User login
Timing, volume of transfusion may not matter in children with severe anemia
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The 28-day mortality was 0.9% in patients who had immediate transfusions and 1.7% in those who did not (hazard ratio, 0.54; P = .19). The 28-day mortality rate was 3.4% in patients who received transfusions of 30 mL/kg and 4.5% in those who received transfusions of 20 mL/kg (HR, 0.76; P = .12). However, the mortality rate was lower in the 30-mL/kg group for children without fevers (HR, 0.43) and higher in the 30-mL/kg group for febrile children (HR, 1.91).
Study details: A phase 3 trial of African children with severe anemia who were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787) and transfusions of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596)
Disclosures: The trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator.
Sources: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Short-course azithromycin no benefit in pediatric asthma admissions
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
REPORTING FROM PHM 2019
FDA approvals permit double-immunoassay approach to Lyme disease diagnosis
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Washington State removes exemption for MMR vaccine
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.
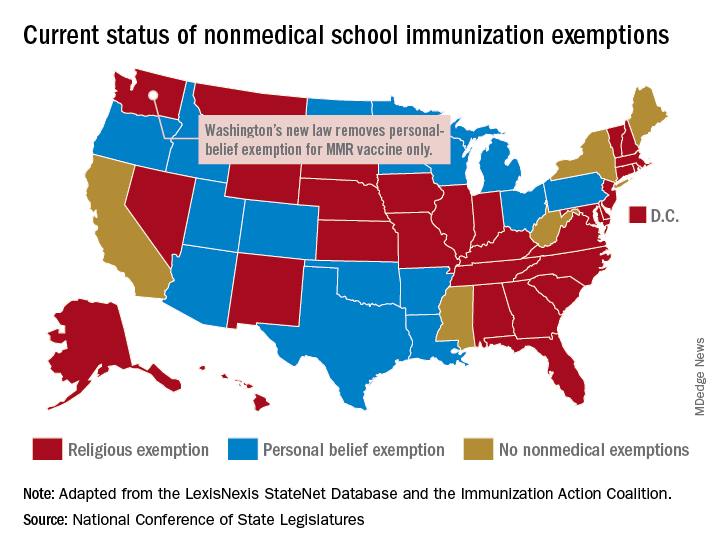
“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Prescriptions for cough, cold medicine dropping for children
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.
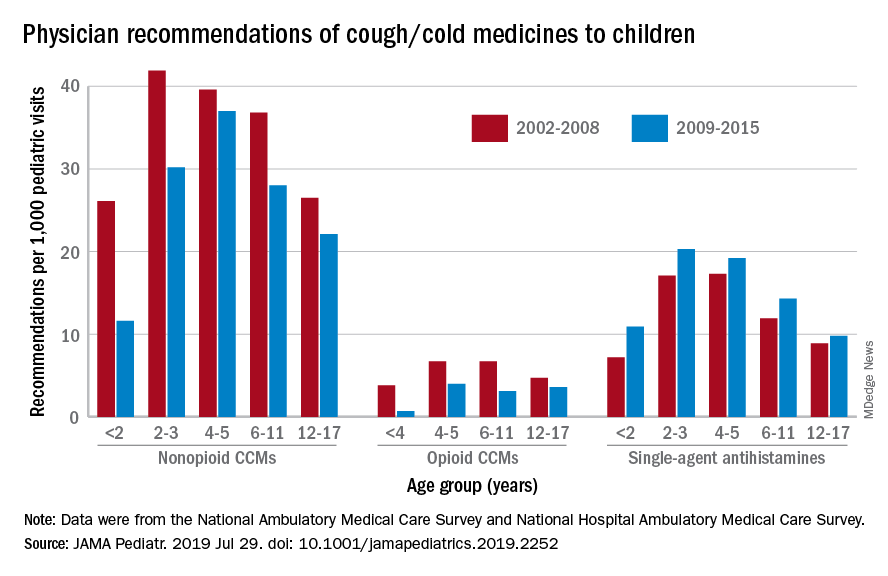
Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
FROM JAMA PEDIATRICS
Pertussis: Comparison studies show DTwP more durable
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
FROM VACCINE
Psychology consult for children’s skin issues can boost adherence, wellness
AUSTIN, TEX. – One day each week, Sasha D. Jaquez, PhD, visits with patients in the dermatology clinic at Dell Children’s Medical Center of Central Texas who wrestle with some aspect of their skin condition, from noncompliance to a recommended treatment regimen to fear of needles when an injection of medicine is required to keep them well.
“Our goal is to help promote the health and development of children, adolescents, and families through the use of evidence-based methods like cognitive-behavioral therapy,” said Dr. Jaquez, who is a pediatric psychologist at the University of Texas, Austin. “We do assessment and treatment of behavioral and emotional difficulties related to their skin condition or medical condition. So if they’re depressed but it’s not related to their skin condition, we will likely refer the patient to a community mental health system.”
During 1-hour visits at the dermatology clinic, Dr. Jaquez uses a mixed approach that includes cognitive-behavioral therapy and motivational interviewing to help patients and family members cope with their problematic behavior or negative thought patterns related to their skin conditions. “We do not have magic wands; we focus on the here and now,” she explained. “We focus on how to move forward in the most efficient way possible by teaching skills, practicing those skills with them in the office, and sending them home to use those skills. I don’t have 100% compliance on this, so if I notice that they’re not doing what I asked of them, we’ll have a conversation about what the barrier is. ‘What is getting in the way?’ I’ll ask. ‘Is this something you’re really wanting, or do you want a magic pill? If you want a magic pill, then our office isn’t where that’s going to come from.’ Sometimes patients aren’t ready to work on feeling better, and that is good for us to know.”
During consultations, she often talks with children and adolescents about how thoughts, feelings, and behaviors are related. She’ll use phrasing like, “The way that you think about something changes the way that you feel, and it changes the way that you act. We have control over our thoughts and behaviors, so if we think it’s going to be a bad day, it’s going to be a bad day. If we think it’s going to be a good day, then we’re going to find the positive aspects in the day and we might let those bad aspects go away. If I do something different [for my skin condition], then I’m going to feel different.”
She recalled the case of a 3-year-old boy with atopic dermatitis who was referred for excessive scratching. His mom stays at home, while dad works and travels frequently. “The parents had differing views on how to treat his medical condition. Mom wanted to do wet wraps while dad wanted to do bleach baths. Their son was getting no treatment because the parents couldn’t agree on anything. Mom noticed that her son scratches when he wants attention and when he’s angry.”
When Dr. Jaquez met with his parents, she encouraged them to agree on a plan to implement at home so that their son would gain some relief. She also advised them to ignore when their son scratches or when he gets angry. “Give him something else to do besides scratch, because if those hands are busy, he won’t be scratching. Let’s change the way this behavior happens. Let’s give him attention all the time instead of just when he’s scratching. That will work very quickly. And it did.”
She makes it a point to talk with patients and their families about living with the stress of a chronic illness like psoriasis or atopic dermatitis. “Let’s figure out, ‘How do we accept that this is how it is, and that they’re going to have to find their own ‘normal?’ ” she said. “I don’t know how many times someone comes into my office and says, ‘I just want to be normal.’ I like to ask patients, ‘what is your normal?’ These kids might have a lower quality of life than a child without a chronic illness, but we want to make sure that they’re living their lives to the fullest. You want to monitor not only adherence [to medication] but also quality of life. Sleep concerns are big. A lot of our kids might not being going to school, or they’re afraid to go to school because they get picked on because people don’t understand their skin condition.”
Dr. Jaquez acknowledged that not all dermatologists have a psychologist on staff or in their referral network, but all are capable of destigmatizing psychological and mental health issues for their patients. “Psychological comorbidities such as depression and anxiety can be associated with certain skin conditions,” she said. “Let them know that this is stressful stuff. Have discussions early, so if the time comes for a referral they won’t think you’re giving up on them. Don’t be afraid to say you have a psychologist that you want to refer to. Say, ‘I have an added team member I would love for you to meet. She’s our psychologist. She works with patients who are having difficulties.’ ”
Giving patients perceived control of their care could also help improve the behavior of concern. For example, when patients with needle phobia require an injection, ask if they would like to lay down, or sit down for the injection. “Giving them this tiny bit of control is going to help them feel more empowered,” she said.
Dr. Jaquez also recommends that clinicians pay attention to nonverbal cues and steer clear of using scare tactics to change their behavior. “Use positive behavioral strategies and try to avoid punishment. Children don’t want to hear ‘stop’ all the time. Parents are tired of saying it, and kids are tired of hearing it. We focus on praising the things that are going well. I advise parents all the time: ‘Catch them being good.’ ”
Dr. Jaquez reported having no financial disclosures.
AUSTIN, TEX. – One day each week, Sasha D. Jaquez, PhD, visits with patients in the dermatology clinic at Dell Children’s Medical Center of Central Texas who wrestle with some aspect of their skin condition, from noncompliance to a recommended treatment regimen to fear of needles when an injection of medicine is required to keep them well.
“Our goal is to help promote the health and development of children, adolescents, and families through the use of evidence-based methods like cognitive-behavioral therapy,” said Dr. Jaquez, who is a pediatric psychologist at the University of Texas, Austin. “We do assessment and treatment of behavioral and emotional difficulties related to their skin condition or medical condition. So if they’re depressed but it’s not related to their skin condition, we will likely refer the patient to a community mental health system.”
During 1-hour visits at the dermatology clinic, Dr. Jaquez uses a mixed approach that includes cognitive-behavioral therapy and motivational interviewing to help patients and family members cope with their problematic behavior or negative thought patterns related to their skin conditions. “We do not have magic wands; we focus on the here and now,” she explained. “We focus on how to move forward in the most efficient way possible by teaching skills, practicing those skills with them in the office, and sending them home to use those skills. I don’t have 100% compliance on this, so if I notice that they’re not doing what I asked of them, we’ll have a conversation about what the barrier is. ‘What is getting in the way?’ I’ll ask. ‘Is this something you’re really wanting, or do you want a magic pill? If you want a magic pill, then our office isn’t where that’s going to come from.’ Sometimes patients aren’t ready to work on feeling better, and that is good for us to know.”
During consultations, she often talks with children and adolescents about how thoughts, feelings, and behaviors are related. She’ll use phrasing like, “The way that you think about something changes the way that you feel, and it changes the way that you act. We have control over our thoughts and behaviors, so if we think it’s going to be a bad day, it’s going to be a bad day. If we think it’s going to be a good day, then we’re going to find the positive aspects in the day and we might let those bad aspects go away. If I do something different [for my skin condition], then I’m going to feel different.”
She recalled the case of a 3-year-old boy with atopic dermatitis who was referred for excessive scratching. His mom stays at home, while dad works and travels frequently. “The parents had differing views on how to treat his medical condition. Mom wanted to do wet wraps while dad wanted to do bleach baths. Their son was getting no treatment because the parents couldn’t agree on anything. Mom noticed that her son scratches when he wants attention and when he’s angry.”
When Dr. Jaquez met with his parents, she encouraged them to agree on a plan to implement at home so that their son would gain some relief. She also advised them to ignore when their son scratches or when he gets angry. “Give him something else to do besides scratch, because if those hands are busy, he won’t be scratching. Let’s change the way this behavior happens. Let’s give him attention all the time instead of just when he’s scratching. That will work very quickly. And it did.”
She makes it a point to talk with patients and their families about living with the stress of a chronic illness like psoriasis or atopic dermatitis. “Let’s figure out, ‘How do we accept that this is how it is, and that they’re going to have to find their own ‘normal?’ ” she said. “I don’t know how many times someone comes into my office and says, ‘I just want to be normal.’ I like to ask patients, ‘what is your normal?’ These kids might have a lower quality of life than a child without a chronic illness, but we want to make sure that they’re living their lives to the fullest. You want to monitor not only adherence [to medication] but also quality of life. Sleep concerns are big. A lot of our kids might not being going to school, or they’re afraid to go to school because they get picked on because people don’t understand their skin condition.”
Dr. Jaquez acknowledged that not all dermatologists have a psychologist on staff or in their referral network, but all are capable of destigmatizing psychological and mental health issues for their patients. “Psychological comorbidities such as depression and anxiety can be associated with certain skin conditions,” she said. “Let them know that this is stressful stuff. Have discussions early, so if the time comes for a referral they won’t think you’re giving up on them. Don’t be afraid to say you have a psychologist that you want to refer to. Say, ‘I have an added team member I would love for you to meet. She’s our psychologist. She works with patients who are having difficulties.’ ”
Giving patients perceived control of their care could also help improve the behavior of concern. For example, when patients with needle phobia require an injection, ask if they would like to lay down, or sit down for the injection. “Giving them this tiny bit of control is going to help them feel more empowered,” she said.
Dr. Jaquez also recommends that clinicians pay attention to nonverbal cues and steer clear of using scare tactics to change their behavior. “Use positive behavioral strategies and try to avoid punishment. Children don’t want to hear ‘stop’ all the time. Parents are tired of saying it, and kids are tired of hearing it. We focus on praising the things that are going well. I advise parents all the time: ‘Catch them being good.’ ”
Dr. Jaquez reported having no financial disclosures.
AUSTIN, TEX. – One day each week, Sasha D. Jaquez, PhD, visits with patients in the dermatology clinic at Dell Children’s Medical Center of Central Texas who wrestle with some aspect of their skin condition, from noncompliance to a recommended treatment regimen to fear of needles when an injection of medicine is required to keep them well.
“Our goal is to help promote the health and development of children, adolescents, and families through the use of evidence-based methods like cognitive-behavioral therapy,” said Dr. Jaquez, who is a pediatric psychologist at the University of Texas, Austin. “We do assessment and treatment of behavioral and emotional difficulties related to their skin condition or medical condition. So if they’re depressed but it’s not related to their skin condition, we will likely refer the patient to a community mental health system.”
During 1-hour visits at the dermatology clinic, Dr. Jaquez uses a mixed approach that includes cognitive-behavioral therapy and motivational interviewing to help patients and family members cope with their problematic behavior or negative thought patterns related to their skin conditions. “We do not have magic wands; we focus on the here and now,” she explained. “We focus on how to move forward in the most efficient way possible by teaching skills, practicing those skills with them in the office, and sending them home to use those skills. I don’t have 100% compliance on this, so if I notice that they’re not doing what I asked of them, we’ll have a conversation about what the barrier is. ‘What is getting in the way?’ I’ll ask. ‘Is this something you’re really wanting, or do you want a magic pill? If you want a magic pill, then our office isn’t where that’s going to come from.’ Sometimes patients aren’t ready to work on feeling better, and that is good for us to know.”
During consultations, she often talks with children and adolescents about how thoughts, feelings, and behaviors are related. She’ll use phrasing like, “The way that you think about something changes the way that you feel, and it changes the way that you act. We have control over our thoughts and behaviors, so if we think it’s going to be a bad day, it’s going to be a bad day. If we think it’s going to be a good day, then we’re going to find the positive aspects in the day and we might let those bad aspects go away. If I do something different [for my skin condition], then I’m going to feel different.”
She recalled the case of a 3-year-old boy with atopic dermatitis who was referred for excessive scratching. His mom stays at home, while dad works and travels frequently. “The parents had differing views on how to treat his medical condition. Mom wanted to do wet wraps while dad wanted to do bleach baths. Their son was getting no treatment because the parents couldn’t agree on anything. Mom noticed that her son scratches when he wants attention and when he’s angry.”
When Dr. Jaquez met with his parents, she encouraged them to agree on a plan to implement at home so that their son would gain some relief. She also advised them to ignore when their son scratches or when he gets angry. “Give him something else to do besides scratch, because if those hands are busy, he won’t be scratching. Let’s change the way this behavior happens. Let’s give him attention all the time instead of just when he’s scratching. That will work very quickly. And it did.”
She makes it a point to talk with patients and their families about living with the stress of a chronic illness like psoriasis or atopic dermatitis. “Let’s figure out, ‘How do we accept that this is how it is, and that they’re going to have to find their own ‘normal?’ ” she said. “I don’t know how many times someone comes into my office and says, ‘I just want to be normal.’ I like to ask patients, ‘what is your normal?’ These kids might have a lower quality of life than a child without a chronic illness, but we want to make sure that they’re living their lives to the fullest. You want to monitor not only adherence [to medication] but also quality of life. Sleep concerns are big. A lot of our kids might not being going to school, or they’re afraid to go to school because they get picked on because people don’t understand their skin condition.”
Dr. Jaquez acknowledged that not all dermatologists have a psychologist on staff or in their referral network, but all are capable of destigmatizing psychological and mental health issues for their patients. “Psychological comorbidities such as depression and anxiety can be associated with certain skin conditions,” she said. “Let them know that this is stressful stuff. Have discussions early, so if the time comes for a referral they won’t think you’re giving up on them. Don’t be afraid to say you have a psychologist that you want to refer to. Say, ‘I have an added team member I would love for you to meet. She’s our psychologist. She works with patients who are having difficulties.’ ”
Giving patients perceived control of their care could also help improve the behavior of concern. For example, when patients with needle phobia require an injection, ask if they would like to lay down, or sit down for the injection. “Giving them this tiny bit of control is going to help them feel more empowered,” she said.
Dr. Jaquez also recommends that clinicians pay attention to nonverbal cues and steer clear of using scare tactics to change their behavior. “Use positive behavioral strategies and try to avoid punishment. Children don’t want to hear ‘stop’ all the time. Parents are tired of saying it, and kids are tired of hearing it. We focus on praising the things that are going well. I advise parents all the time: ‘Catch them being good.’ ”
Dr. Jaquez reported having no financial disclosures.
EXPERT ANALYSIS FROM SPD 2019
Biologics for pediatric psoriasis don’t increase infection risk
AUSTIN, TEX. – Among children with psoriasis, there appears to be no strong evidence that biologic immunomodulating drugs increase the 6-month risk of serious infections, compared with systemic nonbiologics or phototherapy, according to results from the largest population-based study of its kind to date.
However, children with psoriasis face a 64% increased risk of infection, compared with risk-matched pediatric patients without the disease.
“We know that pediatric psoriasis affects up to 1.3% of children, and we know that is associated with multiple potential comorbidities and that it has a significant impact on quality of life for children affected,” lead study author Maria Schneeweiss, MD, said at the annual meeting of the Society for Pediatric Dermatology. “Increasingly, we see that biologic and nonbiologic systemic agents are used to treat moderate to severe pediatric psoriasis. While we have a lot of experience in adult psoriasis and a lot of comparative safety studies of these drugs in adult psoriasis, there are very few population-based studies on the safety of these systemic agents for treating pediatric psoriasis.”
In an effort to evaluate the 6-month risk of serious bacterial and opportunistic infections in children with psoriasis treated with systemic immunomodulatory medications, Dr. Schneeweiss and Joseph F. Merola, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and Jennifer Huang, MD, of Boston Children’s Hospital drew from longitudinal, patient-level U.S. claims data in the MarketScan database between 2003 and 2017. They limited the analysis to patients younger than age 18; those who had a recorded diagnosis of psoriasis; and those who were treated with biologics, nonbiologic immunomodulatory agents, or phototherapy. The researchers used hospital discharge diagnoses to compute the risk of serious bacterial and opportunistic infections, and propensity score matching to determine relative risks.
A total of 54,355 children with psoriasis were identified in the database. Before propensity score matching, 635 patients initiated biologic therapy, 919 initiated nonbiologic systemic agents, and 2,537 initiated phototherapy. Their mean age was 12-14 years and slightly more than half were female. In nonbiologic initiators, the 6-month risk of serious infections was 4.75 per 1,000 patients, while in biologic initiators it was 5.44 per 1,000 patients, resulting in a propensity score–matched ratio of 0.60. There was no statistically significant increased risk when the use of nonbiologics was compared with the use of phototherapy.
Independent of treatment, the risk of infection among psoriasis patients was 1.1 per 1,000 patients, which was 60% higher than matched pediatric patients without psoriasis (risk ratio, 1.64).
“When treating pediatric patients with psoriasis, clinicians should remain mindful that the presence of psoriasis itself may increase the risk of infection in children and adolescents, independent of treatment, but that biologic immunomodulatory agents do not further increase that risk,” Dr. Schneeweiss said in an interview. “Our findings suggest that, while there may be an increased risk of certain infections based on the presence of psoriasis alone, all appropriate treatment options should be discussed with patients in shared decision making with their physician. Patients should understand the risks, benefits, and alternatives to any treatment option but not necessarily be restricted as such and have access to newer, targeted and highly effective therapy as appropriate to each individual case.”
She added that, based on the National Psoriasis Foundation guidance of treat-to-target strategies, “our pediatric patients should be offered the same level of disease control as all psoriasis patients.”
She acknowledged certain limitations of the analysis, including the inability to stratify by disease severity and to determine specific doses of medication used.
The study was funded by the Brigham and Women’s Hospital departments of dermatology and medicine. Dr. Schneeweiss and Dr. Huang reported having no financial disclosures. Dr. Merola reported that he has served as a consultant and/or investigator for numerous pharmaceutical companies.
AUSTIN, TEX. – Among children with psoriasis, there appears to be no strong evidence that biologic immunomodulating drugs increase the 6-month risk of serious infections, compared with systemic nonbiologics or phototherapy, according to results from the largest population-based study of its kind to date.
However, children with psoriasis face a 64% increased risk of infection, compared with risk-matched pediatric patients without the disease.
“We know that pediatric psoriasis affects up to 1.3% of children, and we know that is associated with multiple potential comorbidities and that it has a significant impact on quality of life for children affected,” lead study author Maria Schneeweiss, MD, said at the annual meeting of the Society for Pediatric Dermatology. “Increasingly, we see that biologic and nonbiologic systemic agents are used to treat moderate to severe pediatric psoriasis. While we have a lot of experience in adult psoriasis and a lot of comparative safety studies of these drugs in adult psoriasis, there are very few population-based studies on the safety of these systemic agents for treating pediatric psoriasis.”
In an effort to evaluate the 6-month risk of serious bacterial and opportunistic infections in children with psoriasis treated with systemic immunomodulatory medications, Dr. Schneeweiss and Joseph F. Merola, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and Jennifer Huang, MD, of Boston Children’s Hospital drew from longitudinal, patient-level U.S. claims data in the MarketScan database between 2003 and 2017. They limited the analysis to patients younger than age 18; those who had a recorded diagnosis of psoriasis; and those who were treated with biologics, nonbiologic immunomodulatory agents, or phototherapy. The researchers used hospital discharge diagnoses to compute the risk of serious bacterial and opportunistic infections, and propensity score matching to determine relative risks.
A total of 54,355 children with psoriasis were identified in the database. Before propensity score matching, 635 patients initiated biologic therapy, 919 initiated nonbiologic systemic agents, and 2,537 initiated phototherapy. Their mean age was 12-14 years and slightly more than half were female. In nonbiologic initiators, the 6-month risk of serious infections was 4.75 per 1,000 patients, while in biologic initiators it was 5.44 per 1,000 patients, resulting in a propensity score–matched ratio of 0.60. There was no statistically significant increased risk when the use of nonbiologics was compared with the use of phototherapy.
Independent of treatment, the risk of infection among psoriasis patients was 1.1 per 1,000 patients, which was 60% higher than matched pediatric patients without psoriasis (risk ratio, 1.64).
“When treating pediatric patients with psoriasis, clinicians should remain mindful that the presence of psoriasis itself may increase the risk of infection in children and adolescents, independent of treatment, but that biologic immunomodulatory agents do not further increase that risk,” Dr. Schneeweiss said in an interview. “Our findings suggest that, while there may be an increased risk of certain infections based on the presence of psoriasis alone, all appropriate treatment options should be discussed with patients in shared decision making with their physician. Patients should understand the risks, benefits, and alternatives to any treatment option but not necessarily be restricted as such and have access to newer, targeted and highly effective therapy as appropriate to each individual case.”
She added that, based on the National Psoriasis Foundation guidance of treat-to-target strategies, “our pediatric patients should be offered the same level of disease control as all psoriasis patients.”
She acknowledged certain limitations of the analysis, including the inability to stratify by disease severity and to determine specific doses of medication used.
The study was funded by the Brigham and Women’s Hospital departments of dermatology and medicine. Dr. Schneeweiss and Dr. Huang reported having no financial disclosures. Dr. Merola reported that he has served as a consultant and/or investigator for numerous pharmaceutical companies.
AUSTIN, TEX. – Among children with psoriasis, there appears to be no strong evidence that biologic immunomodulating drugs increase the 6-month risk of serious infections, compared with systemic nonbiologics or phototherapy, according to results from the largest population-based study of its kind to date.
However, children with psoriasis face a 64% increased risk of infection, compared with risk-matched pediatric patients without the disease.
“We know that pediatric psoriasis affects up to 1.3% of children, and we know that is associated with multiple potential comorbidities and that it has a significant impact on quality of life for children affected,” lead study author Maria Schneeweiss, MD, said at the annual meeting of the Society for Pediatric Dermatology. “Increasingly, we see that biologic and nonbiologic systemic agents are used to treat moderate to severe pediatric psoriasis. While we have a lot of experience in adult psoriasis and a lot of comparative safety studies of these drugs in adult psoriasis, there are very few population-based studies on the safety of these systemic agents for treating pediatric psoriasis.”
In an effort to evaluate the 6-month risk of serious bacterial and opportunistic infections in children with psoriasis treated with systemic immunomodulatory medications, Dr. Schneeweiss and Joseph F. Merola, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and Jennifer Huang, MD, of Boston Children’s Hospital drew from longitudinal, patient-level U.S. claims data in the MarketScan database between 2003 and 2017. They limited the analysis to patients younger than age 18; those who had a recorded diagnosis of psoriasis; and those who were treated with biologics, nonbiologic immunomodulatory agents, or phototherapy. The researchers used hospital discharge diagnoses to compute the risk of serious bacterial and opportunistic infections, and propensity score matching to determine relative risks.
A total of 54,355 children with psoriasis were identified in the database. Before propensity score matching, 635 patients initiated biologic therapy, 919 initiated nonbiologic systemic agents, and 2,537 initiated phototherapy. Their mean age was 12-14 years and slightly more than half were female. In nonbiologic initiators, the 6-month risk of serious infections was 4.75 per 1,000 patients, while in biologic initiators it was 5.44 per 1,000 patients, resulting in a propensity score–matched ratio of 0.60. There was no statistically significant increased risk when the use of nonbiologics was compared with the use of phototherapy.
Independent of treatment, the risk of infection among psoriasis patients was 1.1 per 1,000 patients, which was 60% higher than matched pediatric patients without psoriasis (risk ratio, 1.64).
“When treating pediatric patients with psoriasis, clinicians should remain mindful that the presence of psoriasis itself may increase the risk of infection in children and adolescents, independent of treatment, but that biologic immunomodulatory agents do not further increase that risk,” Dr. Schneeweiss said in an interview. “Our findings suggest that, while there may be an increased risk of certain infections based on the presence of psoriasis alone, all appropriate treatment options should be discussed with patients in shared decision making with their physician. Patients should understand the risks, benefits, and alternatives to any treatment option but not necessarily be restricted as such and have access to newer, targeted and highly effective therapy as appropriate to each individual case.”
She added that, based on the National Psoriasis Foundation guidance of treat-to-target strategies, “our pediatric patients should be offered the same level of disease control as all psoriasis patients.”
She acknowledged certain limitations of the analysis, including the inability to stratify by disease severity and to determine specific doses of medication used.
The study was funded by the Brigham and Women’s Hospital departments of dermatology and medicine. Dr. Schneeweiss and Dr. Huang reported having no financial disclosures. Dr. Merola reported that he has served as a consultant and/or investigator for numerous pharmaceutical companies.
REPORTING FROM SPD 2019
Pentavalent DTaP-Hb-Hib vaccine is found noninferior to comparator
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
FROM VACCINE
BRCA2 mutations linked to childhood NHL
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
FROM JAMA ONCOLOGY









