User login
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
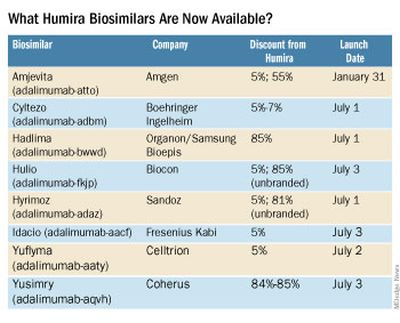
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Phenotypes drive antibiotic response in youth with bronchiectasis
Indigenous children or children with new abnormal auscultatory findings were significantly more likely than children in other categories to respond to oral antibiotics for exacerbations related to bronchiectasis, based on data from more than 200 individuals in New Zealand.
Children and adolescents with bronchiectasis are often treated with antibiotics for respiratory exacerbations, but the effects of antibiotics can vary among individuals, and phenotypic features associated with greater symptom resolution have not been identified, wrote Vikas Goyal, PhD, of the Centre for Children’s Health Research, Brisbane, Australia, and colleagues.
Previous studies have suggested that nearly half of exacerbations in children and adolescents resolve spontaneously after 14 days, and more data are needed to identify which patients are mostly likely to benefit from antibiotics, they noted.
In a study published in the journal Chest, the researchers reviewed secondary data from 217 children and adolescents aged 1-18 years with bronchiectasis enrolled in a pair of randomized, controlled trials comparing oral antibiotics with placebo (known as BEST-1 and BEST-2). The median age of the participants was 6.6 years, 52% were boys, and 41% were Indigenous (defined as Australian First Nations, New Zealand Maori, or Pacific Islander). All participants in the analysis received at least 14 days of oral antibiotics for treatment of nonhospitalized respiratory exacerbations.
Overall, 130 children had resolution of symptoms by day 14, and 87 were nonresponders.
In a multivariate analysis, children who were Indigenous or who had new abnormal auscultatory findings were significantly more likely to respond than children in other categories (odds ratios, 3.59 and 3.85, respectively).
Patients with multiple bronchiectatic lobes at the time of diagnosis and those with higher cough scores at the start of treatment were significantly less likely to respond to antibiotics than patients without these features (OR, 0.66 and 0.55, respectively).
The researchers conducted a further analysis to examine the association between Indigenous ethnicity and treatment response. They found no differences in the other response variables of number of affected lobes at diagnosis and cough scores at the start of treatment between Indigenous and non-Indigenous children.
Given the strong response to antibiotics among Indigenous children, the researchers also conducted a mediation analysis. “Respiratory bacterial pathogens were mediated by Indigenous ethnicity and associated with being an antibiotic ‘responder,’ ” they wrote. For new abnormal chest auscultatory findings, both direct and indirect effects on day 14 response to oral antibiotics were mediated by Indigenous ethnicity. However, neither cough scores at the start of treatment nor the number of affected lobes at diagnosis showed a mediation effect from Indigenous ethnicity.
Among the nonresponders, 59 of 87 resolved symptoms with continuing oral antibiotics over the next 2-4 weeks, and 21 improved without antibiotics.
Additionally, the detection of a respiratory virus at the start of an exacerbation was not associated with antibiotic failure at 14 days, the researchers noted.
The findings were limited by several factors including the use of data from randomized trials that were not designed to address the question in the current study, the researchers noted. Other limitations included incomplete clinical data and lack of data on inflammatory indices, potential antibiotic-resistant pathogens in nonresponders, and the follow-up period of only 14 days, they said.
However, the results suggest a role for patient and exacerbation phenotypes in management of bronchiectasis in clinical practice and promoting antimicrobial stewardship, the researchers wrote. “Although there is benefit in treating exacerbations early to avoid treatment failure and subsequent intravenous antibiotics, future research also needs to identify exacerbations that can be managed without antibiotics,” they concluded.
The BEST-1 and BEST-2 studies were supported by the Australian National Health and Medical Research Council and the NHMRC Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children. Dr. Goyal had no financial conflicts to disclose.
Indigenous children or children with new abnormal auscultatory findings were significantly more likely than children in other categories to respond to oral antibiotics for exacerbations related to bronchiectasis, based on data from more than 200 individuals in New Zealand.
Children and adolescents with bronchiectasis are often treated with antibiotics for respiratory exacerbations, but the effects of antibiotics can vary among individuals, and phenotypic features associated with greater symptom resolution have not been identified, wrote Vikas Goyal, PhD, of the Centre for Children’s Health Research, Brisbane, Australia, and colleagues.
Previous studies have suggested that nearly half of exacerbations in children and adolescents resolve spontaneously after 14 days, and more data are needed to identify which patients are mostly likely to benefit from antibiotics, they noted.
In a study published in the journal Chest, the researchers reviewed secondary data from 217 children and adolescents aged 1-18 years with bronchiectasis enrolled in a pair of randomized, controlled trials comparing oral antibiotics with placebo (known as BEST-1 and BEST-2). The median age of the participants was 6.6 years, 52% were boys, and 41% were Indigenous (defined as Australian First Nations, New Zealand Maori, or Pacific Islander). All participants in the analysis received at least 14 days of oral antibiotics for treatment of nonhospitalized respiratory exacerbations.
Overall, 130 children had resolution of symptoms by day 14, and 87 were nonresponders.
In a multivariate analysis, children who were Indigenous or who had new abnormal auscultatory findings were significantly more likely to respond than children in other categories (odds ratios, 3.59 and 3.85, respectively).
Patients with multiple bronchiectatic lobes at the time of diagnosis and those with higher cough scores at the start of treatment were significantly less likely to respond to antibiotics than patients without these features (OR, 0.66 and 0.55, respectively).
The researchers conducted a further analysis to examine the association between Indigenous ethnicity and treatment response. They found no differences in the other response variables of number of affected lobes at diagnosis and cough scores at the start of treatment between Indigenous and non-Indigenous children.
Given the strong response to antibiotics among Indigenous children, the researchers also conducted a mediation analysis. “Respiratory bacterial pathogens were mediated by Indigenous ethnicity and associated with being an antibiotic ‘responder,’ ” they wrote. For new abnormal chest auscultatory findings, both direct and indirect effects on day 14 response to oral antibiotics were mediated by Indigenous ethnicity. However, neither cough scores at the start of treatment nor the number of affected lobes at diagnosis showed a mediation effect from Indigenous ethnicity.
Among the nonresponders, 59 of 87 resolved symptoms with continuing oral antibiotics over the next 2-4 weeks, and 21 improved without antibiotics.
Additionally, the detection of a respiratory virus at the start of an exacerbation was not associated with antibiotic failure at 14 days, the researchers noted.
The findings were limited by several factors including the use of data from randomized trials that were not designed to address the question in the current study, the researchers noted. Other limitations included incomplete clinical data and lack of data on inflammatory indices, potential antibiotic-resistant pathogens in nonresponders, and the follow-up period of only 14 days, they said.
However, the results suggest a role for patient and exacerbation phenotypes in management of bronchiectasis in clinical practice and promoting antimicrobial stewardship, the researchers wrote. “Although there is benefit in treating exacerbations early to avoid treatment failure and subsequent intravenous antibiotics, future research also needs to identify exacerbations that can be managed without antibiotics,” they concluded.
The BEST-1 and BEST-2 studies were supported by the Australian National Health and Medical Research Council and the NHMRC Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children. Dr. Goyal had no financial conflicts to disclose.
Indigenous children or children with new abnormal auscultatory findings were significantly more likely than children in other categories to respond to oral antibiotics for exacerbations related to bronchiectasis, based on data from more than 200 individuals in New Zealand.
Children and adolescents with bronchiectasis are often treated with antibiotics for respiratory exacerbations, but the effects of antibiotics can vary among individuals, and phenotypic features associated with greater symptom resolution have not been identified, wrote Vikas Goyal, PhD, of the Centre for Children’s Health Research, Brisbane, Australia, and colleagues.
Previous studies have suggested that nearly half of exacerbations in children and adolescents resolve spontaneously after 14 days, and more data are needed to identify which patients are mostly likely to benefit from antibiotics, they noted.
In a study published in the journal Chest, the researchers reviewed secondary data from 217 children and adolescents aged 1-18 years with bronchiectasis enrolled in a pair of randomized, controlled trials comparing oral antibiotics with placebo (known as BEST-1 and BEST-2). The median age of the participants was 6.6 years, 52% were boys, and 41% were Indigenous (defined as Australian First Nations, New Zealand Maori, or Pacific Islander). All participants in the analysis received at least 14 days of oral antibiotics for treatment of nonhospitalized respiratory exacerbations.
Overall, 130 children had resolution of symptoms by day 14, and 87 were nonresponders.
In a multivariate analysis, children who were Indigenous or who had new abnormal auscultatory findings were significantly more likely to respond than children in other categories (odds ratios, 3.59 and 3.85, respectively).
Patients with multiple bronchiectatic lobes at the time of diagnosis and those with higher cough scores at the start of treatment were significantly less likely to respond to antibiotics than patients without these features (OR, 0.66 and 0.55, respectively).
The researchers conducted a further analysis to examine the association between Indigenous ethnicity and treatment response. They found no differences in the other response variables of number of affected lobes at diagnosis and cough scores at the start of treatment between Indigenous and non-Indigenous children.
Given the strong response to antibiotics among Indigenous children, the researchers also conducted a mediation analysis. “Respiratory bacterial pathogens were mediated by Indigenous ethnicity and associated with being an antibiotic ‘responder,’ ” they wrote. For new abnormal chest auscultatory findings, both direct and indirect effects on day 14 response to oral antibiotics were mediated by Indigenous ethnicity. However, neither cough scores at the start of treatment nor the number of affected lobes at diagnosis showed a mediation effect from Indigenous ethnicity.
Among the nonresponders, 59 of 87 resolved symptoms with continuing oral antibiotics over the next 2-4 weeks, and 21 improved without antibiotics.
Additionally, the detection of a respiratory virus at the start of an exacerbation was not associated with antibiotic failure at 14 days, the researchers noted.
The findings were limited by several factors including the use of data from randomized trials that were not designed to address the question in the current study, the researchers noted. Other limitations included incomplete clinical data and lack of data on inflammatory indices, potential antibiotic-resistant pathogens in nonresponders, and the follow-up period of only 14 days, they said.
However, the results suggest a role for patient and exacerbation phenotypes in management of bronchiectasis in clinical practice and promoting antimicrobial stewardship, the researchers wrote. “Although there is benefit in treating exacerbations early to avoid treatment failure and subsequent intravenous antibiotics, future research also needs to identify exacerbations that can be managed without antibiotics,” they concluded.
The BEST-1 and BEST-2 studies were supported by the Australian National Health and Medical Research Council and the NHMRC Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children. Dr. Goyal had no financial conflicts to disclose.
FROM THE JOURNAL CHEST
Goodbye, finger sticks; hello, CGMs
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
In new era of gene therapy, PCPs are ‘boots on the ground’
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
‘Brain fitness program’ may aid memory loss, concussion, ADHD
new research shows.
The program, which consists of targeted cognitive training and EEG-based neurofeedback, coupled with meditation and diet/lifestyle coaching, led to improvements in memory, attention, mood, alertness, and sleep.
The program promotes “neuroplasticity and was equally effective for patients with all three conditions,” program creator Majid Fotuhi, MD, PhD, said in an interview.
Patients with mild to moderate cognitive symptoms often see “remarkable” results within 3 months of consistently following the program, said Dr. Fotuhi, adjunct professor of neuroscience at George Washington University, Washington, and medical director of NeuroGrow Brain Fitness Center, McLean, Va.
“It actually makes intuitive sense that a healthier and stronger brain would function better and that patients of all ages with various cognitive or emotional symptoms would all benefit from improving the biology of their brain,” Dr. Fotuhi added.
The study was published online in the Journal of Alzheimer’s Disease Reports.
Personalized program
The findings are based on 223 children and adults who completed the 12-week NeuroGrow Brain Fitness Program (NeuroGrow BFP), including 71 with ADHD, 88 with PCS, and 64 with memory loss, defined as diagnosed mild cognitive impairment or subjective cognitive decline.
As part of the program, participants undergo a complete neurocognitive evaluation, including tests for verbal memory, complex attention, processing speed, executive functioning, and the Neurocognitive Index.
They also complete questionnaires regarding sleep, mood, diet, exercise, and anxiety/depression, and they undergo quantitative EEG at the beginning and end of the program.
A comparison of before and after neurocognitive test scores showed that all three patient subgroups experienced statistically significant improvements on most measures, the study team reports.
After completing the program, 60%-90% of patients scored higher on cognitive tests and reported having fewer cognitive, sleep, and emotional symptoms.
In all subgroups, the most significant improvement was observed in executive functioning.
“These preliminary findings appear to show that multimodal interventions which are known to increase neuroplasticity in the brain, when personalized, can have benefits for patients with cognitive symptoms from a variety of neurological conditions,” the investigators wrote.
The study’s strengths include a large, community-based sample of patients of different ages who had disruptive symptoms and abnormalities as determined using objective cognitive tests whose progress was monitored by objective and subjective measures.
The chief limitation is the lack of a control or placebo group.
“Though it is difficult to find a comparable group of patients with the exact same profile of cognitive deficits and brain-related symptoms, studying a larger group of patients – and comparing them with a wait-list group – may make it possible to do a more definitive assessment of the NeuroGrow BFP,” the researchers noted.
Dr. Fotuhi said the “secret to the success” of the program is that it involves a full assessment of all cognitive and neurobehavioral symptoms for each patient. This allows for individualized and targeted interventions for specific concerns and symptoms.
He said there is a need to recognize that patients who present to a neurology practice with a single complaint, such as a problem with memory or attention, often have other problems, such as anxiety/depression, stress, insomnia, sedentary lifestyle, obesity, diabetes, sleep apnea, or alcohol overuse.
“Each of these factors can affect their cognitive abilities and need a multimodal set of interventions in order to see full resolution of their cognitive symptoms,” Dr. Fotuhi said.
He has created a series of educational videos to demonstrate the program’s benefits.
The self-pay cost for the NeuroGrow BFP assessment and treatment sessions is approximately $7,000.
Dr. Fotuhi said all of the interventions included in the program are readily available at low cost.
He suggested that health care professionals who lack time or staff for conducting a comprehensive neurocognitive assessment for their patients can provide them with a copy of the Brain Health Index.
“Patients can then be instructed to work on the individual components of their brain health on their own – and measure their brain health index on a weekly basis,” Dr. Fotuhi said. “Private practices or academic centers can use the detailed information I have provided in my paper to develop their own brain fitness program.”
Not ready for prime time
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, noted that “nonpharmacologic interventions can help alleviate some of the symptoms associated with dementia.
“The current study investigates nonpharmacologic interventions in a small number of patients with ADHD, postconcussion syndrome, or memory loss. The researchers found improvements on most measures following the brain rehabilitation program.
“While this is interesting, more work is needed in larger, more diverse cohorts before these programs can be applied broadly. Nonpharmacologic interventions are a helpful tool that need to be studied further in future studies,” Dr. Griffin added.
Funding for the study was provided by the NeuroGrow Brain Fitness Center. Dr. Fotuhi, the owner of NeuroGrow, was involved in data analysis, writing, editing, approval, and decision to publish. Dr. Griffin reported no disclosures.
A version of this article appeared on Medscape.com.
new research shows.
The program, which consists of targeted cognitive training and EEG-based neurofeedback, coupled with meditation and diet/lifestyle coaching, led to improvements in memory, attention, mood, alertness, and sleep.
The program promotes “neuroplasticity and was equally effective for patients with all three conditions,” program creator Majid Fotuhi, MD, PhD, said in an interview.
Patients with mild to moderate cognitive symptoms often see “remarkable” results within 3 months of consistently following the program, said Dr. Fotuhi, adjunct professor of neuroscience at George Washington University, Washington, and medical director of NeuroGrow Brain Fitness Center, McLean, Va.
“It actually makes intuitive sense that a healthier and stronger brain would function better and that patients of all ages with various cognitive or emotional symptoms would all benefit from improving the biology of their brain,” Dr. Fotuhi added.
The study was published online in the Journal of Alzheimer’s Disease Reports.
Personalized program
The findings are based on 223 children and adults who completed the 12-week NeuroGrow Brain Fitness Program (NeuroGrow BFP), including 71 with ADHD, 88 with PCS, and 64 with memory loss, defined as diagnosed mild cognitive impairment or subjective cognitive decline.
As part of the program, participants undergo a complete neurocognitive evaluation, including tests for verbal memory, complex attention, processing speed, executive functioning, and the Neurocognitive Index.
They also complete questionnaires regarding sleep, mood, diet, exercise, and anxiety/depression, and they undergo quantitative EEG at the beginning and end of the program.
A comparison of before and after neurocognitive test scores showed that all three patient subgroups experienced statistically significant improvements on most measures, the study team reports.
After completing the program, 60%-90% of patients scored higher on cognitive tests and reported having fewer cognitive, sleep, and emotional symptoms.
In all subgroups, the most significant improvement was observed in executive functioning.
“These preliminary findings appear to show that multimodal interventions which are known to increase neuroplasticity in the brain, when personalized, can have benefits for patients with cognitive symptoms from a variety of neurological conditions,” the investigators wrote.
The study’s strengths include a large, community-based sample of patients of different ages who had disruptive symptoms and abnormalities as determined using objective cognitive tests whose progress was monitored by objective and subjective measures.
The chief limitation is the lack of a control or placebo group.
“Though it is difficult to find a comparable group of patients with the exact same profile of cognitive deficits and brain-related symptoms, studying a larger group of patients – and comparing them with a wait-list group – may make it possible to do a more definitive assessment of the NeuroGrow BFP,” the researchers noted.
Dr. Fotuhi said the “secret to the success” of the program is that it involves a full assessment of all cognitive and neurobehavioral symptoms for each patient. This allows for individualized and targeted interventions for specific concerns and symptoms.
He said there is a need to recognize that patients who present to a neurology practice with a single complaint, such as a problem with memory or attention, often have other problems, such as anxiety/depression, stress, insomnia, sedentary lifestyle, obesity, diabetes, sleep apnea, or alcohol overuse.
“Each of these factors can affect their cognitive abilities and need a multimodal set of interventions in order to see full resolution of their cognitive symptoms,” Dr. Fotuhi said.
He has created a series of educational videos to demonstrate the program’s benefits.
The self-pay cost for the NeuroGrow BFP assessment and treatment sessions is approximately $7,000.
Dr. Fotuhi said all of the interventions included in the program are readily available at low cost.
He suggested that health care professionals who lack time or staff for conducting a comprehensive neurocognitive assessment for their patients can provide them with a copy of the Brain Health Index.
“Patients can then be instructed to work on the individual components of their brain health on their own – and measure their brain health index on a weekly basis,” Dr. Fotuhi said. “Private practices or academic centers can use the detailed information I have provided in my paper to develop their own brain fitness program.”
Not ready for prime time
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, noted that “nonpharmacologic interventions can help alleviate some of the symptoms associated with dementia.
“The current study investigates nonpharmacologic interventions in a small number of patients with ADHD, postconcussion syndrome, or memory loss. The researchers found improvements on most measures following the brain rehabilitation program.
“While this is interesting, more work is needed in larger, more diverse cohorts before these programs can be applied broadly. Nonpharmacologic interventions are a helpful tool that need to be studied further in future studies,” Dr. Griffin added.
Funding for the study was provided by the NeuroGrow Brain Fitness Center. Dr. Fotuhi, the owner of NeuroGrow, was involved in data analysis, writing, editing, approval, and decision to publish. Dr. Griffin reported no disclosures.
A version of this article appeared on Medscape.com.
new research shows.
The program, which consists of targeted cognitive training and EEG-based neurofeedback, coupled with meditation and diet/lifestyle coaching, led to improvements in memory, attention, mood, alertness, and sleep.
The program promotes “neuroplasticity and was equally effective for patients with all three conditions,” program creator Majid Fotuhi, MD, PhD, said in an interview.
Patients with mild to moderate cognitive symptoms often see “remarkable” results within 3 months of consistently following the program, said Dr. Fotuhi, adjunct professor of neuroscience at George Washington University, Washington, and medical director of NeuroGrow Brain Fitness Center, McLean, Va.
“It actually makes intuitive sense that a healthier and stronger brain would function better and that patients of all ages with various cognitive or emotional symptoms would all benefit from improving the biology of their brain,” Dr. Fotuhi added.
The study was published online in the Journal of Alzheimer’s Disease Reports.
Personalized program
The findings are based on 223 children and adults who completed the 12-week NeuroGrow Brain Fitness Program (NeuroGrow BFP), including 71 with ADHD, 88 with PCS, and 64 with memory loss, defined as diagnosed mild cognitive impairment or subjective cognitive decline.
As part of the program, participants undergo a complete neurocognitive evaluation, including tests for verbal memory, complex attention, processing speed, executive functioning, and the Neurocognitive Index.
They also complete questionnaires regarding sleep, mood, diet, exercise, and anxiety/depression, and they undergo quantitative EEG at the beginning and end of the program.
A comparison of before and after neurocognitive test scores showed that all three patient subgroups experienced statistically significant improvements on most measures, the study team reports.
After completing the program, 60%-90% of patients scored higher on cognitive tests and reported having fewer cognitive, sleep, and emotional symptoms.
In all subgroups, the most significant improvement was observed in executive functioning.
“These preliminary findings appear to show that multimodal interventions which are known to increase neuroplasticity in the brain, when personalized, can have benefits for patients with cognitive symptoms from a variety of neurological conditions,” the investigators wrote.
The study’s strengths include a large, community-based sample of patients of different ages who had disruptive symptoms and abnormalities as determined using objective cognitive tests whose progress was monitored by objective and subjective measures.
The chief limitation is the lack of a control or placebo group.
“Though it is difficult to find a comparable group of patients with the exact same profile of cognitive deficits and brain-related symptoms, studying a larger group of patients – and comparing them with a wait-list group – may make it possible to do a more definitive assessment of the NeuroGrow BFP,” the researchers noted.
Dr. Fotuhi said the “secret to the success” of the program is that it involves a full assessment of all cognitive and neurobehavioral symptoms for each patient. This allows for individualized and targeted interventions for specific concerns and symptoms.
He said there is a need to recognize that patients who present to a neurology practice with a single complaint, such as a problem with memory or attention, often have other problems, such as anxiety/depression, stress, insomnia, sedentary lifestyle, obesity, diabetes, sleep apnea, or alcohol overuse.
“Each of these factors can affect their cognitive abilities and need a multimodal set of interventions in order to see full resolution of their cognitive symptoms,” Dr. Fotuhi said.
He has created a series of educational videos to demonstrate the program’s benefits.
The self-pay cost for the NeuroGrow BFP assessment and treatment sessions is approximately $7,000.
Dr. Fotuhi said all of the interventions included in the program are readily available at low cost.
He suggested that health care professionals who lack time or staff for conducting a comprehensive neurocognitive assessment for their patients can provide them with a copy of the Brain Health Index.
“Patients can then be instructed to work on the individual components of their brain health on their own – and measure their brain health index on a weekly basis,” Dr. Fotuhi said. “Private practices or academic centers can use the detailed information I have provided in my paper to develop their own brain fitness program.”
Not ready for prime time
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, noted that “nonpharmacologic interventions can help alleviate some of the symptoms associated with dementia.
“The current study investigates nonpharmacologic interventions in a small number of patients with ADHD, postconcussion syndrome, or memory loss. The researchers found improvements on most measures following the brain rehabilitation program.
“While this is interesting, more work is needed in larger, more diverse cohorts before these programs can be applied broadly. Nonpharmacologic interventions are a helpful tool that need to be studied further in future studies,” Dr. Griffin added.
Funding for the study was provided by the NeuroGrow Brain Fitness Center. Dr. Fotuhi, the owner of NeuroGrow, was involved in data analysis, writing, editing, approval, and decision to publish. Dr. Griffin reported no disclosures.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE REPORTS
USPSTF maintains ‘insufficient evidence’ for lipid disorder screenings in kids and teens
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
A primer on gender-affirming care for transgender youth
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.
Camp Discovery: A place for children to be comfortable in their own skin
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
Meta-analysis finds increase in type 1 diabetes incidence, ketoacidosis during COVID pandemic
according to a recent meta-analysis.
The review compared 2 years of data from during the pandemic to data from a prepandemic period, and showed a higher incidence of type 1 diabetes in the first year (incidence rate ratio, 1.14) and second year (IRR, 1.27) of the pandemic. The investigators also found an increase in the incidence of diabetic ketoacidosis (DKA) (IRR, 1.26).
The meta-analysis included 17 studies of 38,149 children and adolescents with newly diagnosed type 1 diabetes. “Putting them all together really gave us more confidence to say this is something that we think is real,” study author Rayzel Shulman, MD, PhD, an endocrinologist at The Hospital for Sick Children in Toronto and associate professor of pediatrics at the University of Toronto, said in an interview.
The study was published in JAMA Network Open.
Increased incidence
The investigators reviewed 42 studies, including 17 that examined rates of type 1 diabetes incidence, 10 on type 2 diabetes, and 15 on DKA. The included studies all had a minimum observation period of 12 months during the pandemic and at least 12 months before it. Relative to the prepandemic period, the meta-analysis found higher rates of type 1 diabetes and DKA during the pandemic.
The review was conducted in response to questions about the methodology of study results suggesting an association between the COVID-19 pandemic and the incidence of diabetes, according to Dr. Shulman.
Although this is not the first review of studies on the connection between diabetes and COVID-19, it adds to the literature by extending the study period to 2 years of the pandemic. The longer time frame helps address potential seasonal differences in incidence and increases confidence in the results.
The investigators also sought to look at the incidence of type 2 diabetes in children but found few studies that met the study criteria. Although some studies reported rates of type 2 diabetes, most lacked information about the population, specifically, the “denominator” needed for findings regarding any association with the COVID-19 pandemic.
With greater confidence in the increased incidence of type 1 diabetes, Dr. Shulman emphasized a need to ensure sufficient resources to care for newly diagnosed patients, including education and support for families.
The study’s secondary outcome was the change in incidence rate of DKA among children with newly diagnosed diabetes. Data reported in 15 studies showed a 26% increase in DKA incidence during the first year of the pandemic.
“DKA is a serious and life-threatening condition that is preventable,” said Dr. Shulman. Symptoms of type 1 diabetes include increased thirst and urination, weight loss, and fatigue. If parents or caregivers notice these signs, Dr. Shulman advises them to seek care immediately to reduce the risk of DKA.
Possible mechanisms
In a comment, Elizabeth Sellers, MD, an endocrinologist at the Children’s Hospital Research Institute of Manitoba and associate professor of pediatrics at the University of Manitoba, both in Winnipeg, said the study’s findings on DKA are an important reminder to be attentive to symptoms of diabetes. Dr. Sellers did not participate in the meta-analysis.
One possible explanation for the increase is a hesitancy to seek care among parents and caregivers during the pandemic. “I think we use that information and turn it into a positive,” said Dr. Sellers, by increasing recognition of the symptoms. Dr. Sellers, whose research is included in the review, is part of an initiative by the Canadian Pediatric Endocrine Group to increase diabetes awareness.
The study provides important findings, particularly the second-year results, but is not designed to answer why there has been an increase in diabetes incidence, said Dr. Sellers. “You have to identify the problem first and then you can go back and look at mechanisms.”
The meta-analysis did not seek to draw conclusions about the underlying mechanisms that would explain changes in diabetes incidence but rather indicates a need for further studies to seek a better understanding of the connection. Several theories may be considered, wrote Clemens Kamrath, MD, of the Centre of Child and Adolescent Medicine at Justus Liebig University in Giessen, Germany, and colleagues in an accompanying editorial.
Studies have suggested a direct effect of infections such as COVID-19, whereby the virus damages insulin-producing beta cells. However, the commentary notes these studies do not account for asymptomatic infections among children.
Dr. Kamrath and colleagues also considered the indirect effects of the COVID-19 pandemic, which they indicate may be more likely than direct effects. These indirect effects include autoimmunity and environmental changes that occurred during the pandemic.
Researchers will need to continue monitoring the data to see if the trend persists and continue working to determine the mechanisms, said Dr. Schulman. “I don’t think this is the end of the story.”
The study was supported in part by grant funding from the department of pediatrics at The Hospital for Sick Children. Dr. Shulman, Dr. Sellers, and Dr. Kamrath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a recent meta-analysis.
The review compared 2 years of data from during the pandemic to data from a prepandemic period, and showed a higher incidence of type 1 diabetes in the first year (incidence rate ratio, 1.14) and second year (IRR, 1.27) of the pandemic. The investigators also found an increase in the incidence of diabetic ketoacidosis (DKA) (IRR, 1.26).
The meta-analysis included 17 studies of 38,149 children and adolescents with newly diagnosed type 1 diabetes. “Putting them all together really gave us more confidence to say this is something that we think is real,” study author Rayzel Shulman, MD, PhD, an endocrinologist at The Hospital for Sick Children in Toronto and associate professor of pediatrics at the University of Toronto, said in an interview.
The study was published in JAMA Network Open.
Increased incidence
The investigators reviewed 42 studies, including 17 that examined rates of type 1 diabetes incidence, 10 on type 2 diabetes, and 15 on DKA. The included studies all had a minimum observation period of 12 months during the pandemic and at least 12 months before it. Relative to the prepandemic period, the meta-analysis found higher rates of type 1 diabetes and DKA during the pandemic.
The review was conducted in response to questions about the methodology of study results suggesting an association between the COVID-19 pandemic and the incidence of diabetes, according to Dr. Shulman.
Although this is not the first review of studies on the connection between diabetes and COVID-19, it adds to the literature by extending the study period to 2 years of the pandemic. The longer time frame helps address potential seasonal differences in incidence and increases confidence in the results.
The investigators also sought to look at the incidence of type 2 diabetes in children but found few studies that met the study criteria. Although some studies reported rates of type 2 diabetes, most lacked information about the population, specifically, the “denominator” needed for findings regarding any association with the COVID-19 pandemic.
With greater confidence in the increased incidence of type 1 diabetes, Dr. Shulman emphasized a need to ensure sufficient resources to care for newly diagnosed patients, including education and support for families.
The study’s secondary outcome was the change in incidence rate of DKA among children with newly diagnosed diabetes. Data reported in 15 studies showed a 26% increase in DKA incidence during the first year of the pandemic.
“DKA is a serious and life-threatening condition that is preventable,” said Dr. Shulman. Symptoms of type 1 diabetes include increased thirst and urination, weight loss, and fatigue. If parents or caregivers notice these signs, Dr. Shulman advises them to seek care immediately to reduce the risk of DKA.
Possible mechanisms
In a comment, Elizabeth Sellers, MD, an endocrinologist at the Children’s Hospital Research Institute of Manitoba and associate professor of pediatrics at the University of Manitoba, both in Winnipeg, said the study’s findings on DKA are an important reminder to be attentive to symptoms of diabetes. Dr. Sellers did not participate in the meta-analysis.
One possible explanation for the increase is a hesitancy to seek care among parents and caregivers during the pandemic. “I think we use that information and turn it into a positive,” said Dr. Sellers, by increasing recognition of the symptoms. Dr. Sellers, whose research is included in the review, is part of an initiative by the Canadian Pediatric Endocrine Group to increase diabetes awareness.
The study provides important findings, particularly the second-year results, but is not designed to answer why there has been an increase in diabetes incidence, said Dr. Sellers. “You have to identify the problem first and then you can go back and look at mechanisms.”
The meta-analysis did not seek to draw conclusions about the underlying mechanisms that would explain changes in diabetes incidence but rather indicates a need for further studies to seek a better understanding of the connection. Several theories may be considered, wrote Clemens Kamrath, MD, of the Centre of Child and Adolescent Medicine at Justus Liebig University in Giessen, Germany, and colleagues in an accompanying editorial.
Studies have suggested a direct effect of infections such as COVID-19, whereby the virus damages insulin-producing beta cells. However, the commentary notes these studies do not account for asymptomatic infections among children.
Dr. Kamrath and colleagues also considered the indirect effects of the COVID-19 pandemic, which they indicate may be more likely than direct effects. These indirect effects include autoimmunity and environmental changes that occurred during the pandemic.
Researchers will need to continue monitoring the data to see if the trend persists and continue working to determine the mechanisms, said Dr. Schulman. “I don’t think this is the end of the story.”
The study was supported in part by grant funding from the department of pediatrics at The Hospital for Sick Children. Dr. Shulman, Dr. Sellers, and Dr. Kamrath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a recent meta-analysis.
The review compared 2 years of data from during the pandemic to data from a prepandemic period, and showed a higher incidence of type 1 diabetes in the first year (incidence rate ratio, 1.14) and second year (IRR, 1.27) of the pandemic. The investigators also found an increase in the incidence of diabetic ketoacidosis (DKA) (IRR, 1.26).
The meta-analysis included 17 studies of 38,149 children and adolescents with newly diagnosed type 1 diabetes. “Putting them all together really gave us more confidence to say this is something that we think is real,” study author Rayzel Shulman, MD, PhD, an endocrinologist at The Hospital for Sick Children in Toronto and associate professor of pediatrics at the University of Toronto, said in an interview.
The study was published in JAMA Network Open.
Increased incidence
The investigators reviewed 42 studies, including 17 that examined rates of type 1 diabetes incidence, 10 on type 2 diabetes, and 15 on DKA. The included studies all had a minimum observation period of 12 months during the pandemic and at least 12 months before it. Relative to the prepandemic period, the meta-analysis found higher rates of type 1 diabetes and DKA during the pandemic.
The review was conducted in response to questions about the methodology of study results suggesting an association between the COVID-19 pandemic and the incidence of diabetes, according to Dr. Shulman.
Although this is not the first review of studies on the connection between diabetes and COVID-19, it adds to the literature by extending the study period to 2 years of the pandemic. The longer time frame helps address potential seasonal differences in incidence and increases confidence in the results.
The investigators also sought to look at the incidence of type 2 diabetes in children but found few studies that met the study criteria. Although some studies reported rates of type 2 diabetes, most lacked information about the population, specifically, the “denominator” needed for findings regarding any association with the COVID-19 pandemic.
With greater confidence in the increased incidence of type 1 diabetes, Dr. Shulman emphasized a need to ensure sufficient resources to care for newly diagnosed patients, including education and support for families.
The study’s secondary outcome was the change in incidence rate of DKA among children with newly diagnosed diabetes. Data reported in 15 studies showed a 26% increase in DKA incidence during the first year of the pandemic.
“DKA is a serious and life-threatening condition that is preventable,” said Dr. Shulman. Symptoms of type 1 diabetes include increased thirst and urination, weight loss, and fatigue. If parents or caregivers notice these signs, Dr. Shulman advises them to seek care immediately to reduce the risk of DKA.
Possible mechanisms
In a comment, Elizabeth Sellers, MD, an endocrinologist at the Children’s Hospital Research Institute of Manitoba and associate professor of pediatrics at the University of Manitoba, both in Winnipeg, said the study’s findings on DKA are an important reminder to be attentive to symptoms of diabetes. Dr. Sellers did not participate in the meta-analysis.
One possible explanation for the increase is a hesitancy to seek care among parents and caregivers during the pandemic. “I think we use that information and turn it into a positive,” said Dr. Sellers, by increasing recognition of the symptoms. Dr. Sellers, whose research is included in the review, is part of an initiative by the Canadian Pediatric Endocrine Group to increase diabetes awareness.
The study provides important findings, particularly the second-year results, but is not designed to answer why there has been an increase in diabetes incidence, said Dr. Sellers. “You have to identify the problem first and then you can go back and look at mechanisms.”
The meta-analysis did not seek to draw conclusions about the underlying mechanisms that would explain changes in diabetes incidence but rather indicates a need for further studies to seek a better understanding of the connection. Several theories may be considered, wrote Clemens Kamrath, MD, of the Centre of Child and Adolescent Medicine at Justus Liebig University in Giessen, Germany, and colleagues in an accompanying editorial.
Studies have suggested a direct effect of infections such as COVID-19, whereby the virus damages insulin-producing beta cells. However, the commentary notes these studies do not account for asymptomatic infections among children.
Dr. Kamrath and colleagues also considered the indirect effects of the COVID-19 pandemic, which they indicate may be more likely than direct effects. These indirect effects include autoimmunity and environmental changes that occurred during the pandemic.
Researchers will need to continue monitoring the data to see if the trend persists and continue working to determine the mechanisms, said Dr. Schulman. “I don’t think this is the end of the story.”
The study was supported in part by grant funding from the department of pediatrics at The Hospital for Sick Children. Dr. Shulman, Dr. Sellers, and Dr. Kamrath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
More children missing developmental milestones: Survey
Nearly 9 out of every 100 U.S. children are now diagnosed with a developmental disability, according to updated figures from the CDC.
Developmental disabilities include autism, intellectual disabilities such as Down syndrome, and a range of other diagnoses related to missing developmental milestones in how a child plays, learns, or speaks.
The newly reported increase amounts to just over 1 percentage point from 2019 to 2021. In 2019, the rate of developmental disability diagnoses was about 7 in 100 children. The latest figures are from 2021 data, published this week after the CDC finished analyzing responses to the National Health Survey.
Among children ages 3-17 years old in 2021, the survey showed that:
- 1.7% had an intellectual disability.
- 3.1% had autism spectrum disorder.
- 6.1% had a diagnosis of “other developmental delay.”
No significant change was seen from 2019 to 2021 in how common it was for survey respondents to report children having autism or an intellectual disability. The overall increase was driven by a jump in reports from parents that a doctor or health professional told them their child had “any other developmental delay,” excluding autism spectrum disorder or an intellectual disability.
“A lot of times developmental delays might be temporary diagnoses that evolve into something like autism, potentially, or intellectual disability. But also a lot of times children do age out of those,” lead report author and CDC statistician Benjamin Zablotsky, PhD, told CBS News.
The CDC offers an app called Milestone Tracker to help parents watch for signs of developmental delays, in addition to operating a public health education program called “Learn the Signs. Act Early.”
The new report showed that boys were nearly twice as likely as girls to have any developmental delay, a pattern that was magnified when looking specifically at autism diagnoses. Boys were more than three times as likely as girls to be diagnosed with autism spectrum disorder. The rate of autism among boys was 4.7%, compared with 1.5% among girls.
While these latest survey results showed consistent rates of autism from 2019 to 2021, a different CDC report earlier this year showed an alarming jump in the rate of autism spectrum disorder among 8-year-olds. That report, which compared data from 2008 to 2020, showed the rate of autism among 8-year-olds rose during those 12 years from 1 in 88 kids to 1 in 36 kids.
The two analyses also differed in their findings regarding prevalence of autism when looking at children by race and ethnicity. The report from earlier this year showed that Black and Hispanic children were more likely to be diagnosed with autism, compared with White children. This latest report did not find any differences in the prevalence of autism based on a child’s race or ethnicity.
A version of this article appeared on WebMD.com.
Nearly 9 out of every 100 U.S. children are now diagnosed with a developmental disability, according to updated figures from the CDC.
Developmental disabilities include autism, intellectual disabilities such as Down syndrome, and a range of other diagnoses related to missing developmental milestones in how a child plays, learns, or speaks.
The newly reported increase amounts to just over 1 percentage point from 2019 to 2021. In 2019, the rate of developmental disability diagnoses was about 7 in 100 children. The latest figures are from 2021 data, published this week after the CDC finished analyzing responses to the National Health Survey.
Among children ages 3-17 years old in 2021, the survey showed that:
- 1.7% had an intellectual disability.
- 3.1% had autism spectrum disorder.
- 6.1% had a diagnosis of “other developmental delay.”
No significant change was seen from 2019 to 2021 in how common it was for survey respondents to report children having autism or an intellectual disability. The overall increase was driven by a jump in reports from parents that a doctor or health professional told them their child had “any other developmental delay,” excluding autism spectrum disorder or an intellectual disability.
“A lot of times developmental delays might be temporary diagnoses that evolve into something like autism, potentially, or intellectual disability. But also a lot of times children do age out of those,” lead report author and CDC statistician Benjamin Zablotsky, PhD, told CBS News.
The CDC offers an app called Milestone Tracker to help parents watch for signs of developmental delays, in addition to operating a public health education program called “Learn the Signs. Act Early.”
The new report showed that boys were nearly twice as likely as girls to have any developmental delay, a pattern that was magnified when looking specifically at autism diagnoses. Boys were more than three times as likely as girls to be diagnosed with autism spectrum disorder. The rate of autism among boys was 4.7%, compared with 1.5% among girls.
While these latest survey results showed consistent rates of autism from 2019 to 2021, a different CDC report earlier this year showed an alarming jump in the rate of autism spectrum disorder among 8-year-olds. That report, which compared data from 2008 to 2020, showed the rate of autism among 8-year-olds rose during those 12 years from 1 in 88 kids to 1 in 36 kids.
The two analyses also differed in their findings regarding prevalence of autism when looking at children by race and ethnicity. The report from earlier this year showed that Black and Hispanic children were more likely to be diagnosed with autism, compared with White children. This latest report did not find any differences in the prevalence of autism based on a child’s race or ethnicity.
A version of this article appeared on WebMD.com.
Nearly 9 out of every 100 U.S. children are now diagnosed with a developmental disability, according to updated figures from the CDC.
Developmental disabilities include autism, intellectual disabilities such as Down syndrome, and a range of other diagnoses related to missing developmental milestones in how a child plays, learns, or speaks.
The newly reported increase amounts to just over 1 percentage point from 2019 to 2021. In 2019, the rate of developmental disability diagnoses was about 7 in 100 children. The latest figures are from 2021 data, published this week after the CDC finished analyzing responses to the National Health Survey.
Among children ages 3-17 years old in 2021, the survey showed that:
- 1.7% had an intellectual disability.
- 3.1% had autism spectrum disorder.
- 6.1% had a diagnosis of “other developmental delay.”
No significant change was seen from 2019 to 2021 in how common it was for survey respondents to report children having autism or an intellectual disability. The overall increase was driven by a jump in reports from parents that a doctor or health professional told them their child had “any other developmental delay,” excluding autism spectrum disorder or an intellectual disability.
“A lot of times developmental delays might be temporary diagnoses that evolve into something like autism, potentially, or intellectual disability. But also a lot of times children do age out of those,” lead report author and CDC statistician Benjamin Zablotsky, PhD, told CBS News.
The CDC offers an app called Milestone Tracker to help parents watch for signs of developmental delays, in addition to operating a public health education program called “Learn the Signs. Act Early.”
The new report showed that boys were nearly twice as likely as girls to have any developmental delay, a pattern that was magnified when looking specifically at autism diagnoses. Boys were more than three times as likely as girls to be diagnosed with autism spectrum disorder. The rate of autism among boys was 4.7%, compared with 1.5% among girls.
While these latest survey results showed consistent rates of autism from 2019 to 2021, a different CDC report earlier this year showed an alarming jump in the rate of autism spectrum disorder among 8-year-olds. That report, which compared data from 2008 to 2020, showed the rate of autism among 8-year-olds rose during those 12 years from 1 in 88 kids to 1 in 36 kids.
The two analyses also differed in their findings regarding prevalence of autism when looking at children by race and ethnicity. The report from earlier this year showed that Black and Hispanic children were more likely to be diagnosed with autism, compared with White children. This latest report did not find any differences in the prevalence of autism based on a child’s race or ethnicity.
A version of this article appeared on WebMD.com.