User login
Researchers use AI to diagnose infantile hemangioma
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY
Saururus chinensis
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Novel platform harnesses 3D laser technology for skin treatments
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
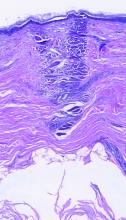
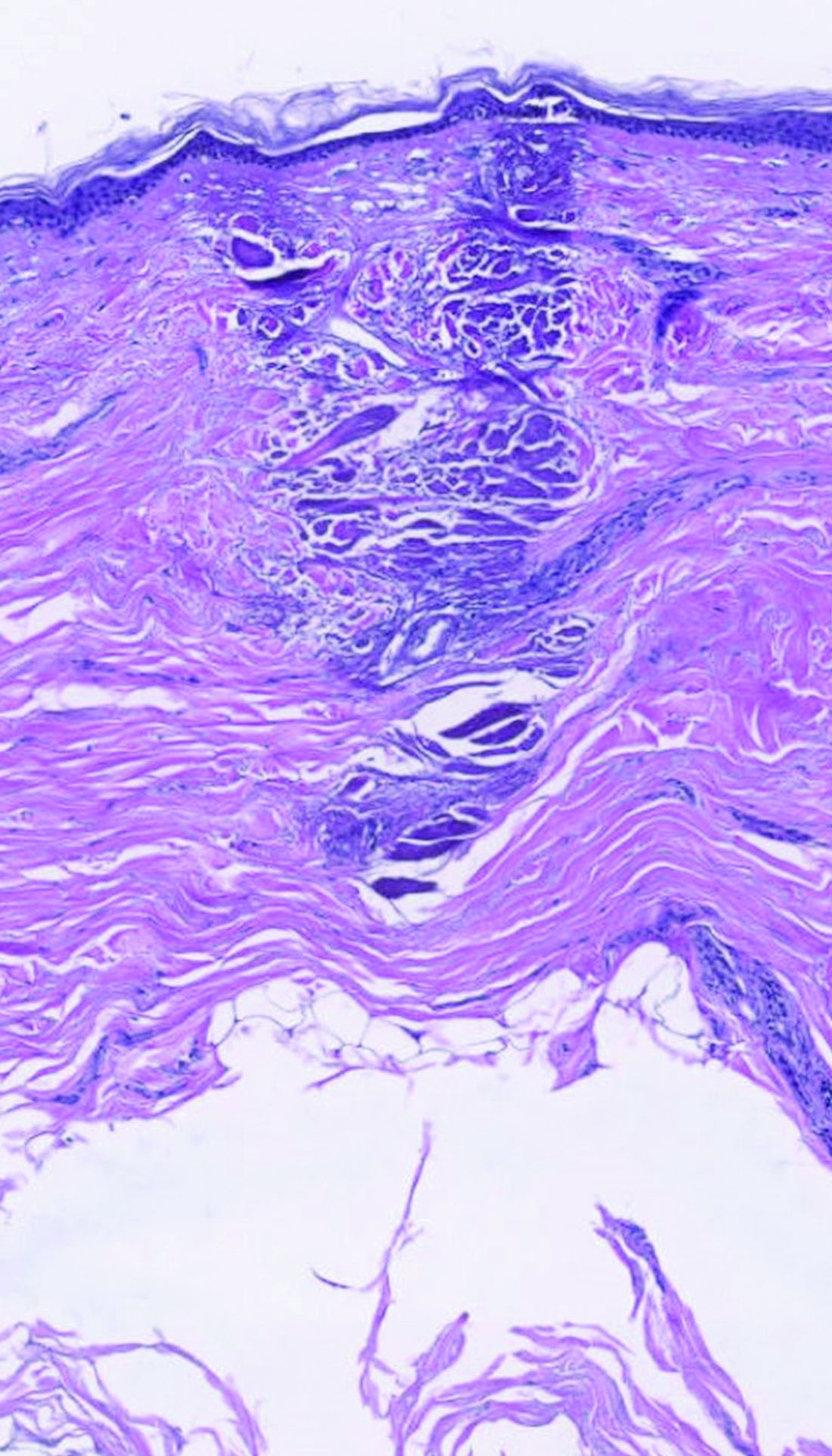
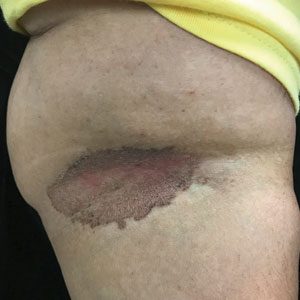
Erythrasma
THE COMPARISON
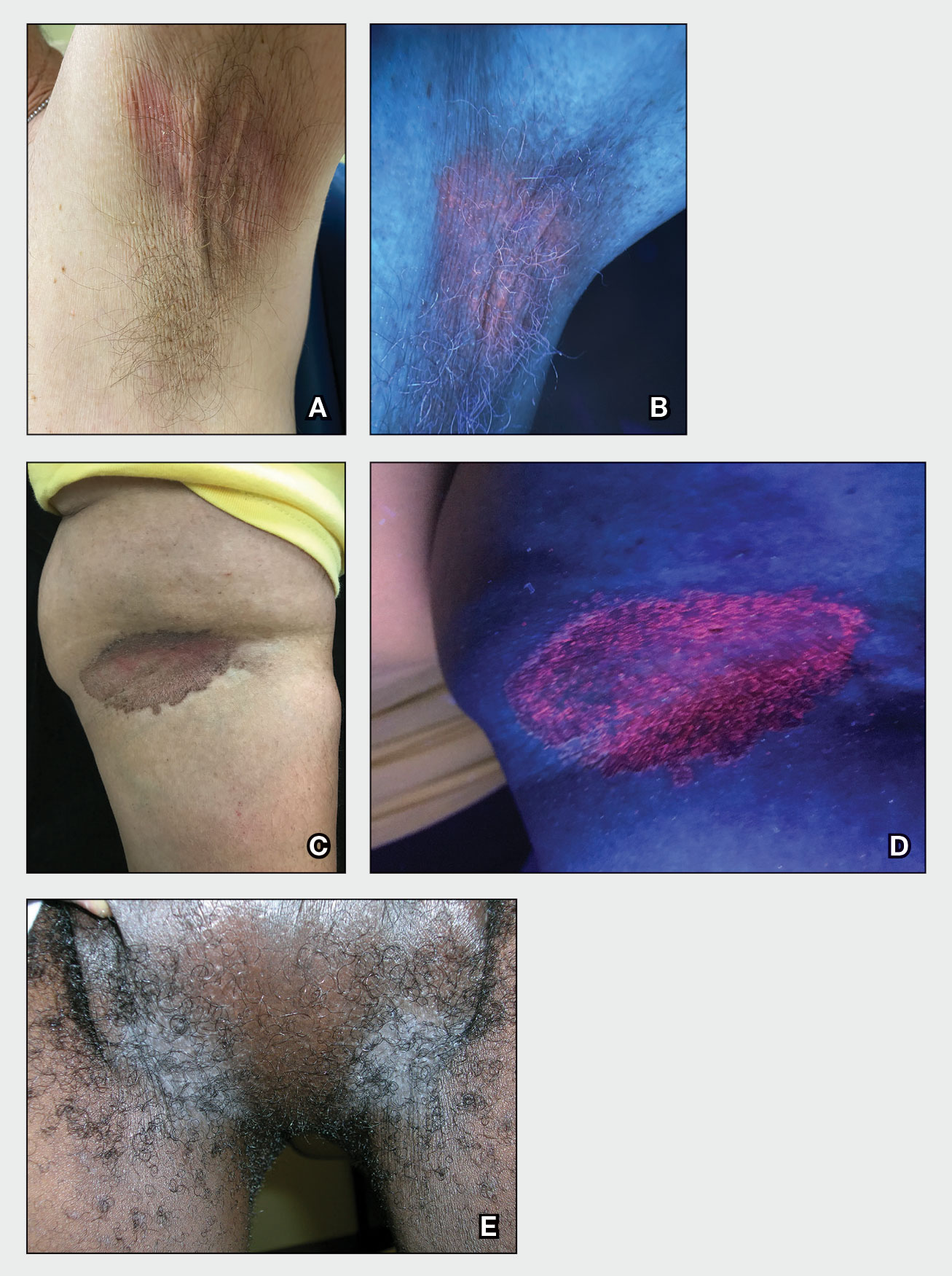
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
ICD-10 code can identify patients with melasma for future study
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
FROM JAMA DERMATOLOGY
Lego introduces first character with vitiligo
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Genital Lentiginosis: A Benign Pigmentary Abnormality Often Raising Concern for Melanoma
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.
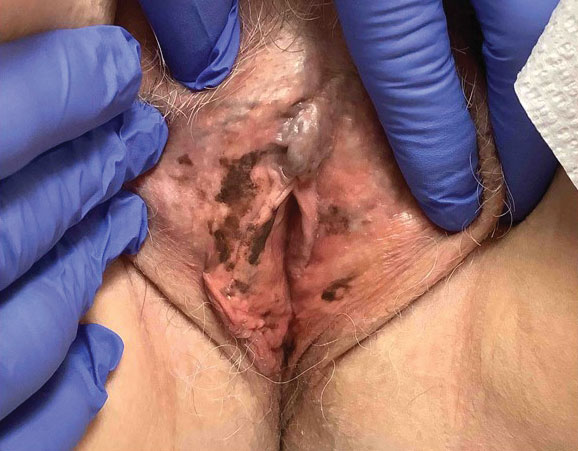
Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.
A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.
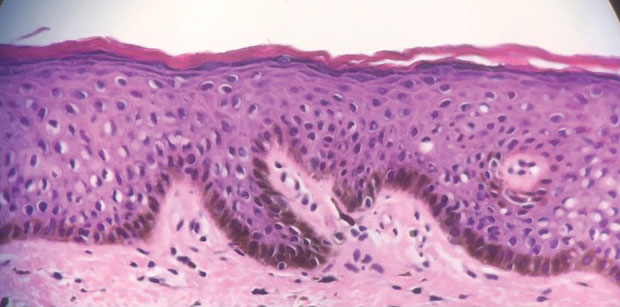
Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
Practice Points
- The irregular appearance of genital lentiginosis—multifocal, asymmetric, irregular, and darkly pigmented patches—often raises concern for melanoma but is benign.
- Certain genetic conditions can present with genital lentiginosis.
- Dermoscopic assessment of the lesion color is highly helpful in narrowing the differential diagnosis; seeing no gray, red, blue, or white makes melanoma less likely.
- Be aware of genital lentigines and their characteristic presentation in adulthood to avoid unwarranted concern and unneeded surgery.
Itchy Red-Brown Spots on a Child
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.
Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
A 5-year-old boy presented with red-brown spots diffusely spread over the body that were present since birth. There were no subjective symptoms, except for rare instances of flushing, itching, and urtication following hot baths and abrasive scrubs. Dermatologic examination revealed widespread brown polymorphic macules and papules of varying sizes on the forehead, neck, torso, and extremities. Physical examination was otherwise normal.
JAK inhibitors show no excess cardiovascular safety signal in French nationwide cohort
Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib (Olumiant) may pose no greater risk than does adalimumab (Humira and biosimilars) for major adverse cardiovascular events (MACEs) or venous thromboembolism (VTE) on the basis of a nationwide cohort study.
The French data, which included almost 16,000 patients with rheumatoid arthritis, revealed similar safety across subgroups, including older patients with at least one preexisting cardiovascular risk factor, reported lead author Léa Hoisnard, MD, of Henri Mondor Hospital, Paris, and colleagues.
These findings arrive 1 year after the U.S. Food and Drug Administration imposed class-wide boxed warnings on three Janus kinase (JAK) inhibitors, citing increased risks for both cancer and serious cardiac events detected by the open-label, randomized ORAL Surveillance postmarketing trial, which compared tofacitinib against adalimumab and etanercept.
More recently, the observational STAR-RA study, relying upon private insurance and Medicare claims in the United States, found no significant increase in cardiovascular events among patients taking tofacitinib, adding some uncertainty to the conversation.
“In this context, observational studies of unselected populations outside of North America are still needed to assess other JAK inhibitor agents,” Dr. Hoisnard and colleagues write in Annals of the Rheumatic Diseases.
Their retrospective study included 8,481 patients who received baricitinib or tofacitinib, and 7,354 patients who received adalimumab. Almost all patients in the tofacitinib group received 5 mg twice daily instead of 10 mg twice daily (99.4% vs. 0.6%), so cardiovascular safety was assessed only for the 5-mg dose. Baricitinib was prescribed at 4-mg and 2-mg doses (79.5% vs. 20.5%), allowing inclusion of both dose levels. The investigators accounted for a range of covariates, including concurrent therapy, comorbidities, and other patient characteristics.
Median follow-up durations were 440 days in the JAK inhibitor group and 344 days in the adalimumab group. The JAK inhibitor group had numerically more MACEs than did the adalimumab group, but the difference in risk was not statistically significant (54 vs. 35 MACEs; weighted hazard ratio, 1.0; 95% confidence interval, 0.7-1.5; P = .99). Similarly, more patients taking JAK inhibitors had VTEs, but relative risk was, again, not significant (75 vs. 32 VTEs; HRw, 1.1; 95% CI, 0.7-1.6; P = .63).
These findings were consistent for all subgroups, including patients aged 50 years or older and patients aged 65 years or older, although the investigators noted that statistical power was lacking for subgroup analyses.
Findings from Echo ORAL Surveillance
“I think the baricitinib data are important,” Kevin Winthrop, MD, MPH, professor of infectious diseases and epidemiology at Oregon Health & Science University, Portland, told this news organization. “There’s no difference between 2 mg and 4 mg [dose levels] in this analysis. And there doesn’t really seem to be a difference between baricitinib and tofacitinib. Most of the results are pretty consistent with ORAL Surveillance, which was a randomized, controlled trial.”
Dr. Winthrop, who has been active in JAK inhibitor clinical trials, recently coauthored an article in Nature Reviews Rheumatology encouraging clinicians to remember that the cardiovascular risks of JAK inhibitors are relative to adalimumab, and safety should be framed within the context of risk-to-benefit ratios.
He and his coauthor also called into question the FDA’s “better to be safe than sorry” approach, which resulted in boxed warnings across all JAK inhibitors, despite differences in target specificity.
“There are pros and cons of taking that approach,” Dr. Winthrop said in an interview. “The FDA might ultimately be right. Certainly, these drugs appear similar for some types of events, like herpes zoster, for example. But whether they’re similar with regard to malignancy or cardiovascular events, I don’t think we know.”
Dr. Winthrop noted that deucravacitinib was recently approved for psoriasis sans boxed warning, suggesting inconsistency in the FDA’s approach. The agent headlines as a “TYK2 inhibitor,” but TYK2 is a member of the JAK family.
“I don’t know why the FDA decided to treat them differently,” Dr. Winthrop said.
Boxed warnings encourage caution, lock treatment sequence
Michael Thakor, MD, of Arthritis & Rheumatology Clinic of Northern Colorado, Fort Collins, supports the boxed warnings because they encourage caution and transparency.
“It forces you to have that discussion with your patient, which may take some time, but it’s actually a very good thing,” Dr. Thakor said in an interview. “Some patients will say, ‘Oh my gosh, I don’t want to take that drug.’ But most patients, considering the level of risk that you’re talking about, are actually okay going ahead with the medication.”
If these risks aren’t discussed, he noted, patient trust may falter.
“They’re going to go online, and they’re going to be reading about it,” Dr. Thakor said. “And then they tend to get more spooked. They also may question your advice from then on, if you’re not telling them the possible risk.”
Reflecting on the present study, Dr. Thakor said that the findings initially appeared reassuring, but he became concerned about the lack of power and how adverse events trended higher in the JAK inhibitor group, particularly for VTEs, most of which occurred with baricitinib. This latter finding is challenging to interpret, however, because the 4-mg dose is not used in the United States, he added.
Dr. Thakor described how JAK inhibitors once seemed poised to assume a frontline role in RA until the boxed warnings came out. These safety concerns don’t take JAK inhibitors off the table, he said, but they do keep the class further down the treatment sequence, and the present data don’t alter this picture in daily practice.
“If I had a patient who was over the age of 50 with at least one cardiovascular risk factor, I might have a little bit of concern, but if they need their RA treated, I would definitely discuss the possibility of using a JAK inhibitor,” Dr. Thakor said. “If the patient is comfortable with it, then I would feel comfortable going ahead.”
The investigators disclosed no outside funding or conflicts of interest. Dr. Winthrop disclosed relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, and others. Dr. Thakor disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib (Olumiant) may pose no greater risk than does adalimumab (Humira and biosimilars) for major adverse cardiovascular events (MACEs) or venous thromboembolism (VTE) on the basis of a nationwide cohort study.
The French data, which included almost 16,000 patients with rheumatoid arthritis, revealed similar safety across subgroups, including older patients with at least one preexisting cardiovascular risk factor, reported lead author Léa Hoisnard, MD, of Henri Mondor Hospital, Paris, and colleagues.
These findings arrive 1 year after the U.S. Food and Drug Administration imposed class-wide boxed warnings on three Janus kinase (JAK) inhibitors, citing increased risks for both cancer and serious cardiac events detected by the open-label, randomized ORAL Surveillance postmarketing trial, which compared tofacitinib against adalimumab and etanercept.
More recently, the observational STAR-RA study, relying upon private insurance and Medicare claims in the United States, found no significant increase in cardiovascular events among patients taking tofacitinib, adding some uncertainty to the conversation.
“In this context, observational studies of unselected populations outside of North America are still needed to assess other JAK inhibitor agents,” Dr. Hoisnard and colleagues write in Annals of the Rheumatic Diseases.
Their retrospective study included 8,481 patients who received baricitinib or tofacitinib, and 7,354 patients who received adalimumab. Almost all patients in the tofacitinib group received 5 mg twice daily instead of 10 mg twice daily (99.4% vs. 0.6%), so cardiovascular safety was assessed only for the 5-mg dose. Baricitinib was prescribed at 4-mg and 2-mg doses (79.5% vs. 20.5%), allowing inclusion of both dose levels. The investigators accounted for a range of covariates, including concurrent therapy, comorbidities, and other patient characteristics.
Median follow-up durations were 440 days in the JAK inhibitor group and 344 days in the adalimumab group. The JAK inhibitor group had numerically more MACEs than did the adalimumab group, but the difference in risk was not statistically significant (54 vs. 35 MACEs; weighted hazard ratio, 1.0; 95% confidence interval, 0.7-1.5; P = .99). Similarly, more patients taking JAK inhibitors had VTEs, but relative risk was, again, not significant (75 vs. 32 VTEs; HRw, 1.1; 95% CI, 0.7-1.6; P = .63).
These findings were consistent for all subgroups, including patients aged 50 years or older and patients aged 65 years or older, although the investigators noted that statistical power was lacking for subgroup analyses.
Findings from Echo ORAL Surveillance
“I think the baricitinib data are important,” Kevin Winthrop, MD, MPH, professor of infectious diseases and epidemiology at Oregon Health & Science University, Portland, told this news organization. “There’s no difference between 2 mg and 4 mg [dose levels] in this analysis. And there doesn’t really seem to be a difference between baricitinib and tofacitinib. Most of the results are pretty consistent with ORAL Surveillance, which was a randomized, controlled trial.”
Dr. Winthrop, who has been active in JAK inhibitor clinical trials, recently coauthored an article in Nature Reviews Rheumatology encouraging clinicians to remember that the cardiovascular risks of JAK inhibitors are relative to adalimumab, and safety should be framed within the context of risk-to-benefit ratios.
He and his coauthor also called into question the FDA’s “better to be safe than sorry” approach, which resulted in boxed warnings across all JAK inhibitors, despite differences in target specificity.
“There are pros and cons of taking that approach,” Dr. Winthrop said in an interview. “The FDA might ultimately be right. Certainly, these drugs appear similar for some types of events, like herpes zoster, for example. But whether they’re similar with regard to malignancy or cardiovascular events, I don’t think we know.”
Dr. Winthrop noted that deucravacitinib was recently approved for psoriasis sans boxed warning, suggesting inconsistency in the FDA’s approach. The agent headlines as a “TYK2 inhibitor,” but TYK2 is a member of the JAK family.
“I don’t know why the FDA decided to treat them differently,” Dr. Winthrop said.
Boxed warnings encourage caution, lock treatment sequence
Michael Thakor, MD, of Arthritis & Rheumatology Clinic of Northern Colorado, Fort Collins, supports the boxed warnings because they encourage caution and transparency.
“It forces you to have that discussion with your patient, which may take some time, but it’s actually a very good thing,” Dr. Thakor said in an interview. “Some patients will say, ‘Oh my gosh, I don’t want to take that drug.’ But most patients, considering the level of risk that you’re talking about, are actually okay going ahead with the medication.”
If these risks aren’t discussed, he noted, patient trust may falter.
“They’re going to go online, and they’re going to be reading about it,” Dr. Thakor said. “And then they tend to get more spooked. They also may question your advice from then on, if you’re not telling them the possible risk.”
Reflecting on the present study, Dr. Thakor said that the findings initially appeared reassuring, but he became concerned about the lack of power and how adverse events trended higher in the JAK inhibitor group, particularly for VTEs, most of which occurred with baricitinib. This latter finding is challenging to interpret, however, because the 4-mg dose is not used in the United States, he added.
Dr. Thakor described how JAK inhibitors once seemed poised to assume a frontline role in RA until the boxed warnings came out. These safety concerns don’t take JAK inhibitors off the table, he said, but they do keep the class further down the treatment sequence, and the present data don’t alter this picture in daily practice.
“If I had a patient who was over the age of 50 with at least one cardiovascular risk factor, I might have a little bit of concern, but if they need their RA treated, I would definitely discuss the possibility of using a JAK inhibitor,” Dr. Thakor said. “If the patient is comfortable with it, then I would feel comfortable going ahead.”
The investigators disclosed no outside funding or conflicts of interest. Dr. Winthrop disclosed relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, and others. Dr. Thakor disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib (Olumiant) may pose no greater risk than does adalimumab (Humira and biosimilars) for major adverse cardiovascular events (MACEs) or venous thromboembolism (VTE) on the basis of a nationwide cohort study.
The French data, which included almost 16,000 patients with rheumatoid arthritis, revealed similar safety across subgroups, including older patients with at least one preexisting cardiovascular risk factor, reported lead author Léa Hoisnard, MD, of Henri Mondor Hospital, Paris, and colleagues.
These findings arrive 1 year after the U.S. Food and Drug Administration imposed class-wide boxed warnings on three Janus kinase (JAK) inhibitors, citing increased risks for both cancer and serious cardiac events detected by the open-label, randomized ORAL Surveillance postmarketing trial, which compared tofacitinib against adalimumab and etanercept.
More recently, the observational STAR-RA study, relying upon private insurance and Medicare claims in the United States, found no significant increase in cardiovascular events among patients taking tofacitinib, adding some uncertainty to the conversation.
“In this context, observational studies of unselected populations outside of North America are still needed to assess other JAK inhibitor agents,” Dr. Hoisnard and colleagues write in Annals of the Rheumatic Diseases.
Their retrospective study included 8,481 patients who received baricitinib or tofacitinib, and 7,354 patients who received adalimumab. Almost all patients in the tofacitinib group received 5 mg twice daily instead of 10 mg twice daily (99.4% vs. 0.6%), so cardiovascular safety was assessed only for the 5-mg dose. Baricitinib was prescribed at 4-mg and 2-mg doses (79.5% vs. 20.5%), allowing inclusion of both dose levels. The investigators accounted for a range of covariates, including concurrent therapy, comorbidities, and other patient characteristics.
Median follow-up durations were 440 days in the JAK inhibitor group and 344 days in the adalimumab group. The JAK inhibitor group had numerically more MACEs than did the adalimumab group, but the difference in risk was not statistically significant (54 vs. 35 MACEs; weighted hazard ratio, 1.0; 95% confidence interval, 0.7-1.5; P = .99). Similarly, more patients taking JAK inhibitors had VTEs, but relative risk was, again, not significant (75 vs. 32 VTEs; HRw, 1.1; 95% CI, 0.7-1.6; P = .63).
These findings were consistent for all subgroups, including patients aged 50 years or older and patients aged 65 years or older, although the investigators noted that statistical power was lacking for subgroup analyses.
Findings from Echo ORAL Surveillance
“I think the baricitinib data are important,” Kevin Winthrop, MD, MPH, professor of infectious diseases and epidemiology at Oregon Health & Science University, Portland, told this news organization. “There’s no difference between 2 mg and 4 mg [dose levels] in this analysis. And there doesn’t really seem to be a difference between baricitinib and tofacitinib. Most of the results are pretty consistent with ORAL Surveillance, which was a randomized, controlled trial.”
Dr. Winthrop, who has been active in JAK inhibitor clinical trials, recently coauthored an article in Nature Reviews Rheumatology encouraging clinicians to remember that the cardiovascular risks of JAK inhibitors are relative to adalimumab, and safety should be framed within the context of risk-to-benefit ratios.
He and his coauthor also called into question the FDA’s “better to be safe than sorry” approach, which resulted in boxed warnings across all JAK inhibitors, despite differences in target specificity.
“There are pros and cons of taking that approach,” Dr. Winthrop said in an interview. “The FDA might ultimately be right. Certainly, these drugs appear similar for some types of events, like herpes zoster, for example. But whether they’re similar with regard to malignancy or cardiovascular events, I don’t think we know.”
Dr. Winthrop noted that deucravacitinib was recently approved for psoriasis sans boxed warning, suggesting inconsistency in the FDA’s approach. The agent headlines as a “TYK2 inhibitor,” but TYK2 is a member of the JAK family.
“I don’t know why the FDA decided to treat them differently,” Dr. Winthrop said.
Boxed warnings encourage caution, lock treatment sequence
Michael Thakor, MD, of Arthritis & Rheumatology Clinic of Northern Colorado, Fort Collins, supports the boxed warnings because they encourage caution and transparency.
“It forces you to have that discussion with your patient, which may take some time, but it’s actually a very good thing,” Dr. Thakor said in an interview. “Some patients will say, ‘Oh my gosh, I don’t want to take that drug.’ But most patients, considering the level of risk that you’re talking about, are actually okay going ahead with the medication.”
If these risks aren’t discussed, he noted, patient trust may falter.
“They’re going to go online, and they’re going to be reading about it,” Dr. Thakor said. “And then they tend to get more spooked. They also may question your advice from then on, if you’re not telling them the possible risk.”
Reflecting on the present study, Dr. Thakor said that the findings initially appeared reassuring, but he became concerned about the lack of power and how adverse events trended higher in the JAK inhibitor group, particularly for VTEs, most of which occurred with baricitinib. This latter finding is challenging to interpret, however, because the 4-mg dose is not used in the United States, he added.
Dr. Thakor described how JAK inhibitors once seemed poised to assume a frontline role in RA until the boxed warnings came out. These safety concerns don’t take JAK inhibitors off the table, he said, but they do keep the class further down the treatment sequence, and the present data don’t alter this picture in daily practice.
“If I had a patient who was over the age of 50 with at least one cardiovascular risk factor, I might have a little bit of concern, but if they need their RA treated, I would definitely discuss the possibility of using a JAK inhibitor,” Dr. Thakor said. “If the patient is comfortable with it, then I would feel comfortable going ahead.”
The investigators disclosed no outside funding or conflicts of interest. Dr. Winthrop disclosed relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, and others. Dr. Thakor disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES