User login
Australia’s rotavirus outbreak wasn’t caused by vaccine effectiveness decline
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
FROM PEDIATRICS
My patient tells me that they are transgender – now what?
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
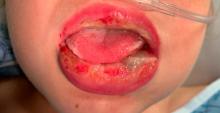
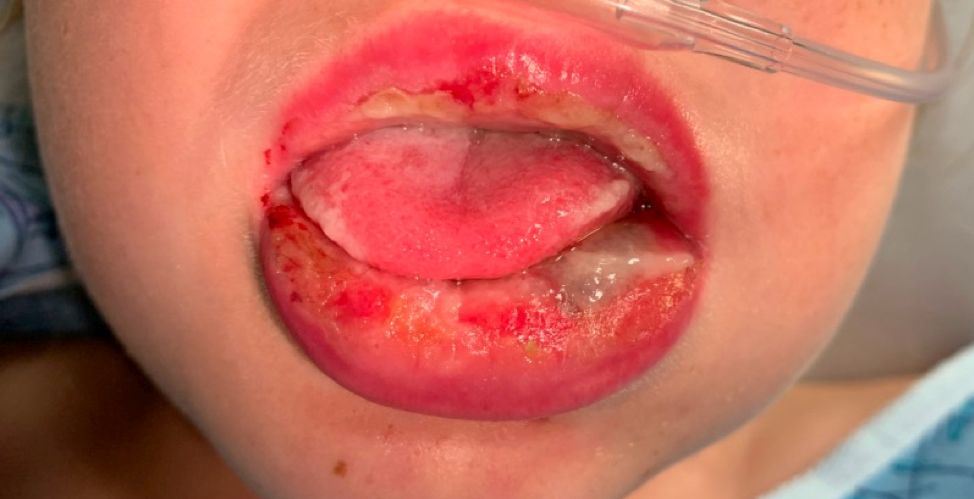
Pneumonia with tender, dry, crusted lips
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Benefits of peanut desensitization may not last
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
FROM THE LANCET
Presumptive style of conversation boosts HPV vaccination rates in adolescents
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
FROM PEDIATRICS
Key clinical point: A presumptive style of conversation and a two-dose vaccination schedule can increase HPV vaccination rates in adolescents.
Major finding: Overall, 65% of pediatricians and 42% of FPs reported using a presumptive style to discuss HPV vaccination.
Study details: National survey of 302 pediatricians and 228 family physicians conducted July-September 2018.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
Source: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
Rates of off-label prescribing for children continue to increase
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
FROM PEDIATRICS
Key clinical point:
Major finding: At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% CI, 17.7%-19.3%).
Study details: A retrospective study of serial, cross-sectional data from the National Ambulatory Medical Care Surveys (2006-2015).
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
Source: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Newer drugs provide superior disease activity control in pediatric MS
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
REPORTING FROM ECTRIMS 2019
Peanut allergy pill gets thumbs-up from FDA advisory panel
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
FROM THE FDA ALLERGENIC PRODUCTS ADVISORY COMMITTEE MEETING
FDA approves mepolizumab for severe eosinophilic asthma in younger kids
according to a release from GlaxoSmithKline, which developed the drug. This is the first targeted biologic approved for this condition in this age group.
The approval is supported by both an open-label study in children aged 6-11 years and evidence from other trials conducted in adults and adolescents. The 52-week, long-term study in these younger patients investigated pharmacokinetics, pharmacodynamics, and safety, the last of which was shown to be similar to that seen in older patients.
Hypersensitivity reactions, such as anaphylaxis, rash, and bronchospasm, have been associated with mepolizumab. It should not be used to treat acute bronchospasm or status asthmaticus, nor should systemic or inhaled corticosteroids be stopped abruptly after initiating mepolizumab treatment. Common adverse events include headache, injection-site reactions, back pain, and fatigue. Injection site reactions (such as pain, erythema, and itching) occurred in 8% of mepolizumab patients treated with 100 mg of the drug versus 3% of placebo patients.
The monoclonal antibody targeting interleukin-5 was first approved for severe eosinophilic asthma in 2015 for ages 12 years and older and in ages 6 years and older in the European Union in August 2018. It inhibits IL-5 from binding to eosinophils, which reduces the presence of eosinophils in blood without completely eliminating them.
according to a release from GlaxoSmithKline, which developed the drug. This is the first targeted biologic approved for this condition in this age group.
The approval is supported by both an open-label study in children aged 6-11 years and evidence from other trials conducted in adults and adolescents. The 52-week, long-term study in these younger patients investigated pharmacokinetics, pharmacodynamics, and safety, the last of which was shown to be similar to that seen in older patients.
Hypersensitivity reactions, such as anaphylaxis, rash, and bronchospasm, have been associated with mepolizumab. It should not be used to treat acute bronchospasm or status asthmaticus, nor should systemic or inhaled corticosteroids be stopped abruptly after initiating mepolizumab treatment. Common adverse events include headache, injection-site reactions, back pain, and fatigue. Injection site reactions (such as pain, erythema, and itching) occurred in 8% of mepolizumab patients treated with 100 mg of the drug versus 3% of placebo patients.
The monoclonal antibody targeting interleukin-5 was first approved for severe eosinophilic asthma in 2015 for ages 12 years and older and in ages 6 years and older in the European Union in August 2018. It inhibits IL-5 from binding to eosinophils, which reduces the presence of eosinophils in blood without completely eliminating them.
according to a release from GlaxoSmithKline, which developed the drug. This is the first targeted biologic approved for this condition in this age group.
The approval is supported by both an open-label study in children aged 6-11 years and evidence from other trials conducted in adults and adolescents. The 52-week, long-term study in these younger patients investigated pharmacokinetics, pharmacodynamics, and safety, the last of which was shown to be similar to that seen in older patients.
Hypersensitivity reactions, such as anaphylaxis, rash, and bronchospasm, have been associated with mepolizumab. It should not be used to treat acute bronchospasm or status asthmaticus, nor should systemic or inhaled corticosteroids be stopped abruptly after initiating mepolizumab treatment. Common adverse events include headache, injection-site reactions, back pain, and fatigue. Injection site reactions (such as pain, erythema, and itching) occurred in 8% of mepolizumab patients treated with 100 mg of the drug versus 3% of placebo patients.
The monoclonal antibody targeting interleukin-5 was first approved for severe eosinophilic asthma in 2015 for ages 12 years and older and in ages 6 years and older in the European Union in August 2018. It inhibits IL-5 from binding to eosinophils, which reduces the presence of eosinophils in blood without completely eliminating them.
Encourage participation in team sports
Participation in sports, competitive team sports in particular, is very good for the physical well-being and emotional development of children and adolescents. Specifically, there is growing evidence that sports promote healthy development socially and emotionally, protecting against drug use, poor body image, and against psychiatric illness in youth.
Sustaining academic productivity and team sports is demanding. By the middle of autumn, the amount of homework can begin to wear on teenagers, and the burden of getting them to practices and games can wear on parents. It can be very tempting for youth and their parents to drop team sports in high school, and turn their time and effort more completely to the serious work of school. But advocating for your patients and their parents to protect the time for team sports participation will pay dividends in the health and well-being of your patients and may even support rather than detract from academic performance.
The benefits of regular exercise for physical health are well established. Most teenagers do not get the recommended 60 minutes daily of moderate to vigorous physical activity. Participating in a team sport enforces this level of activity, in ways that parents typically don’t have to enforce. This level of physical activity typically promotes healthy eating and a healthy weight. Daily exercise promotes adequate, restful sleep, one of the most critical (and usually compromised) components of adolescent health. These exercise habits are easier to maintain into adulthood – when they protect against cardiovascular and inflammatory diseases – if they have been established early.
Beyond physical health, participation in team sports has been shown to promote good mental health and protect against psychiatric illnesses. They generally are less likely to use drugs and more likely to have a healthy body image than are their nonathlete peers. It is worth noting that the mental health benefits of team sports are even more robust than the benefits of solitary exercise in teenagers,1 possibly because of the social connections to peers and adults that grow out of them.
In the Monitoring the Future surveys (biannual national surveys of high school student health and behaviors funded by the National Institutes of Health) from 2010 to 2015, teenagers who participated in team sports were more likely to describe higher self-esteem and lower levels of loneliness. It is important to note that it has been difficult to establish the causal direction of the association between team sports and mental health in youth. We need more prospective randomized controlled trials to assert that the benefit is not simply an artifact of healthier youth choosing to participate in sports, but actually an active consequence of that choice. For now, though, we can say with confidence that physical activity promotes good mental health in youth and may protect against mental illness.
While student athletes benefit from the opportunity to develop deep social connections – ones forged in the intense setting of competition, collaboration, and sustained teamwork – they also benefit from strong mentorship connections with adults, including coaches, trainers, and even the parents of teammates who participate in all of the efforts that go into team sports in youth. While it might seem that all of the mental and physical benefits must be offset by lower academic performance, it turns out that is not the case. It is well established that regular exercise promotes healthy cognitive function, including processing speed, working memory, and even creativity. According to data from the Monitoring the Future survey, adolescents who participated in team sports were more likely to have As and to plan on attending a 4-year college than were their nonathlete peers.
Beyond the physiologic and social benefits of exercise, team sports provide adolescents with a powerful opportunity to get comfortable with failure. Even the best athletes cannot win all the time, and sports are unique in building failure into the work. Practice is almost entirely about failure, gradually getting better at something that is difficult. While everyone aims to win, they also prepare to struggle and lose. Athletes must learn how to persevere through a match that they are losing, and then pick themselves up and prepare again for the next match. When young people get comfortable with facing and managing challenges, managing setbacks and failure, they are ready to face the larger challenges, setbacks, and failures of adult life.
Team sports enable young people to learn what they are actually capable of managing – they build resilience. This promotion of resilience is illustrated in recent research that demonstrated that team sports may be especially protective for young people who have experienced trauma (adverse childhood experiences, or “ACEs”). Researchers at the University of California, Los Angeles, followed teenagers with and without high ACE scores into their mid 20s. They found that those with high ACE scores who participated in team sports as adolescents were 24% less likely to have depression and 30% less likely to have anxiety diagnoses as adults, compared with their peers who did not participate in team sports.2
Of course, the details matter in team sports. If your patients are participating and they or their parents are worried about spending so much time on something other than homework, talk to them about all of these exceptional benefits of team sports. But the culture of the team matters also. Some teams may be focused on winning at all costs, or have a practice culture that is humiliating or bullying. Some teams may have a culture of partying after games, with binge drinking and drug use. Ask your patients about whether they feel they are respected members of the team, and if effort and sportsmanship are valued as well as performance. Do they trust their coaches? Do they believe their coaches know and care about them? If your patients are not participating in a team sport, encourage them to find one (or more) that engage their interests. The benefits of track and field, crew, and tennis can be just as robust as the benefits of football or soccer. Speak with your patients and their parents about the payoff for their physical, mental, and developmental health the time and effort they are putting into a team sport can provide.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Int J Nutr Phys Act. 2013 Aug 15. doi: 10.1186/1479-5868-10-98.
2. JAMA Pediatr. 2019 Jul 1;173(7):681-8.
Participation in sports, competitive team sports in particular, is very good for the physical well-being and emotional development of children and adolescents. Specifically, there is growing evidence that sports promote healthy development socially and emotionally, protecting against drug use, poor body image, and against psychiatric illness in youth.
Sustaining academic productivity and team sports is demanding. By the middle of autumn, the amount of homework can begin to wear on teenagers, and the burden of getting them to practices and games can wear on parents. It can be very tempting for youth and their parents to drop team sports in high school, and turn their time and effort more completely to the serious work of school. But advocating for your patients and their parents to protect the time for team sports participation will pay dividends in the health and well-being of your patients and may even support rather than detract from academic performance.
The benefits of regular exercise for physical health are well established. Most teenagers do not get the recommended 60 minutes daily of moderate to vigorous physical activity. Participating in a team sport enforces this level of activity, in ways that parents typically don’t have to enforce. This level of physical activity typically promotes healthy eating and a healthy weight. Daily exercise promotes adequate, restful sleep, one of the most critical (and usually compromised) components of adolescent health. These exercise habits are easier to maintain into adulthood – when they protect against cardiovascular and inflammatory diseases – if they have been established early.
Beyond physical health, participation in team sports has been shown to promote good mental health and protect against psychiatric illnesses. They generally are less likely to use drugs and more likely to have a healthy body image than are their nonathlete peers. It is worth noting that the mental health benefits of team sports are even more robust than the benefits of solitary exercise in teenagers,1 possibly because of the social connections to peers and adults that grow out of them.
In the Monitoring the Future surveys (biannual national surveys of high school student health and behaviors funded by the National Institutes of Health) from 2010 to 2015, teenagers who participated in team sports were more likely to describe higher self-esteem and lower levels of loneliness. It is important to note that it has been difficult to establish the causal direction of the association between team sports and mental health in youth. We need more prospective randomized controlled trials to assert that the benefit is not simply an artifact of healthier youth choosing to participate in sports, but actually an active consequence of that choice. For now, though, we can say with confidence that physical activity promotes good mental health in youth and may protect against mental illness.
While student athletes benefit from the opportunity to develop deep social connections – ones forged in the intense setting of competition, collaboration, and sustained teamwork – they also benefit from strong mentorship connections with adults, including coaches, trainers, and even the parents of teammates who participate in all of the efforts that go into team sports in youth. While it might seem that all of the mental and physical benefits must be offset by lower academic performance, it turns out that is not the case. It is well established that regular exercise promotes healthy cognitive function, including processing speed, working memory, and even creativity. According to data from the Monitoring the Future survey, adolescents who participated in team sports were more likely to have As and to plan on attending a 4-year college than were their nonathlete peers.
Beyond the physiologic and social benefits of exercise, team sports provide adolescents with a powerful opportunity to get comfortable with failure. Even the best athletes cannot win all the time, and sports are unique in building failure into the work. Practice is almost entirely about failure, gradually getting better at something that is difficult. While everyone aims to win, they also prepare to struggle and lose. Athletes must learn how to persevere through a match that they are losing, and then pick themselves up and prepare again for the next match. When young people get comfortable with facing and managing challenges, managing setbacks and failure, they are ready to face the larger challenges, setbacks, and failures of adult life.
Team sports enable young people to learn what they are actually capable of managing – they build resilience. This promotion of resilience is illustrated in recent research that demonstrated that team sports may be especially protective for young people who have experienced trauma (adverse childhood experiences, or “ACEs”). Researchers at the University of California, Los Angeles, followed teenagers with and without high ACE scores into their mid 20s. They found that those with high ACE scores who participated in team sports as adolescents were 24% less likely to have depression and 30% less likely to have anxiety diagnoses as adults, compared with their peers who did not participate in team sports.2
Of course, the details matter in team sports. If your patients are participating and they or their parents are worried about spending so much time on something other than homework, talk to them about all of these exceptional benefits of team sports. But the culture of the team matters also. Some teams may be focused on winning at all costs, or have a practice culture that is humiliating or bullying. Some teams may have a culture of partying after games, with binge drinking and drug use. Ask your patients about whether they feel they are respected members of the team, and if effort and sportsmanship are valued as well as performance. Do they trust their coaches? Do they believe their coaches know and care about them? If your patients are not participating in a team sport, encourage them to find one (or more) that engage their interests. The benefits of track and field, crew, and tennis can be just as robust as the benefits of football or soccer. Speak with your patients and their parents about the payoff for their physical, mental, and developmental health the time and effort they are putting into a team sport can provide.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Int J Nutr Phys Act. 2013 Aug 15. doi: 10.1186/1479-5868-10-98.
2. JAMA Pediatr. 2019 Jul 1;173(7):681-8.
Participation in sports, competitive team sports in particular, is very good for the physical well-being and emotional development of children and adolescents. Specifically, there is growing evidence that sports promote healthy development socially and emotionally, protecting against drug use, poor body image, and against psychiatric illness in youth.
Sustaining academic productivity and team sports is demanding. By the middle of autumn, the amount of homework can begin to wear on teenagers, and the burden of getting them to practices and games can wear on parents. It can be very tempting for youth and their parents to drop team sports in high school, and turn their time and effort more completely to the serious work of school. But advocating for your patients and their parents to protect the time for team sports participation will pay dividends in the health and well-being of your patients and may even support rather than detract from academic performance.
The benefits of regular exercise for physical health are well established. Most teenagers do not get the recommended 60 minutes daily of moderate to vigorous physical activity. Participating in a team sport enforces this level of activity, in ways that parents typically don’t have to enforce. This level of physical activity typically promotes healthy eating and a healthy weight. Daily exercise promotes adequate, restful sleep, one of the most critical (and usually compromised) components of adolescent health. These exercise habits are easier to maintain into adulthood – when they protect against cardiovascular and inflammatory diseases – if they have been established early.
Beyond physical health, participation in team sports has been shown to promote good mental health and protect against psychiatric illnesses. They generally are less likely to use drugs and more likely to have a healthy body image than are their nonathlete peers. It is worth noting that the mental health benefits of team sports are even more robust than the benefits of solitary exercise in teenagers,1 possibly because of the social connections to peers and adults that grow out of them.
In the Monitoring the Future surveys (biannual national surveys of high school student health and behaviors funded by the National Institutes of Health) from 2010 to 2015, teenagers who participated in team sports were more likely to describe higher self-esteem and lower levels of loneliness. It is important to note that it has been difficult to establish the causal direction of the association between team sports and mental health in youth. We need more prospective randomized controlled trials to assert that the benefit is not simply an artifact of healthier youth choosing to participate in sports, but actually an active consequence of that choice. For now, though, we can say with confidence that physical activity promotes good mental health in youth and may protect against mental illness.
While student athletes benefit from the opportunity to develop deep social connections – ones forged in the intense setting of competition, collaboration, and sustained teamwork – they also benefit from strong mentorship connections with adults, including coaches, trainers, and even the parents of teammates who participate in all of the efforts that go into team sports in youth. While it might seem that all of the mental and physical benefits must be offset by lower academic performance, it turns out that is not the case. It is well established that regular exercise promotes healthy cognitive function, including processing speed, working memory, and even creativity. According to data from the Monitoring the Future survey, adolescents who participated in team sports were more likely to have As and to plan on attending a 4-year college than were their nonathlete peers.
Beyond the physiologic and social benefits of exercise, team sports provide adolescents with a powerful opportunity to get comfortable with failure. Even the best athletes cannot win all the time, and sports are unique in building failure into the work. Practice is almost entirely about failure, gradually getting better at something that is difficult. While everyone aims to win, they also prepare to struggle and lose. Athletes must learn how to persevere through a match that they are losing, and then pick themselves up and prepare again for the next match. When young people get comfortable with facing and managing challenges, managing setbacks and failure, they are ready to face the larger challenges, setbacks, and failures of adult life.
Team sports enable young people to learn what they are actually capable of managing – they build resilience. This promotion of resilience is illustrated in recent research that demonstrated that team sports may be especially protective for young people who have experienced trauma (adverse childhood experiences, or “ACEs”). Researchers at the University of California, Los Angeles, followed teenagers with and without high ACE scores into their mid 20s. They found that those with high ACE scores who participated in team sports as adolescents were 24% less likely to have depression and 30% less likely to have anxiety diagnoses as adults, compared with their peers who did not participate in team sports.2
Of course, the details matter in team sports. If your patients are participating and they or their parents are worried about spending so much time on something other than homework, talk to them about all of these exceptional benefits of team sports. But the culture of the team matters also. Some teams may be focused on winning at all costs, or have a practice culture that is humiliating or bullying. Some teams may have a culture of partying after games, with binge drinking and drug use. Ask your patients about whether they feel they are respected members of the team, and if effort and sportsmanship are valued as well as performance. Do they trust their coaches? Do they believe their coaches know and care about them? If your patients are not participating in a team sport, encourage them to find one (or more) that engage their interests. The benefits of track and field, crew, and tennis can be just as robust as the benefits of football or soccer. Speak with your patients and their parents about the payoff for their physical, mental, and developmental health the time and effort they are putting into a team sport can provide.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Int J Nutr Phys Act. 2013 Aug 15. doi: 10.1186/1479-5868-10-98.
2. JAMA Pediatr. 2019 Jul 1;173(7):681-8.