenges, said Mary Roessel, MD, a Santa Fe, N.M.–based psychiatrist who specializes in cultural psychiatry. Native and Indigenous residents often have a general mistrust of outsiders and a stigma against mental illnesses, Dr. Roessel said in an interview.
“One of the problems is being able to identify when a person has attempted suicide in some of these small, private, Pueblo communities because they are very closed,” she said. “At times, we don’t get the information to go in and help them. They’re trying to address or deal with the problem themselves.”
To address the many barriers of rural New Mexico, ASPYR works hard to recognize, identify, and support preexisting community resources that are often neglected in needs assessment and stakeholder identification, Dr. Kriechman said. This can include food banks, church care committees, youth advocacy groups, local caregiving, and spiritual traditions, among others. Frequently, many community caregivers and agencies have not connected or communicated with one another and often are unaware of all they have to offer, he said.
“We try to build capacity through community trainings, which include a widely diverse group of providers, advocates, and supports,” he continued. “Our trainings involve highlighting and building upon local and cultural practices and traditions of healing, caregiving, and support. A significant part of our onsite training involves assembling a representative group of local providers in health care, behavioral health care, peer & community support and advocacy, education, first responders to community crises, and government and nonprofit agencies, then facilitating a community conversation between the panel and training attendees about how best to move forward in a synergistic and systemic manner to support youth safety and resilience.”
Peers support peers
While ASPYR encompasses elements of other suicide prevention models, two unique cornerstones of the program are its emphasis on resilience and promotion of peer support. The strength-based, youth-directed approach includes creating a youth-directed safety plan, enlisting peers as support and reducing access to lethal means.
Regarding the youth safety plan, Dr. Kriechman explained that, rather than being prescribed and instructed in expert-selected and expert-driven coping skills, youth are offered a menu of options that most speak to their strengths, values, experience, and preferences. Young people also select a peer who, if they wish, accompanies them to sessions, and supports and coaches them at home.
“Peers are often more influential than parents, siblings, family members, and adults regarding youth behavior,” Dr. Kriechman said. “Most often, it is a peer that a youth-at-risk turns to for support, counsel, role models, and understanding. Youth who wish to offer their peers support can quickly be trained to provide early identification of youth at risk, motivational support to seek help, and a ‘warm hand-off’ to community resources.”
In addition, a Youth Advisory Council established as part of the program draws from young people across New Mexico to participate in state and national conferences, and conduct outreach efforts to peers.
ASPYR Youth council member Serenity Gomez, a senior at the Public Academy for Performing Arts in Albuquerque, became interested in ASPYR after volunteering for the American Foundation for Suicide Prevention in 2016. As a youth council member, Ms. Gomez said she helps create projects to raise suicide awareness, whether through posters, stickers, social media, poetry, or songs.
“My experience as a youth council member has really opened my eyes and has made me more motivated to help others,” she said in an interview. “It has also showed me that talking about suicide doesn’t always have to be a slideshow of facts. You can reach people through music, poetry, storytelling, and so much more. Many people are afraid to talk about suicide because it’s such a scary idea, but if we all talk about it and bring more awareness, then we can find the support everyone needs. In ASPYR, specifically, I hope to reach youth and help all youth learn to support each other.”
Since ASPYR launched in 2017, the program has provided both onsite and online trainings to hundreds of New Mexicans, and has helped rural and frontier communities start working on collaborative approaches to promoting youth safety and resilience, Dr. Kriechman said. Following community consultations, numerous rural communities have since formed systems of care to identify, support, and treat youth at risk. In addition to the youth council, an Advisory Community Council has also been established that welcomes any New Mexico resident interested in working on the mission of preventing youth suicide.
For example, the program shifts from “no-suicide contracts” to safety planning, focusing on reasons for living rather than reasons for dying, and shifting from prescribing coping skills to strengthening preexisting coping skills in young people.
“An ultimate hope for ASPYR is emphasizing that recovery from any of life’s challenges is far more than symptom reduction or agency collaboration,” Dr. Kriechman said. “It is the understanding that a life of value and meaning, the instillation of hope and support for the unique strengths, competencies, skills, and understandings of each individual, is honored, respected, and supported.”
Talking to overweight children
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Taking vaccines to the next level via mucosal immunity
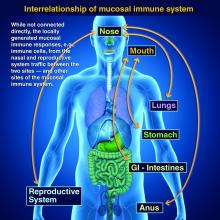
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Novel gene therapies show promise for sickle cell cure
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.
Baby beverage 101: New recommendations on what young children should drink
entirely. A coalition of health and nutrition organizations offered all this and more in a new set of sometimes surprising recommendations about beverage consumption in children from birth to age 5 years.
The recommendations “are based on the best available evidence, combined with sound expert judgment, and provide consistent messages that can be used by a variety of stakeholders to improve the beverage intake patterns of young children,” the report authors wrote. “It is imperative to capitalize on early childhood as a critical window of opportunity during which dietary patterns are both impressionable and capable of setting the stage for lifelong eating behaviors.”
The Academy of Nutrition and Dietetics, the American Academy of Pediatric Dentistry, the American Academy of Pediatrics, and the American Heart Association released their recommendations titled “Healthy Beverage Consumption in Early Childhood” in a report published Sept. 18.
The organizations convened expert panels and analyzed research to develop consensus beverage recommendations. Here are some highlights from the report, the writing of which was led by Megan Lott, MPH, RDN, of Healthy Eating Research and Duke Global Health Institute at Duke University, Durham, N.C.
- Breast milk or formula. This is what babies should be drinking for the first year of life.
- Plain drinking water. None is needed in the first 6 months of life. Introduce 0.5-1.0 cups/day over the next 6 months in cups and during meals when solid food is introduced. Then 1-4 cups/day are recommended from age 1-3 years, then 1.5-5 cups by age 4-5 years.
- Plain, pasteurized milk. None during the first year, then 2-3 cups a day of whole milk at age 12-24 months; if weight gain is excessive or family history is positive for obesity, dyslipidemia, or other cardiovascular disease, the pediatrician may recommend skim or lowfat milk at 12-24 months. Then provide up to 2 cups (age 2-3 years) then 2.5 cups (age 4-5 years) per day of skim /fat-free milk or low fat/1% milk
- 100% juice. None until 12 months. Then it can be provided, although whole fruit is preferred. No more than 0.5 cup per day until age 4-5 years, when no more than 0.5-0.75 cup per day is recommended.
- Plant-based milks and non-dairy beverages. None from age 0-1 year. And at ages 1-5 years, they only should be consumed when medically indicated, such as when a child has an allergy or per a preferred diet such as a vegan one. The panel didn’t recommend them as full replacements for dairy milk from age 12-24 months, and it noted that “consumption of these beverages as a full replacement for dairy milk should be undertaken in consultation with a health care provider so that adequate intake of key nutrients commonly obtained from dairy milk can be considered in dietary planning.”
- Other beverages. Flavored milk (like chocolate milk), “toddler milk,” sugar-sweetened drinks (including soft drinks, flavored water, and various fruit drinks), low-calorie sweetened drinks, and caffeinated drinks are not recommended at any age from 0-5 years. Toddler milk products “offer no unique nutritional value beyond what a nutritionally adequate diet provides and may contribute added sugars to the diet and undermine sustained breastfeeding,” the report authors said.
Lillian M. Beard, MD, said in an interview, “Pediatricians and other child health providers have major roles of influence in parents’ choices of foods and beverages for their infants and young children.
“Following these first-ever consensus recommendations on healthy drinks will alter family grocery shopping patterns, and not only improve the overall health of children under age 5 years, but also may improve the health outcomes of everyone in the household.” Dr. Beard is a clinical professor of pediatrics at George Washington University, Washington. She was not one of the report authors and was asked to comment on the report.
The report was supported by the Healthy Eating Research, a national program of the Robert Wood Johnson Foundation.
SOURCE: Lott M et al. Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations. Technical Scientific Report. (Durham, NC: Healthy Eating Research, 2019).
entirely. A coalition of health and nutrition organizations offered all this and more in a new set of sometimes surprising recommendations about beverage consumption in children from birth to age 5 years.
The recommendations “are based on the best available evidence, combined with sound expert judgment, and provide consistent messages that can be used by a variety of stakeholders to improve the beverage intake patterns of young children,” the report authors wrote. “It is imperative to capitalize on early childhood as a critical window of opportunity during which dietary patterns are both impressionable and capable of setting the stage for lifelong eating behaviors.”
The Academy of Nutrition and Dietetics, the American Academy of Pediatric Dentistry, the American Academy of Pediatrics, and the American Heart Association released their recommendations titled “Healthy Beverage Consumption in Early Childhood” in a report published Sept. 18.
The organizations convened expert panels and analyzed research to develop consensus beverage recommendations. Here are some highlights from the report, the writing of which was led by Megan Lott, MPH, RDN, of Healthy Eating Research and Duke Global Health Institute at Duke University, Durham, N.C.
- Breast milk or formula. This is what babies should be drinking for the first year of life.
- Plain drinking water. None is needed in the first 6 months of life. Introduce 0.5-1.0 cups/day over the next 6 months in cups and during meals when solid food is introduced. Then 1-4 cups/day are recommended from age 1-3 years, then 1.5-5 cups by age 4-5 years.
- Plain, pasteurized milk. None during the first year, then 2-3 cups a day of whole milk at age 12-24 months; if weight gain is excessive or family history is positive for obesity, dyslipidemia, or other cardiovascular disease, the pediatrician may recommend skim or lowfat milk at 12-24 months. Then provide up to 2 cups (age 2-3 years) then 2.5 cups (age 4-5 years) per day of skim /fat-free milk or low fat/1% milk
- 100% juice. None until 12 months. Then it can be provided, although whole fruit is preferred. No more than 0.5 cup per day until age 4-5 years, when no more than 0.5-0.75 cup per day is recommended.
- Plant-based milks and non-dairy beverages. None from age 0-1 year. And at ages 1-5 years, they only should be consumed when medically indicated, such as when a child has an allergy or per a preferred diet such as a vegan one. The panel didn’t recommend them as full replacements for dairy milk from age 12-24 months, and it noted that “consumption of these beverages as a full replacement for dairy milk should be undertaken in consultation with a health care provider so that adequate intake of key nutrients commonly obtained from dairy milk can be considered in dietary planning.”
- Other beverages. Flavored milk (like chocolate milk), “toddler milk,” sugar-sweetened drinks (including soft drinks, flavored water, and various fruit drinks), low-calorie sweetened drinks, and caffeinated drinks are not recommended at any age from 0-5 years. Toddler milk products “offer no unique nutritional value beyond what a nutritionally adequate diet provides and may contribute added sugars to the diet and undermine sustained breastfeeding,” the report authors said.
Lillian M. Beard, MD, said in an interview, “Pediatricians and other child health providers have major roles of influence in parents’ choices of foods and beverages for their infants and young children.
“Following these first-ever consensus recommendations on healthy drinks will alter family grocery shopping patterns, and not only improve the overall health of children under age 5 years, but also may improve the health outcomes of everyone in the household.” Dr. Beard is a clinical professor of pediatrics at George Washington University, Washington. She was not one of the report authors and was asked to comment on the report.
The report was supported by the Healthy Eating Research, a national program of the Robert Wood Johnson Foundation.
SOURCE: Lott M et al. Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations. Technical Scientific Report. (Durham, NC: Healthy Eating Research, 2019).
entirely. A coalition of health and nutrition organizations offered all this and more in a new set of sometimes surprising recommendations about beverage consumption in children from birth to age 5 years.
The recommendations “are based on the best available evidence, combined with sound expert judgment, and provide consistent messages that can be used by a variety of stakeholders to improve the beverage intake patterns of young children,” the report authors wrote. “It is imperative to capitalize on early childhood as a critical window of opportunity during which dietary patterns are both impressionable and capable of setting the stage for lifelong eating behaviors.”
The Academy of Nutrition and Dietetics, the American Academy of Pediatric Dentistry, the American Academy of Pediatrics, and the American Heart Association released their recommendations titled “Healthy Beverage Consumption in Early Childhood” in a report published Sept. 18.
The organizations convened expert panels and analyzed research to develop consensus beverage recommendations. Here are some highlights from the report, the writing of which was led by Megan Lott, MPH, RDN, of Healthy Eating Research and Duke Global Health Institute at Duke University, Durham, N.C.
- Breast milk or formula. This is what babies should be drinking for the first year of life.
- Plain drinking water. None is needed in the first 6 months of life. Introduce 0.5-1.0 cups/day over the next 6 months in cups and during meals when solid food is introduced. Then 1-4 cups/day are recommended from age 1-3 years, then 1.5-5 cups by age 4-5 years.
- Plain, pasteurized milk. None during the first year, then 2-3 cups a day of whole milk at age 12-24 months; if weight gain is excessive or family history is positive for obesity, dyslipidemia, or other cardiovascular disease, the pediatrician may recommend skim or lowfat milk at 12-24 months. Then provide up to 2 cups (age 2-3 years) then 2.5 cups (age 4-5 years) per day of skim /fat-free milk or low fat/1% milk
- 100% juice. None until 12 months. Then it can be provided, although whole fruit is preferred. No more than 0.5 cup per day until age 4-5 years, when no more than 0.5-0.75 cup per day is recommended.
- Plant-based milks and non-dairy beverages. None from age 0-1 year. And at ages 1-5 years, they only should be consumed when medically indicated, such as when a child has an allergy or per a preferred diet such as a vegan one. The panel didn’t recommend them as full replacements for dairy milk from age 12-24 months, and it noted that “consumption of these beverages as a full replacement for dairy milk should be undertaken in consultation with a health care provider so that adequate intake of key nutrients commonly obtained from dairy milk can be considered in dietary planning.”
- Other beverages. Flavored milk (like chocolate milk), “toddler milk,” sugar-sweetened drinks (including soft drinks, flavored water, and various fruit drinks), low-calorie sweetened drinks, and caffeinated drinks are not recommended at any age from 0-5 years. Toddler milk products “offer no unique nutritional value beyond what a nutritionally adequate diet provides and may contribute added sugars to the diet and undermine sustained breastfeeding,” the report authors said.
Lillian M. Beard, MD, said in an interview, “Pediatricians and other child health providers have major roles of influence in parents’ choices of foods and beverages for their infants and young children.
“Following these first-ever consensus recommendations on healthy drinks will alter family grocery shopping patterns, and not only improve the overall health of children under age 5 years, but also may improve the health outcomes of everyone in the household.” Dr. Beard is a clinical professor of pediatrics at George Washington University, Washington. She was not one of the report authors and was asked to comment on the report.
The report was supported by the Healthy Eating Research, a national program of the Robert Wood Johnson Foundation.
SOURCE: Lott M et al. Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations. Technical Scientific Report. (Durham, NC: Healthy Eating Research, 2019).
Gastrostomy tube placement associated with higher pneumonia recurrence in children with neurologic impairment
according to findings published in Pediatrics.
Five of the remaining seven strategies – gastrostomy tube placement, chest physiotherapy, outpatient antibiotics before hospitalization, and clinic visit before and after index hospitalization – were associated with increased recurrence, Jody L. Lin, MD, of the department of pediatrics at Stanford (Calif.) University, and colleagues reported. Oral secretion management and gastric acid suppression were associated with increased risk, but to a lesser extent.
The researchers examined the outcomes of the prevention strategies because, although children with neurologic impairment are more susceptible to community-acquired pneumonia, current guidelines are based mostly on expert opinion. The study included 3,632 children aged 21 years or younger with neurologic impairment and at least one hospitalization for pneumonia, who were enrolled in the California Children’s Services program between July 1, 2009, and June 30, 2014.
Propensity-score matching based on factors such as age, sex, household income, as well as characteristics of index hospitalization, showed decreased odds of recurrence only with receipt of dental care (adjusted odds ratio, 0.64; 95% confidence interval, 0.49-0.85), whereas increased odds were seen with other recommended prevention strategies, such as chest physiotherapy (aOR, 2.03; 95% CI, 1.29-3.20), receipt of antibiotics before hospitalization (aOR, 1.42; 95% CI, 1.06-1.92), and clinic visit before (aOR, 1.30; 95% CI, 1.11-1.52) and after index hospitalization (aOR, 1.72; 95% CI, 1.35-2.20).
The greatest increased odds, however, were seen with new gastrostomy tube placement (aOR, 2.15; 95% CI, 1.63-2.85).
The investigators noted that the biggest limitation of this study was the potential for residual confounding by indication even after adjustment, whereby certain interventions were provided to patients deemed more clinically severe to begin with. A strength of the study is its longitudinal nature.
“Our results suggest that more attention should be paid to dental health for children with [neurologic impairment],” the researchers wrote, although they noted that dental care “remains the most common unmet health care need” for children with special health care needs.
The findings also “support a clinical trial of dental care for prevention of severe pneumonia in children with [neurologic impairment] and do not support the widespread use of gastrostomy tubes for that purpose,” they added.
The study was funded by the National Institutes of Health. Dr. Lin received support from the NIH and the Clinical Excellence Research Center. The authors reported that they had no conflicts of interest.
[email protected]
SOURCE: Lin JL et al. Pediatrics. 2019 Sep 19. doi: 10.1542/peds.2019-0543.
according to findings published in Pediatrics.
Five of the remaining seven strategies – gastrostomy tube placement, chest physiotherapy, outpatient antibiotics before hospitalization, and clinic visit before and after index hospitalization – were associated with increased recurrence, Jody L. Lin, MD, of the department of pediatrics at Stanford (Calif.) University, and colleagues reported. Oral secretion management and gastric acid suppression were associated with increased risk, but to a lesser extent.
The researchers examined the outcomes of the prevention strategies because, although children with neurologic impairment are more susceptible to community-acquired pneumonia, current guidelines are based mostly on expert opinion. The study included 3,632 children aged 21 years or younger with neurologic impairment and at least one hospitalization for pneumonia, who were enrolled in the California Children’s Services program between July 1, 2009, and June 30, 2014.
Propensity-score matching based on factors such as age, sex, household income, as well as characteristics of index hospitalization, showed decreased odds of recurrence only with receipt of dental care (adjusted odds ratio, 0.64; 95% confidence interval, 0.49-0.85), whereas increased odds were seen with other recommended prevention strategies, such as chest physiotherapy (aOR, 2.03; 95% CI, 1.29-3.20), receipt of antibiotics before hospitalization (aOR, 1.42; 95% CI, 1.06-1.92), and clinic visit before (aOR, 1.30; 95% CI, 1.11-1.52) and after index hospitalization (aOR, 1.72; 95% CI, 1.35-2.20).
The greatest increased odds, however, were seen with new gastrostomy tube placement (aOR, 2.15; 95% CI, 1.63-2.85).
The investigators noted that the biggest limitation of this study was the potential for residual confounding by indication even after adjustment, whereby certain interventions were provided to patients deemed more clinically severe to begin with. A strength of the study is its longitudinal nature.
“Our results suggest that more attention should be paid to dental health for children with [neurologic impairment],” the researchers wrote, although they noted that dental care “remains the most common unmet health care need” for children with special health care needs.
The findings also “support a clinical trial of dental care for prevention of severe pneumonia in children with [neurologic impairment] and do not support the widespread use of gastrostomy tubes for that purpose,” they added.
The study was funded by the National Institutes of Health. Dr. Lin received support from the NIH and the Clinical Excellence Research Center. The authors reported that they had no conflicts of interest.
[email protected]
SOURCE: Lin JL et al. Pediatrics. 2019 Sep 19. doi: 10.1542/peds.2019-0543.
according to findings published in Pediatrics.
Five of the remaining seven strategies – gastrostomy tube placement, chest physiotherapy, outpatient antibiotics before hospitalization, and clinic visit before and after index hospitalization – were associated with increased recurrence, Jody L. Lin, MD, of the department of pediatrics at Stanford (Calif.) University, and colleagues reported. Oral secretion management and gastric acid suppression were associated with increased risk, but to a lesser extent.
The researchers examined the outcomes of the prevention strategies because, although children with neurologic impairment are more susceptible to community-acquired pneumonia, current guidelines are based mostly on expert opinion. The study included 3,632 children aged 21 years or younger with neurologic impairment and at least one hospitalization for pneumonia, who were enrolled in the California Children’s Services program between July 1, 2009, and June 30, 2014.
Propensity-score matching based on factors such as age, sex, household income, as well as characteristics of index hospitalization, showed decreased odds of recurrence only with receipt of dental care (adjusted odds ratio, 0.64; 95% confidence interval, 0.49-0.85), whereas increased odds were seen with other recommended prevention strategies, such as chest physiotherapy (aOR, 2.03; 95% CI, 1.29-3.20), receipt of antibiotics before hospitalization (aOR, 1.42; 95% CI, 1.06-1.92), and clinic visit before (aOR, 1.30; 95% CI, 1.11-1.52) and after index hospitalization (aOR, 1.72; 95% CI, 1.35-2.20).
The greatest increased odds, however, were seen with new gastrostomy tube placement (aOR, 2.15; 95% CI, 1.63-2.85).
The investigators noted that the biggest limitation of this study was the potential for residual confounding by indication even after adjustment, whereby certain interventions were provided to patients deemed more clinically severe to begin with. A strength of the study is its longitudinal nature.
“Our results suggest that more attention should be paid to dental health for children with [neurologic impairment],” the researchers wrote, although they noted that dental care “remains the most common unmet health care need” for children with special health care needs.
The findings also “support a clinical trial of dental care for prevention of severe pneumonia in children with [neurologic impairment] and do not support the widespread use of gastrostomy tubes for that purpose,” they added.
The study was funded by the National Institutes of Health. Dr. Lin received support from the NIH and the Clinical Excellence Research Center. The authors reported that they had no conflicts of interest.
[email protected]
SOURCE: Lin JL et al. Pediatrics. 2019 Sep 19. doi: 10.1542/peds.2019-0543.
FROM PEDIATRICS
Key clinical point: Gastrostomy tube placement is associated with higher pneumonia recurrence in children with neurologic impairment, and dental care is linked to decreased recurrence.
Major finding: There was an increased odds of pneumonia recurrence with new gastrostomy tube placement (adjusted odds ratio, 2.15; 95% confidence interval, 1.63-2.85) and decreased odds with dental care (aOR, 0.64; 95% CI, 0.49-0.85).
Study details: A comparative effectiveness study of a retrospective cohort of 3,632 children with neurologic impairment and at least one hospitalization for pneumonia, enrolled in California Children’s Services from July 1, 2009, to June 30, 2014.
Disclosures: The study was funded by the National Institutes of Health. Dr. Lin received support from the NIH and the Clinical Excellence Research Center. The authors reported that they had no conflicts of interest.
Source: Lin JL et al. Pediatrics. 2019 Sep 19. doi: 10.1542/peds.2019-0543.
Growing vaping habit may lead to nicotine addiction in adolescents
and in 2019 almost 12% of high school seniors reported that they were vaping every day, according to data from the Monitoring the Future surveys.
Daily use – defined as vaping on 20 or more of the previous 30 days – was reported by 6.9% of 10th-grade and 1.9% of 8th-grade respondents in the 2019 survey, which was the first time use in these age groups was assessed. “The substantial levels of daily vaping suggest the development of nicotine addiction,” Richard Miech, PhD, and associates said Sept. 18 in the New England Journal of Medicine.
From 2017 to 2019, e-cigarette use over the previous 30 days increased from 11.0% to 25.4% among 12th graders, from 8.2% to 20.2% in 10th graders, and from 3.5% to 9.0% of 8th graders, suggesting that “current efforts by the vaping industry, government agencies, and schools have thus far proved insufficient to stop the rapid spread of nicotine vaping among adolescents,” the investigators wrote.
By 2019, over 40% of 12th-grade students reported ever using e-cigarettes, along with more than 36% of 10th graders and almost 21% of 8th graders. Corresponding figures for past 12-month use were 35.1%, 31.1%, and 16.1%, they reported.
“New efforts are needed to protect youth from using nicotine during adolescence, when the developing brain is particularly susceptible to permanent changes from nicotine use and when almost all nicotine addiction is established,” the investigators wrote.
The analysis was funded by a grant from the National Institute on Drug Abuse to Dr. Miech.
SOURCE: Miech R et al. N Engl J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739.
and in 2019 almost 12% of high school seniors reported that they were vaping every day, according to data from the Monitoring the Future surveys.

Daily use – defined as vaping on 20 or more of the previous 30 days – was reported by 6.9% of 10th-grade and 1.9% of 8th-grade respondents in the 2019 survey, which was the first time use in these age groups was assessed. “The substantial levels of daily vaping suggest the development of nicotine addiction,” Richard Miech, PhD, and associates said Sept. 18 in the New England Journal of Medicine.
From 2017 to 2019, e-cigarette use over the previous 30 days increased from 11.0% to 25.4% among 12th graders, from 8.2% to 20.2% in 10th graders, and from 3.5% to 9.0% of 8th graders, suggesting that “current efforts by the vaping industry, government agencies, and schools have thus far proved insufficient to stop the rapid spread of nicotine vaping among adolescents,” the investigators wrote.
By 2019, over 40% of 12th-grade students reported ever using e-cigarettes, along with more than 36% of 10th graders and almost 21% of 8th graders. Corresponding figures for past 12-month use were 35.1%, 31.1%, and 16.1%, they reported.
“New efforts are needed to protect youth from using nicotine during adolescence, when the developing brain is particularly susceptible to permanent changes from nicotine use and when almost all nicotine addiction is established,” the investigators wrote.
The analysis was funded by a grant from the National Institute on Drug Abuse to Dr. Miech.
SOURCE: Miech R et al. N Engl J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739.
and in 2019 almost 12% of high school seniors reported that they were vaping every day, according to data from the Monitoring the Future surveys.

Daily use – defined as vaping on 20 or more of the previous 30 days – was reported by 6.9% of 10th-grade and 1.9% of 8th-grade respondents in the 2019 survey, which was the first time use in these age groups was assessed. “The substantial levels of daily vaping suggest the development of nicotine addiction,” Richard Miech, PhD, and associates said Sept. 18 in the New England Journal of Medicine.
From 2017 to 2019, e-cigarette use over the previous 30 days increased from 11.0% to 25.4% among 12th graders, from 8.2% to 20.2% in 10th graders, and from 3.5% to 9.0% of 8th graders, suggesting that “current efforts by the vaping industry, government agencies, and schools have thus far proved insufficient to stop the rapid spread of nicotine vaping among adolescents,” the investigators wrote.
By 2019, over 40% of 12th-grade students reported ever using e-cigarettes, along with more than 36% of 10th graders and almost 21% of 8th graders. Corresponding figures for past 12-month use were 35.1%, 31.1%, and 16.1%, they reported.
“New efforts are needed to protect youth from using nicotine during adolescence, when the developing brain is particularly susceptible to permanent changes from nicotine use and when almost all nicotine addiction is established,” the investigators wrote.
The analysis was funded by a grant from the National Institute on Drug Abuse to Dr. Miech.
SOURCE: Miech R et al. N Engl J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adolescents who use e-cigarettes every day may be developing nicotine addiction.
Major finding: In 2019, almost 12% of high school seniors were vaping every day.
Study details: Monitoring the Future surveys nationally representative samples of 8th-, 10th-, and 12th-grade students each year.
Disclosures: The analysis was funded by a grant from the National Institute on Drug Abuse to Dr. Miech.
Source: Miech R et al. N Engl J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739.
‘Fast MRI’ may be option in TBI screening for children
“Fast MRI,” which allows scans to be taken quickly without sedation, is a “reasonable alternative” to screen certain younger children for traumatic brain injury, a new study found.
The fast MRI option has “the potential to eliminate ionizing radiation exposure for thousands of children each year,” the study authors wrote in Pediatrics. “The ability to complete imaging in about 6 minutes, without the need for anesthesia or sedation, suggests that fast MRI is appropriate even in acute settings, where patient throughput is a priority.”
Daniel M. Lindberg, MD, of the University of Colorado at Denver, Aurora, and associates wrote that children make between 600,000 and 1.6 million ED visits in the United States each year for evaluation of possible traumatic brain injury (TBI). While the incidence of clinically significant injury from TBI is low, 20%-70% of these children are exposed to potentially dangerous radiation as they undergo CT.
The new study focuses on fast MRI. Unlike traditional MRI, it doesn’t require children to remain motionless – typically with the help of sedation – to be scanned.
The researchers performed fast MRI in 223 children aged younger than 6 years (median age, 12.6 months; interquartile range, 4.7-32.6) who sought emergency care at a level 1 pediatric trauma center from 2015 to 2018. They had all had CT scans performed.
CT identified TBI in 111 (50%) of the subjects, while fast MRI identified it in 103 (sensitivity, 92.8%; 95% confidence interval, 86.3-96.8). Fast MRI missed six participants with isolated skull fractures and two with subarachnoid hemorrhage; CT missed five participants with subdural hematomas, parenchymal contusions, and subarachnoid hemorrhage.
While the researchers hoped for a higher sensitivity level, they wrote that “we feel that the benefit of avoiding radiation exposure outweighs the concern for missed injury.”
In a commentary, Brett Burstein, MDCM, PhD, MPH, and Christine Saint-Martin, MDCM, MSc, of Montreal Children’s Hospital and McGill University Health Center, also in Montreal, wrote that the study is “well conducted.”
However, they noted that “the reported feasibility reflects a highly selected cohort of stable patients in whom fast MRI is already likely to succeed. Feasibility results in a more generalizable population of head-injured children cannot be extrapolated.”
And, they added, “fast MRI was unavailable for 65 of 299 consenting, eligible patients because of lack of overnight staffing. Although not included among the outcome definitions of imaging time, this would be an important ‘feasibility’ consideration in most centers.”
Dr. Burstein and Dr. Saint-Martin wrote that “centers migrating toward this modality for neuroimaging children with head injuries should still use clinical judgment and highly sensitive, validated clinical decision rules when determining the need for any neuroimaging for head-injured children.”
The study was funded by the Colorado Traumatic Brain Injury Trust Fund (MindSource) and the Colorado Clinical and Translational Sciences Institute. The study and commentary authors reported no relevant financial disclosures.
SOURCES: Lindberg DM et al. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-0419; Burstein B, Saint-Martin C. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-2387.
“Fast MRI,” which allows scans to be taken quickly without sedation, is a “reasonable alternative” to screen certain younger children for traumatic brain injury, a new study found.
The fast MRI option has “the potential to eliminate ionizing radiation exposure for thousands of children each year,” the study authors wrote in Pediatrics. “The ability to complete imaging in about 6 minutes, without the need for anesthesia or sedation, suggests that fast MRI is appropriate even in acute settings, where patient throughput is a priority.”
Daniel M. Lindberg, MD, of the University of Colorado at Denver, Aurora, and associates wrote that children make between 600,000 and 1.6 million ED visits in the United States each year for evaluation of possible traumatic brain injury (TBI). While the incidence of clinically significant injury from TBI is low, 20%-70% of these children are exposed to potentially dangerous radiation as they undergo CT.
The new study focuses on fast MRI. Unlike traditional MRI, it doesn’t require children to remain motionless – typically with the help of sedation – to be scanned.
The researchers performed fast MRI in 223 children aged younger than 6 years (median age, 12.6 months; interquartile range, 4.7-32.6) who sought emergency care at a level 1 pediatric trauma center from 2015 to 2018. They had all had CT scans performed.
CT identified TBI in 111 (50%) of the subjects, while fast MRI identified it in 103 (sensitivity, 92.8%; 95% confidence interval, 86.3-96.8). Fast MRI missed six participants with isolated skull fractures and two with subarachnoid hemorrhage; CT missed five participants with subdural hematomas, parenchymal contusions, and subarachnoid hemorrhage.
While the researchers hoped for a higher sensitivity level, they wrote that “we feel that the benefit of avoiding radiation exposure outweighs the concern for missed injury.”
In a commentary, Brett Burstein, MDCM, PhD, MPH, and Christine Saint-Martin, MDCM, MSc, of Montreal Children’s Hospital and McGill University Health Center, also in Montreal, wrote that the study is “well conducted.”
However, they noted that “the reported feasibility reflects a highly selected cohort of stable patients in whom fast MRI is already likely to succeed. Feasibility results in a more generalizable population of head-injured children cannot be extrapolated.”
And, they added, “fast MRI was unavailable for 65 of 299 consenting, eligible patients because of lack of overnight staffing. Although not included among the outcome definitions of imaging time, this would be an important ‘feasibility’ consideration in most centers.”
Dr. Burstein and Dr. Saint-Martin wrote that “centers migrating toward this modality for neuroimaging children with head injuries should still use clinical judgment and highly sensitive, validated clinical decision rules when determining the need for any neuroimaging for head-injured children.”
The study was funded by the Colorado Traumatic Brain Injury Trust Fund (MindSource) and the Colorado Clinical and Translational Sciences Institute. The study and commentary authors reported no relevant financial disclosures.
SOURCES: Lindberg DM et al. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-0419; Burstein B, Saint-Martin C. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-2387.
“Fast MRI,” which allows scans to be taken quickly without sedation, is a “reasonable alternative” to screen certain younger children for traumatic brain injury, a new study found.
The fast MRI option has “the potential to eliminate ionizing radiation exposure for thousands of children each year,” the study authors wrote in Pediatrics. “The ability to complete imaging in about 6 minutes, without the need for anesthesia or sedation, suggests that fast MRI is appropriate even in acute settings, where patient throughput is a priority.”
Daniel M. Lindberg, MD, of the University of Colorado at Denver, Aurora, and associates wrote that children make between 600,000 and 1.6 million ED visits in the United States each year for evaluation of possible traumatic brain injury (TBI). While the incidence of clinically significant injury from TBI is low, 20%-70% of these children are exposed to potentially dangerous radiation as they undergo CT.
The new study focuses on fast MRI. Unlike traditional MRI, it doesn’t require children to remain motionless – typically with the help of sedation – to be scanned.
The researchers performed fast MRI in 223 children aged younger than 6 years (median age, 12.6 months; interquartile range, 4.7-32.6) who sought emergency care at a level 1 pediatric trauma center from 2015 to 2018. They had all had CT scans performed.
CT identified TBI in 111 (50%) of the subjects, while fast MRI identified it in 103 (sensitivity, 92.8%; 95% confidence interval, 86.3-96.8). Fast MRI missed six participants with isolated skull fractures and two with subarachnoid hemorrhage; CT missed five participants with subdural hematomas, parenchymal contusions, and subarachnoid hemorrhage.
While the researchers hoped for a higher sensitivity level, they wrote that “we feel that the benefit of avoiding radiation exposure outweighs the concern for missed injury.”
In a commentary, Brett Burstein, MDCM, PhD, MPH, and Christine Saint-Martin, MDCM, MSc, of Montreal Children’s Hospital and McGill University Health Center, also in Montreal, wrote that the study is “well conducted.”
However, they noted that “the reported feasibility reflects a highly selected cohort of stable patients in whom fast MRI is already likely to succeed. Feasibility results in a more generalizable population of head-injured children cannot be extrapolated.”
And, they added, “fast MRI was unavailable for 65 of 299 consenting, eligible patients because of lack of overnight staffing. Although not included among the outcome definitions of imaging time, this would be an important ‘feasibility’ consideration in most centers.”
Dr. Burstein and Dr. Saint-Martin wrote that “centers migrating toward this modality for neuroimaging children with head injuries should still use clinical judgment and highly sensitive, validated clinical decision rules when determining the need for any neuroimaging for head-injured children.”
The study was funded by the Colorado Traumatic Brain Injury Trust Fund (MindSource) and the Colorado Clinical and Translational Sciences Institute. The study and commentary authors reported no relevant financial disclosures.
SOURCES: Lindberg DM et al. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-0419; Burstein B, Saint-Martin C. Pediatrics. 2019 Sep 18. doi: 10.1542/peds.2019-2387.
FROM PEDIATRICS
Juvenile dermatomyositis derails growth and pubertal development
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
FROM ARTHRITIS CARE & RESEARCH