User login
Periorbital Orange Spots
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
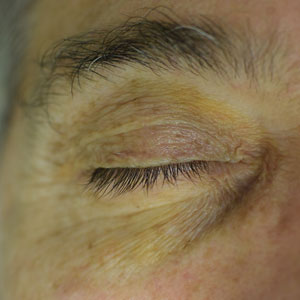
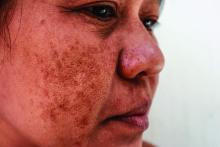
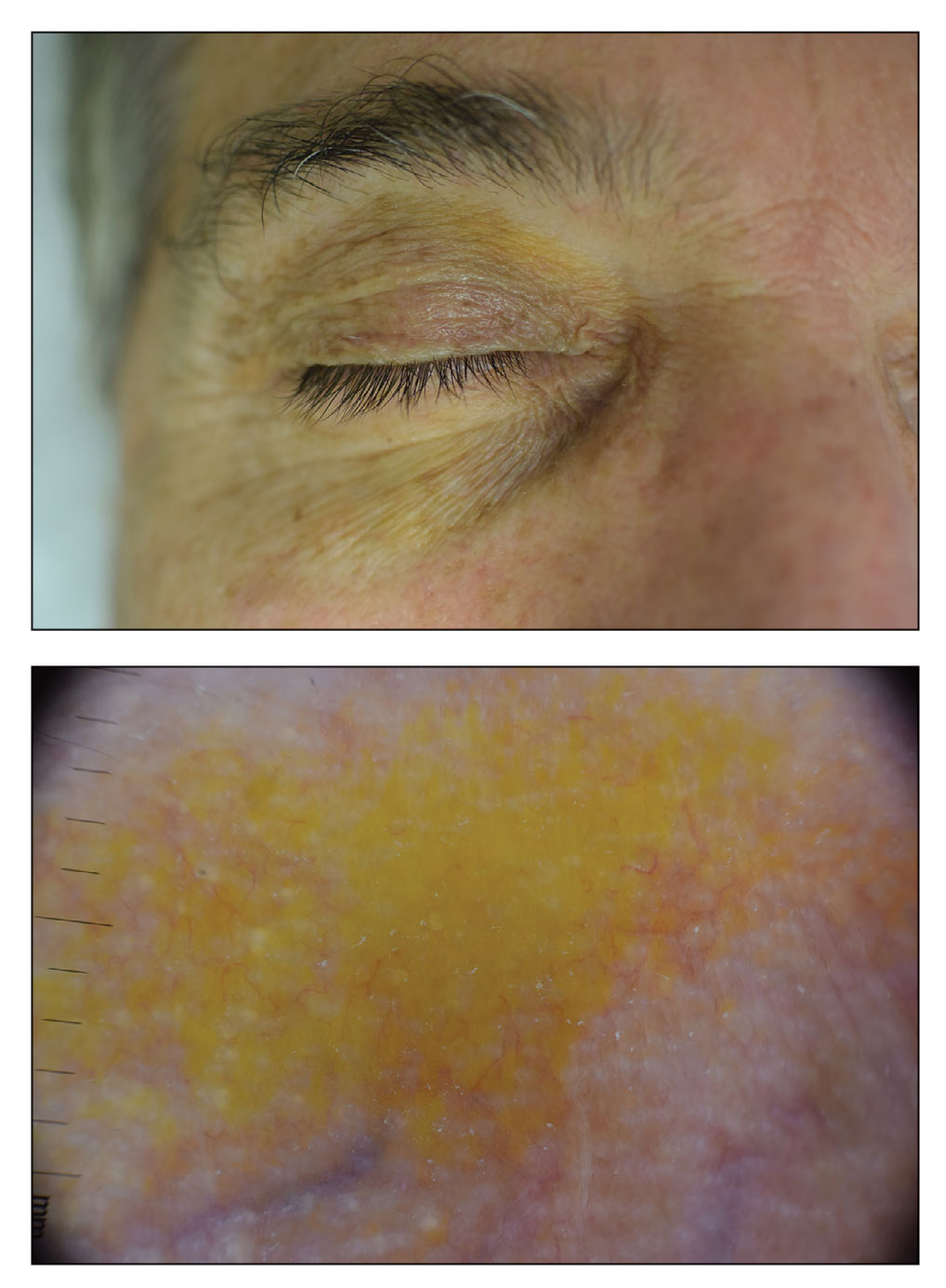
A 63-year-old White man with a history of melanoma presented to our dermatology clinic for evaluation of gradually worsening yellow discoloration around the eyes of 2 years’ duration. Physical examination revealed periorbital yellow-orange patches (top). The discolorations were nonelevated and nonpalpable. Dermoscopy revealed yellow blotches with sparing of the hair follicles (bottom). The remainder of the skin examination was unremarkable.
Vitiligo
THE COMPARISON
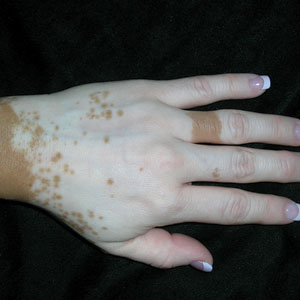
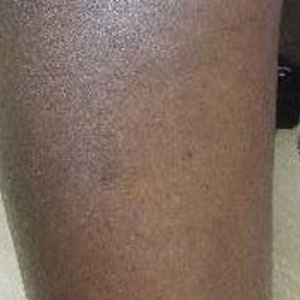
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Camellia japonica
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
Pigmentary disorder experts welcome research explosion
ORLANDO – , a panel of experts said in a session on this topic at the ODAC Dermatology, Aesthetic & Surgical Conference.
The arrival of ruxolitinib cream, a topical JAK inhibitor – and oral JAK inhibitors, including ritlecitinib, a JAK3/TEC (tyrosine kinase expressed in hepatocellular carcinoma) inhibitor in clinical trials – is a welcome development for treatment of vitiligo, said John E. Harris, MD, PhD, chair of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester. Also in the pipeline is a kit for melanocyte-keratinocyte transplantation, which involves transplanting epidermal cells from one part of the body to another. This can be a challenging procedure but a kit would make it easier for a wider range of practitioners. (Topical ruxolitinib was approved by the Food and Drug Administration for treating nonsegmental vitiligo in July, 2022.)
“In the last 10 years, it’s just blown up and people care about vitiligo now,” Dr. Harris said, noting that vitiligo is more than a cosmetic issue, like gray hair or wrinkles. “Vitiligo is an autoimmune disease and now is being treated as such.”
Nada Elbuluk, MD, MSc, associate professor of dermatology at the University of Southern California, Los Angeles, said she’s pleased at the increasing availability of treatment options for hyperpigmentation, aside from hydroquinone, which is associated with an increased risk of adverse effects.
“We have more and more nonhydroquinone agents ... which is really nice because it expands our treatment armamentarium and what we can use to cycle people off of hydroquinone,” she said.
Some of these options include tranexamic acid and products containing azelaic acid or vitamin C.
Iltefat H. Hamzavi, MD, senior staff physician at Henry Ford Health System, Detroit, said that pigmentary disorders are receiving the recognition they deserve.
“I’m excited just about the intersection of society and science, the awareness that pigmentary abnormalities mean something, and they mean something across our society,” he said.
Dr. Elbuluk said that hyperpigmentation has “profound effects on quality of life” for patients.
“They are often more bothered by the darkening of the skin than the primary process that caused it,” she said. “It’s not uncommon that the chief complaint will say ‘dark spots’ and I walk in a room and it’s a patient who has acne. They don’t even say they’re here for acne. They just put ‘dark spots’ [down] because that’s what bothers them. That’s what lasts for so long after the acne is gone.”
The experts offered suggestions for managing these cases. Among her tips, Dr. Elbuluk said that for hyperpigmentation, physicians should not be afraid to biopsy the face – but suggested small, 2-millimeter specimens. In addition, “you can get common conditions in uncommon places,” she noted. “If you see something that looks like melasma off the face, it actually could be, so keep that in your differential.”
Dr. Hamzavi, who spoke about hypopigmentation disorders, said clinicians need to use an algorithm for diagnosis, considering features such as localized or diffuse, scale or no scale, as well as patient history, and other factors. For instance, a hypopigmented area that is localized and has a reddish central papule might lead a clinician to a diagnosis of hypopigmented sarcoidosis.
Using the algorithms, “you actually have to categorize these and then use your own experience. ... All of these elements can help you become a really good taxonomist – ultimately that’s what physicians are.”
He said it’s also important to know when it’s time to reconsider a diagnosis, such as when patients do not respond to traditional treatments. “If they don’t respond, re-categorize,” he said.
Speaking about vitiligo, Dr. Harris said it’s crucial to differentiate active vitiligo from inactive vitiligo and if it’s active, steps need to be taken to keep it from worsening..
Four signs of active vitiligo are a “confetti” pattern of clustered tiny macules of depigmentation, which will coalesce quickly into huge patches; tri-chrome vitiligo that includes a hypopigmented zone; linear areas of depigmentation (Koebner’s phenomenon) that look like scratches on the skin; and inflammatory vitiligo, with an erythematous ring around the edges of a depigmented area.
Dr. Harris disclosed ties with Incyte, Pfizer, Abbvie, Genzyme/Sanofi and other companies. Dr. Elbuluk disclosed ties with Avita, Incyte, Beiersdorf, and other companies. Dr. Hamzavi disclosed ties with AbbVie, Pfizer, Incyte, and other companies.
ORLANDO – , a panel of experts said in a session on this topic at the ODAC Dermatology, Aesthetic & Surgical Conference.
The arrival of ruxolitinib cream, a topical JAK inhibitor – and oral JAK inhibitors, including ritlecitinib, a JAK3/TEC (tyrosine kinase expressed in hepatocellular carcinoma) inhibitor in clinical trials – is a welcome development for treatment of vitiligo, said John E. Harris, MD, PhD, chair of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester. Also in the pipeline is a kit for melanocyte-keratinocyte transplantation, which involves transplanting epidermal cells from one part of the body to another. This can be a challenging procedure but a kit would make it easier for a wider range of practitioners. (Topical ruxolitinib was approved by the Food and Drug Administration for treating nonsegmental vitiligo in July, 2022.)
“In the last 10 years, it’s just blown up and people care about vitiligo now,” Dr. Harris said, noting that vitiligo is more than a cosmetic issue, like gray hair or wrinkles. “Vitiligo is an autoimmune disease and now is being treated as such.”
Nada Elbuluk, MD, MSc, associate professor of dermatology at the University of Southern California, Los Angeles, said she’s pleased at the increasing availability of treatment options for hyperpigmentation, aside from hydroquinone, which is associated with an increased risk of adverse effects.
“We have more and more nonhydroquinone agents ... which is really nice because it expands our treatment armamentarium and what we can use to cycle people off of hydroquinone,” she said.
Some of these options include tranexamic acid and products containing azelaic acid or vitamin C.
Iltefat H. Hamzavi, MD, senior staff physician at Henry Ford Health System, Detroit, said that pigmentary disorders are receiving the recognition they deserve.
“I’m excited just about the intersection of society and science, the awareness that pigmentary abnormalities mean something, and they mean something across our society,” he said.
Dr. Elbuluk said that hyperpigmentation has “profound effects on quality of life” for patients.
“They are often more bothered by the darkening of the skin than the primary process that caused it,” she said. “It’s not uncommon that the chief complaint will say ‘dark spots’ and I walk in a room and it’s a patient who has acne. They don’t even say they’re here for acne. They just put ‘dark spots’ [down] because that’s what bothers them. That’s what lasts for so long after the acne is gone.”
The experts offered suggestions for managing these cases. Among her tips, Dr. Elbuluk said that for hyperpigmentation, physicians should not be afraid to biopsy the face – but suggested small, 2-millimeter specimens. In addition, “you can get common conditions in uncommon places,” she noted. “If you see something that looks like melasma off the face, it actually could be, so keep that in your differential.”
Dr. Hamzavi, who spoke about hypopigmentation disorders, said clinicians need to use an algorithm for diagnosis, considering features such as localized or diffuse, scale or no scale, as well as patient history, and other factors. For instance, a hypopigmented area that is localized and has a reddish central papule might lead a clinician to a diagnosis of hypopigmented sarcoidosis.
Using the algorithms, “you actually have to categorize these and then use your own experience. ... All of these elements can help you become a really good taxonomist – ultimately that’s what physicians are.”
He said it’s also important to know when it’s time to reconsider a diagnosis, such as when patients do not respond to traditional treatments. “If they don’t respond, re-categorize,” he said.
Speaking about vitiligo, Dr. Harris said it’s crucial to differentiate active vitiligo from inactive vitiligo and if it’s active, steps need to be taken to keep it from worsening..
Four signs of active vitiligo are a “confetti” pattern of clustered tiny macules of depigmentation, which will coalesce quickly into huge patches; tri-chrome vitiligo that includes a hypopigmented zone; linear areas of depigmentation (Koebner’s phenomenon) that look like scratches on the skin; and inflammatory vitiligo, with an erythematous ring around the edges of a depigmented area.
Dr. Harris disclosed ties with Incyte, Pfizer, Abbvie, Genzyme/Sanofi and other companies. Dr. Elbuluk disclosed ties with Avita, Incyte, Beiersdorf, and other companies. Dr. Hamzavi disclosed ties with AbbVie, Pfizer, Incyte, and other companies.
ORLANDO – , a panel of experts said in a session on this topic at the ODAC Dermatology, Aesthetic & Surgical Conference.
The arrival of ruxolitinib cream, a topical JAK inhibitor – and oral JAK inhibitors, including ritlecitinib, a JAK3/TEC (tyrosine kinase expressed in hepatocellular carcinoma) inhibitor in clinical trials – is a welcome development for treatment of vitiligo, said John E. Harris, MD, PhD, chair of dermatology and director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester. Also in the pipeline is a kit for melanocyte-keratinocyte transplantation, which involves transplanting epidermal cells from one part of the body to another. This can be a challenging procedure but a kit would make it easier for a wider range of practitioners. (Topical ruxolitinib was approved by the Food and Drug Administration for treating nonsegmental vitiligo in July, 2022.)
“In the last 10 years, it’s just blown up and people care about vitiligo now,” Dr. Harris said, noting that vitiligo is more than a cosmetic issue, like gray hair or wrinkles. “Vitiligo is an autoimmune disease and now is being treated as such.”
Nada Elbuluk, MD, MSc, associate professor of dermatology at the University of Southern California, Los Angeles, said she’s pleased at the increasing availability of treatment options for hyperpigmentation, aside from hydroquinone, which is associated with an increased risk of adverse effects.
“We have more and more nonhydroquinone agents ... which is really nice because it expands our treatment armamentarium and what we can use to cycle people off of hydroquinone,” she said.
Some of these options include tranexamic acid and products containing azelaic acid or vitamin C.
Iltefat H. Hamzavi, MD, senior staff physician at Henry Ford Health System, Detroit, said that pigmentary disorders are receiving the recognition they deserve.
“I’m excited just about the intersection of society and science, the awareness that pigmentary abnormalities mean something, and they mean something across our society,” he said.
Dr. Elbuluk said that hyperpigmentation has “profound effects on quality of life” for patients.
“They are often more bothered by the darkening of the skin than the primary process that caused it,” she said. “It’s not uncommon that the chief complaint will say ‘dark spots’ and I walk in a room and it’s a patient who has acne. They don’t even say they’re here for acne. They just put ‘dark spots’ [down] because that’s what bothers them. That’s what lasts for so long after the acne is gone.”
The experts offered suggestions for managing these cases. Among her tips, Dr. Elbuluk said that for hyperpigmentation, physicians should not be afraid to biopsy the face – but suggested small, 2-millimeter specimens. In addition, “you can get common conditions in uncommon places,” she noted. “If you see something that looks like melasma off the face, it actually could be, so keep that in your differential.”
Dr. Hamzavi, who spoke about hypopigmentation disorders, said clinicians need to use an algorithm for diagnosis, considering features such as localized or diffuse, scale or no scale, as well as patient history, and other factors. For instance, a hypopigmented area that is localized and has a reddish central papule might lead a clinician to a diagnosis of hypopigmented sarcoidosis.
Using the algorithms, “you actually have to categorize these and then use your own experience. ... All of these elements can help you become a really good taxonomist – ultimately that’s what physicians are.”
He said it’s also important to know when it’s time to reconsider a diagnosis, such as when patients do not respond to traditional treatments. “If they don’t respond, re-categorize,” he said.
Speaking about vitiligo, Dr. Harris said it’s crucial to differentiate active vitiligo from inactive vitiligo and if it’s active, steps need to be taken to keep it from worsening..
Four signs of active vitiligo are a “confetti” pattern of clustered tiny macules of depigmentation, which will coalesce quickly into huge patches; tri-chrome vitiligo that includes a hypopigmented zone; linear areas of depigmentation (Koebner’s phenomenon) that look like scratches on the skin; and inflammatory vitiligo, with an erythematous ring around the edges of a depigmented area.
Dr. Harris disclosed ties with Incyte, Pfizer, Abbvie, Genzyme/Sanofi and other companies. Dr. Elbuluk disclosed ties with Avita, Incyte, Beiersdorf, and other companies. Dr. Hamzavi disclosed ties with AbbVie, Pfizer, Incyte, and other companies.
AT ODAC 2023
Microneedling With Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.
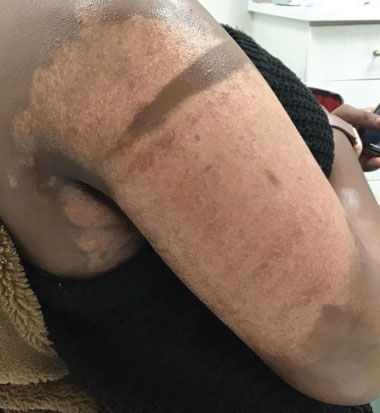
Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).
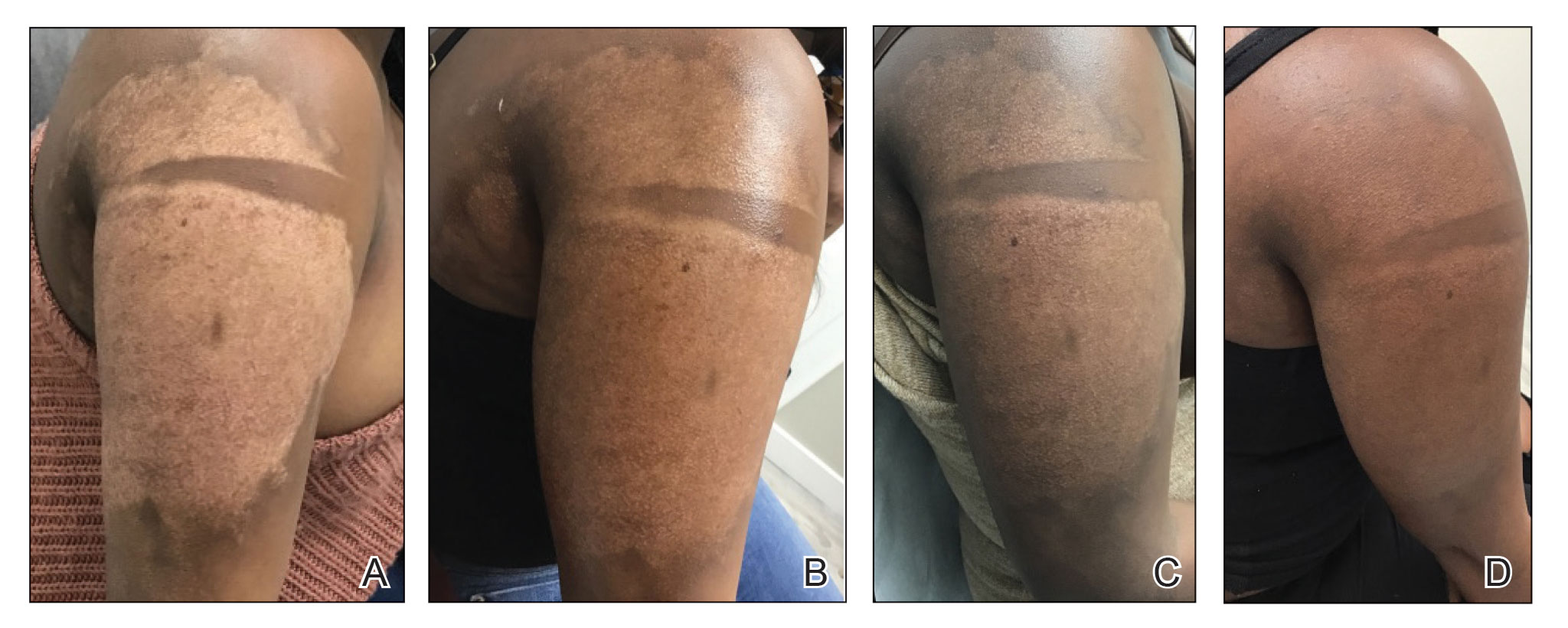
We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.
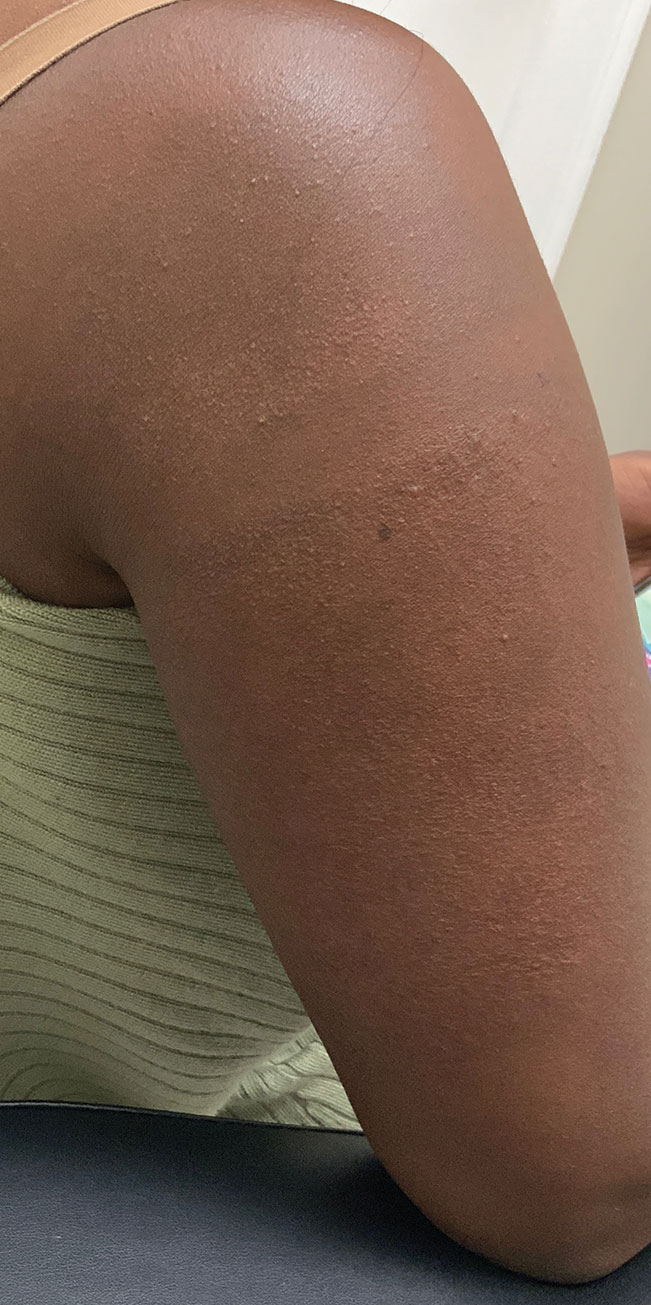
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
PRACTICE POINTS
- Microneedling is a percutaneous collagen induction therapy that also may be used in drug delivery.
- Hypopigmentation can cause considerable distress for patients with skin of color.
- Percutaneous drug delivery of bimatoprost may be helpful in skin repigmentation.
A toddler presents with a dark line on a fingernail
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Examination findings reveal a 2-mm brown longitudinal band on the radial aspect of the right thumbnail that does not extend into the proximal or lateral nailfolds. The rest of the skin and nail exam is unremarkable.
Long-term maintenance required in melasma patients
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
AT MOAS 2022
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
New treatments aim to tame vitiligo
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
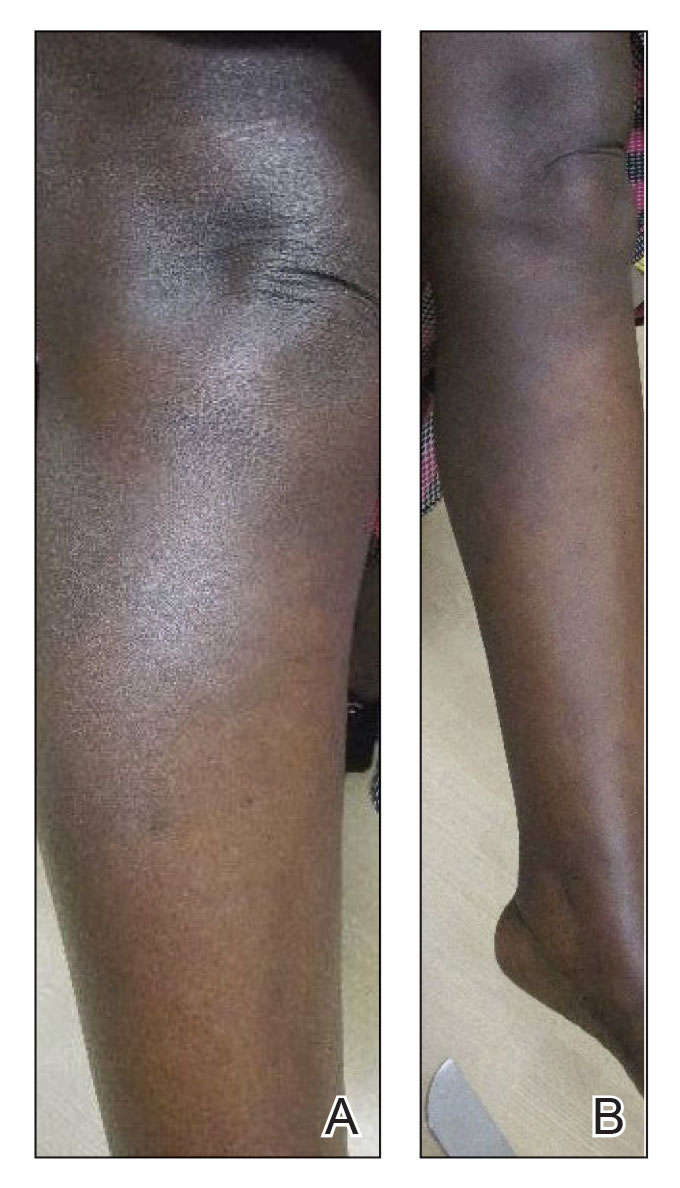
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.