User login
Achieving Promotion for Junior Faculty in Academic Medicine: An Interview With Experts
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Working Hard or Work Addiction — Have You Crossed the Line?
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
How These Young MDs Impressed the Hell Out of Their Bosses
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
Are Women Better Doctors Than Men?
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.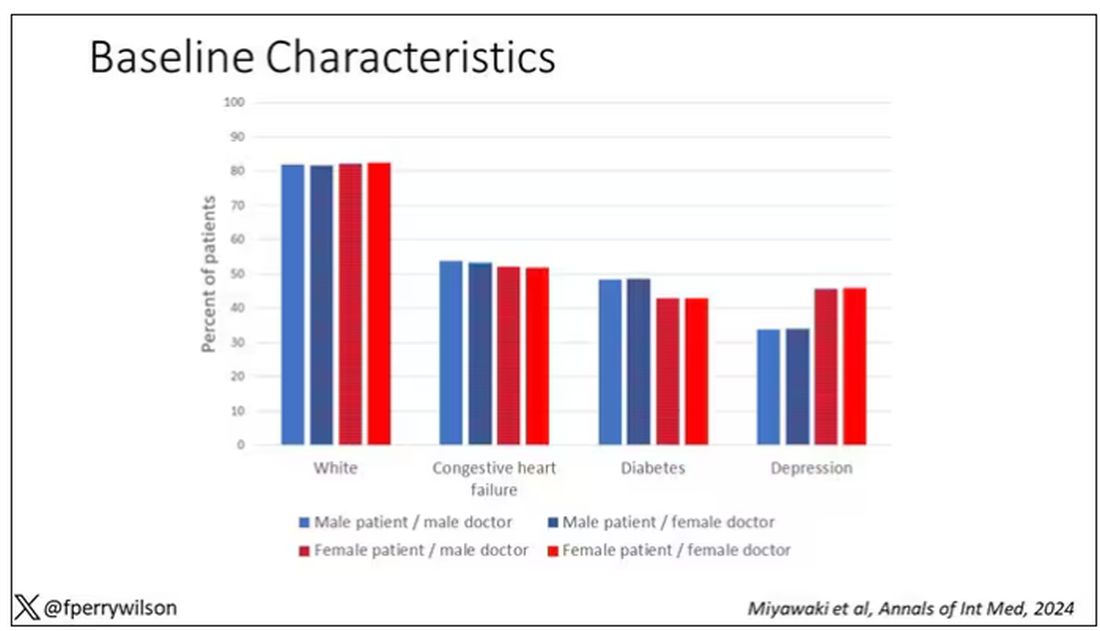
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 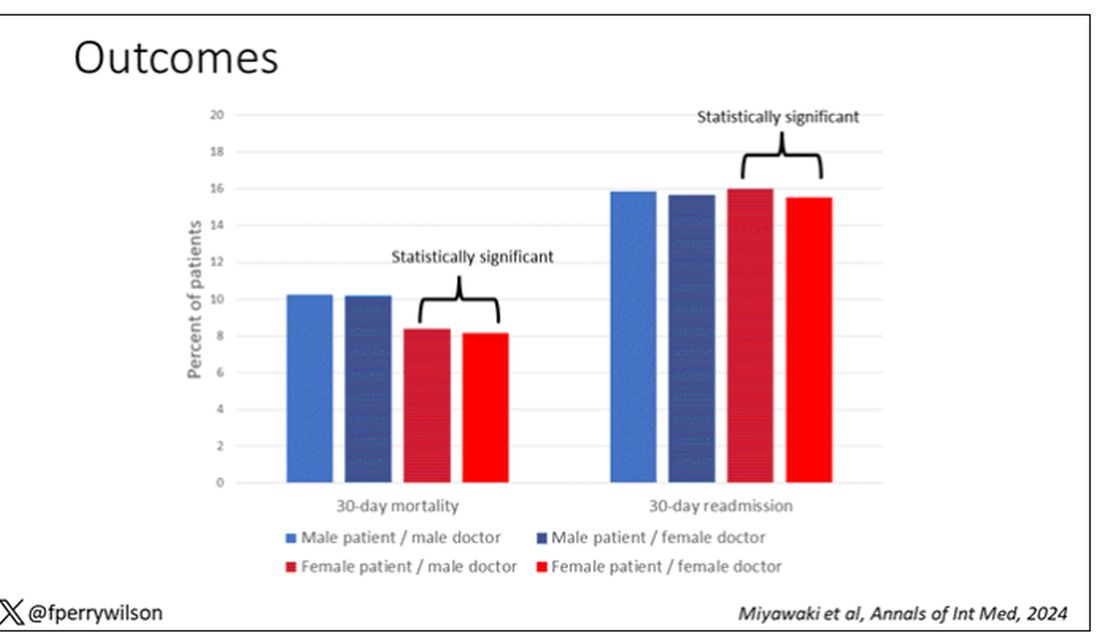
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA Approves New Bladder Cancer Drug
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
CRC Screening in Primary Care: The Blood Test Option
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
Weighing the Benefits of Integrating AI-based Clinical Notes Into Your Practice
Picture a healthcare system where physicians aren’t bogged down by excessive charting but are instead fully present with their patients, offering undivided attention and personalized care. In a recent X post, Stuart Blitz, COO and co-founder of Hone Health, sparked a thought-provoking conversation. “The problem with US healthcare is physicians are burned out since they spend way too much time charting, not enough with patients,” he wrote. “If you created a health system that did zero charting, you’d attract the best physicians and all patients would go there. Who is working on this?”
This resonates with many in the medical community, myself included, because the strain of extensive documentation detracts from patient care. Having worked in both large and small healthcare systems, I know the burden of extensive charting is a palpable challenge, often detracting from the time we can devote to our patients.
The first part of this two-part series examines the overarching benefits of artificial intelligence (AI)–based clinical documentation in modern healthcare, a field witnessing a paradigm shift thanks to advancements in AI.
Transformative Evolution of Clinical Documentation
The transition from manual documentation to AI-driven solutions marks a significant shift in the field, with a number of products in development including Nuance, Abridge, Ambience, ScribeAmerica, 3M, and DeepScribe. These tools use ambient clinical intelligence (ACI) to automate documentation, capturing patient conversations and translating them into structured clinical summaries. This innovation aligns with the vision of reducing charting burdens and enhancing patient-physician interactions.
How does it work? ACI refers to a sophisticated form of AI applied in healthcare settings, particularly focusing on enhancing the clinical documentation process without disrupting the natural flow of the consultation. Here’s a technical yet practical breakdown of ACI and the algorithms it typically employs:
Data capture and processing: ACI systems employ various sensors and processing units, typically integrated into clinical settings. These sensors, like microphones and cameras, gather diverse data such as audio from patient-doctor dialogues and visual cues. This information is then processed in real-time or near–real-time.
Natural language processing (NLP): A core component of ACI is advanced NLP algorithms. These algorithms analyze the captured audio data, transcribing spoken words into text. NLP goes beyond mere transcription; it involves understanding context, extracting relevant medical information (like symptoms, diagnoses, and treatment plans), and interpreting the nuances of human language.
Deep learning: Machine learning, particularly deep-learning techniques, are employed to improve the accuracy of ACI systems continually. These algorithms can learn from vast datasets of clinical interactions, enhancing their ability to transcribe and interpret future conversations accurately. As they learn, they become better at understanding different accents, complex medical terms, and variations in speech patterns.
Integration with electronic health records (EHRs): ACI systems are often designed to integrate seamlessly with existing EHR systems. They can automatically populate patient records with information from patient-clinician interactions, reducing manual entry and potential errors.
Customization and personalization: Many ACI systems offer customizable templates or allow clinicians to tailor documentation workflows. This flexibility ensures that the output aligns with the specific needs and preferences of healthcare providers.
Ethical and privacy considerations: ACI systems must navigate significant ethical and privacy concerns, especially related to patient consent and data security. These systems need to comply with healthcare privacy regulations such as HIPAA. They need to securely manage sensitive patient data and restrict access to authorized personnel only.
Broad-Spectrum Benefits of AI in Documentation
- Reducing clinician burnout: By automating the documentation process, AI tools like DAX Copilot alleviate a significant contributor to physician burnout, enabling clinicians to focus more on patient care.
- Enhanced patient care: With AI handling documentation, clinicians can engage more with their patients, leading to improved care quality and patient satisfaction.
- Data accuracy and quality: AI-driven documentation captures detailed patient encounters accurately, ensuring high-quality and comprehensive medical records.
- Response to the growing need for efficient healthcare: AI-based documentation is a direct response to the growing call for more efficient healthcare practices, where clinicians spend less time on paperwork and more with patients.
The shift toward AI-based clinical documentation represents a critical step in addressing the inefficiencies in healthcare systems. It’s a move towards a more patient-centered approach, where clinicians can focus more on patient care by reducing the time spent on excessive charting. Hopefully, we can integrate these solutions into our clinics at a large enough scale to make such an impact.
In the next column, we will explore in-depth insights from Kenneth Harper at Nuance on the technical implementation of these tools, with DAX as an example.
I would love to read your comments on AI in clinical trials as well as other AI-related topics. Write me at [email protected] or find me on X @DrBonillaOnc.
Dr. Loaiza-Bonilla is the co-founder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr Loaiza-Bonilla serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has served as a consultant for Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, and Guardant; served as a speaker or a member of a speakers bureau for Amgen, Guardant, Eisai, Ipsen, Natera, Merck, Bristol-Myers Squibb, and AstraZeneca. He holds a 5% or greater equity interest in Massive Bio.
A version of this article appeared on Medscape.com.
Picture a healthcare system where physicians aren’t bogged down by excessive charting but are instead fully present with their patients, offering undivided attention and personalized care. In a recent X post, Stuart Blitz, COO and co-founder of Hone Health, sparked a thought-provoking conversation. “The problem with US healthcare is physicians are burned out since they spend way too much time charting, not enough with patients,” he wrote. “If you created a health system that did zero charting, you’d attract the best physicians and all patients would go there. Who is working on this?”
This resonates with many in the medical community, myself included, because the strain of extensive documentation detracts from patient care. Having worked in both large and small healthcare systems, I know the burden of extensive charting is a palpable challenge, often detracting from the time we can devote to our patients.
The first part of this two-part series examines the overarching benefits of artificial intelligence (AI)–based clinical documentation in modern healthcare, a field witnessing a paradigm shift thanks to advancements in AI.
Transformative Evolution of Clinical Documentation
The transition from manual documentation to AI-driven solutions marks a significant shift in the field, with a number of products in development including Nuance, Abridge, Ambience, ScribeAmerica, 3M, and DeepScribe. These tools use ambient clinical intelligence (ACI) to automate documentation, capturing patient conversations and translating them into structured clinical summaries. This innovation aligns with the vision of reducing charting burdens and enhancing patient-physician interactions.
How does it work? ACI refers to a sophisticated form of AI applied in healthcare settings, particularly focusing on enhancing the clinical documentation process without disrupting the natural flow of the consultation. Here’s a technical yet practical breakdown of ACI and the algorithms it typically employs:
Data capture and processing: ACI systems employ various sensors and processing units, typically integrated into clinical settings. These sensors, like microphones and cameras, gather diverse data such as audio from patient-doctor dialogues and visual cues. This information is then processed in real-time or near–real-time.
Natural language processing (NLP): A core component of ACI is advanced NLP algorithms. These algorithms analyze the captured audio data, transcribing spoken words into text. NLP goes beyond mere transcription; it involves understanding context, extracting relevant medical information (like symptoms, diagnoses, and treatment plans), and interpreting the nuances of human language.
Deep learning: Machine learning, particularly deep-learning techniques, are employed to improve the accuracy of ACI systems continually. These algorithms can learn from vast datasets of clinical interactions, enhancing their ability to transcribe and interpret future conversations accurately. As they learn, they become better at understanding different accents, complex medical terms, and variations in speech patterns.
Integration with electronic health records (EHRs): ACI systems are often designed to integrate seamlessly with existing EHR systems. They can automatically populate patient records with information from patient-clinician interactions, reducing manual entry and potential errors.
Customization and personalization: Many ACI systems offer customizable templates or allow clinicians to tailor documentation workflows. This flexibility ensures that the output aligns with the specific needs and preferences of healthcare providers.
Ethical and privacy considerations: ACI systems must navigate significant ethical and privacy concerns, especially related to patient consent and data security. These systems need to comply with healthcare privacy regulations such as HIPAA. They need to securely manage sensitive patient data and restrict access to authorized personnel only.
Broad-Spectrum Benefits of AI in Documentation
- Reducing clinician burnout: By automating the documentation process, AI tools like DAX Copilot alleviate a significant contributor to physician burnout, enabling clinicians to focus more on patient care.
- Enhanced patient care: With AI handling documentation, clinicians can engage more with their patients, leading to improved care quality and patient satisfaction.
- Data accuracy and quality: AI-driven documentation captures detailed patient encounters accurately, ensuring high-quality and comprehensive medical records.
- Response to the growing need for efficient healthcare: AI-based documentation is a direct response to the growing call for more efficient healthcare practices, where clinicians spend less time on paperwork and more with patients.
The shift toward AI-based clinical documentation represents a critical step in addressing the inefficiencies in healthcare systems. It’s a move towards a more patient-centered approach, where clinicians can focus more on patient care by reducing the time spent on excessive charting. Hopefully, we can integrate these solutions into our clinics at a large enough scale to make such an impact.
In the next column, we will explore in-depth insights from Kenneth Harper at Nuance on the technical implementation of these tools, with DAX as an example.
I would love to read your comments on AI in clinical trials as well as other AI-related topics. Write me at [email protected] or find me on X @DrBonillaOnc.
Dr. Loaiza-Bonilla is the co-founder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr Loaiza-Bonilla serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has served as a consultant for Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, and Guardant; served as a speaker or a member of a speakers bureau for Amgen, Guardant, Eisai, Ipsen, Natera, Merck, Bristol-Myers Squibb, and AstraZeneca. He holds a 5% or greater equity interest in Massive Bio.
A version of this article appeared on Medscape.com.
Picture a healthcare system where physicians aren’t bogged down by excessive charting but are instead fully present with their patients, offering undivided attention and personalized care. In a recent X post, Stuart Blitz, COO and co-founder of Hone Health, sparked a thought-provoking conversation. “The problem with US healthcare is physicians are burned out since they spend way too much time charting, not enough with patients,” he wrote. “If you created a health system that did zero charting, you’d attract the best physicians and all patients would go there. Who is working on this?”
This resonates with many in the medical community, myself included, because the strain of extensive documentation detracts from patient care. Having worked in both large and small healthcare systems, I know the burden of extensive charting is a palpable challenge, often detracting from the time we can devote to our patients.
The first part of this two-part series examines the overarching benefits of artificial intelligence (AI)–based clinical documentation in modern healthcare, a field witnessing a paradigm shift thanks to advancements in AI.
Transformative Evolution of Clinical Documentation
The transition from manual documentation to AI-driven solutions marks a significant shift in the field, with a number of products in development including Nuance, Abridge, Ambience, ScribeAmerica, 3M, and DeepScribe. These tools use ambient clinical intelligence (ACI) to automate documentation, capturing patient conversations and translating them into structured clinical summaries. This innovation aligns with the vision of reducing charting burdens and enhancing patient-physician interactions.
How does it work? ACI refers to a sophisticated form of AI applied in healthcare settings, particularly focusing on enhancing the clinical documentation process without disrupting the natural flow of the consultation. Here’s a technical yet practical breakdown of ACI and the algorithms it typically employs:
Data capture and processing: ACI systems employ various sensors and processing units, typically integrated into clinical settings. These sensors, like microphones and cameras, gather diverse data such as audio from patient-doctor dialogues and visual cues. This information is then processed in real-time or near–real-time.
Natural language processing (NLP): A core component of ACI is advanced NLP algorithms. These algorithms analyze the captured audio data, transcribing spoken words into text. NLP goes beyond mere transcription; it involves understanding context, extracting relevant medical information (like symptoms, diagnoses, and treatment plans), and interpreting the nuances of human language.
Deep learning: Machine learning, particularly deep-learning techniques, are employed to improve the accuracy of ACI systems continually. These algorithms can learn from vast datasets of clinical interactions, enhancing their ability to transcribe and interpret future conversations accurately. As they learn, they become better at understanding different accents, complex medical terms, and variations in speech patterns.
Integration with electronic health records (EHRs): ACI systems are often designed to integrate seamlessly with existing EHR systems. They can automatically populate patient records with information from patient-clinician interactions, reducing manual entry and potential errors.
Customization and personalization: Many ACI systems offer customizable templates or allow clinicians to tailor documentation workflows. This flexibility ensures that the output aligns with the specific needs and preferences of healthcare providers.
Ethical and privacy considerations: ACI systems must navigate significant ethical and privacy concerns, especially related to patient consent and data security. These systems need to comply with healthcare privacy regulations such as HIPAA. They need to securely manage sensitive patient data and restrict access to authorized personnel only.
Broad-Spectrum Benefits of AI in Documentation
- Reducing clinician burnout: By automating the documentation process, AI tools like DAX Copilot alleviate a significant contributor to physician burnout, enabling clinicians to focus more on patient care.
- Enhanced patient care: With AI handling documentation, clinicians can engage more with their patients, leading to improved care quality and patient satisfaction.
- Data accuracy and quality: AI-driven documentation captures detailed patient encounters accurately, ensuring high-quality and comprehensive medical records.
- Response to the growing need for efficient healthcare: AI-based documentation is a direct response to the growing call for more efficient healthcare practices, where clinicians spend less time on paperwork and more with patients.
The shift toward AI-based clinical documentation represents a critical step in addressing the inefficiencies in healthcare systems. It’s a move towards a more patient-centered approach, where clinicians can focus more on patient care by reducing the time spent on excessive charting. Hopefully, we can integrate these solutions into our clinics at a large enough scale to make such an impact.
In the next column, we will explore in-depth insights from Kenneth Harper at Nuance on the technical implementation of these tools, with DAX as an example.
I would love to read your comments on AI in clinical trials as well as other AI-related topics. Write me at [email protected] or find me on X @DrBonillaOnc.
Dr. Loaiza-Bonilla is the co-founder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr Loaiza-Bonilla serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has served as a consultant for Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, and Guardant; served as a speaker or a member of a speakers bureau for Amgen, Guardant, Eisai, Ipsen, Natera, Merck, Bristol-Myers Squibb, and AstraZeneca. He holds a 5% or greater equity interest in Massive Bio.
A version of this article appeared on Medscape.com.
New Federal Rule Delivers Workplace Support, Time Off for Pregnant Docs
Pregnant physicians may receive more workplace accommodations and protection against discrimination thanks to an updated rule from the US Equal Employment Opportunity Commission (EEOC). The guidelines could prevent women from losing critical career momentum.
The Pregnant Workers Fairness Act (PWFA) aims to help workers balance professional demands with healthy pregnancies. It requires employers to provide reasonable accommodations for a “worker’s known limitations,” including physical or mental conditions associated with “pregnancy, childbirth, or related medical conditions.”
Reasonable accommodations vary but may involve time off to attend healthcare appointments or recover from childbirth, extra breaks during a shift, shorter work hours, or the ability to sit instead of stand. Private and public sector employers, including state and local governments, federal agencies, and employment agencies, must abide by the new guidelines unless they can provide evidence that doing so will cause undue hardship.
Female doctors have historically encountered significant barriers to family planning. Years of training cause them to delay having children, often leading to higher rates of infertility, miscarriage, and pregnancy complications than in the general population.
Some specialties, like surgeons, are particularly at risk, with 42% reporting at least one pregnancy loss. Most surgeons work their regular schedules until delivery despite desiring workload reductions, commonly citing unsupportive workplaces as a reason for not seeking accommodations.
Trauma surgeon Qaali Hussein, MD, became pregnant with her first child during her intern year in 2008. She told this news organization that her residency program didn’t even have a maternity policy at the time, and her male supervisor was certain that motherhood would end her surgical career.
She shared how “women usually waited until the end of their training to get pregnant. No one had ever gotten pregnant during the program and returned from maternity leave. I was the first to do so, so there wasn’t a policy or any program support to say, ‘What can we do to help?’ ”
Dr. Hussein used her vacation and sick time, returning to work 4 weeks after delivery. She had five more children, including twins her chief year and another baby during fellowship training in 2014.
Each subsequent pregnancy was met with the same response from program leadership, she recalled. “They’d say, ‘This is it. You may have been able to do the first and second child, but this one will be impossible.’ ”
After the PWFA regulations first became enforceable in June, the EEOC accepted public feedback. The guidelines received nearly 100,000 comments, spurred mainly by the inclusion of abortion care as a qualifying condition for which an employee could receive accommodations. About 54,000 comments called for abortion to be excluded from the final rule, and 40,000 supported keeping the clause.
The EEOC issued the final rule on April 15. It includes abortion care. However, the updated rule “does not require any employee to have — or not to have — an abortion, does not require taxpayers to pay for any abortions, and does not compel health care providers to provide any abortions,” the unpublished version of the final rule said. It is scheduled to take effect 60 days after its publication in the Federal Register on April 19.
Increasing Support for Doctor-Moms
The PWFA supplements other EEOC protections, such as pregnancy discrimination under Title VII of the Civil Rights Act of 1964 and access to reasonable accommodations under the Americans with Disabilities Act. In addition, it builds upon Department of Labor regulations, like the PUMP Act for breastfeeding employees and the Family and Medical Leave Act, which provides 12 weeks of unpaid, job-protected leave for the arrival of a child or certain medical conditions.
FMLA applies only to employees who have worked full-time for at least 12 months for an employer with 50 or more employees. Meanwhile, the unpaid, job-protected leave under the PWFA has no waiting period, lowers the required number of employees to 15, and permits accommodations for up to 40 weeks.
Employers are encouraged to honor “common and simple” requests, like using a closer parking space or pumping or nursing at work, without requiring a doctor’s note, the rule said.
Efforts to improve family leave policies for physicians and residents have been gaining traction. In 2021, the American Board of Medical Specialties began requiring its member boards with training programs lasting 2 or more years to allow at least 6 weeks off for parental, caregiver, and medical leave. This time can be taken without exhausting vacation or sick leave or requiring an extension in training. Over half of the 24 member boards permit leave beyond 6 weeks, including the American Boards of Allergy and Immunology, Emergency Medicine, Family Medicine, Radiology, and Surgery.
Estefania Oliveros, MD, MSc, cardiologist and assistant professor at the Lewis Katz School of Medicine at Temple University, Philadelphia, told this news organization that the Accreditation Council for Graduate Medical Education also requires that residents and fellows receive 6 weeks of paid leave.
“We add to that vacation time, so it gives them at least 8 weeks,” she said. The school has created spaces for nursing mothers — something neither she nor Dr. Hussein had access to when breastfeeding — and encourages the attendings to be proactive in excusing pregnant fellows for appointments.
This differs significantly from her fellowship training experience 6 years ago at another institution, where she worked without accommodations until the day before her cesarean delivery. Dr. Oliveros had to use all her vacation time for recovery, returning to the program after 4 weeks instead of the recommended 6.
“And that’s the story you hear all the time. Not because people are ill-intended; I just don’t think the system is designed to accommodate women, so we lose a lot of talent that way,” said Dr. Oliveros, whose 2019 survey in the Journal of the American College of Cardiology called for more support and protections for pregnant doctors.
Both doctors believe the PWFA will be beneficial but only if leadership in the field takes up the cause.
“The cultures of these institutions determine whether women feel safe or even confident enough to have children in medical school or residency,” said Dr. Hussein.
A version of this article appeared on Medscape.com.
Pregnant physicians may receive more workplace accommodations and protection against discrimination thanks to an updated rule from the US Equal Employment Opportunity Commission (EEOC). The guidelines could prevent women from losing critical career momentum.
The Pregnant Workers Fairness Act (PWFA) aims to help workers balance professional demands with healthy pregnancies. It requires employers to provide reasonable accommodations for a “worker’s known limitations,” including physical or mental conditions associated with “pregnancy, childbirth, or related medical conditions.”
Reasonable accommodations vary but may involve time off to attend healthcare appointments or recover from childbirth, extra breaks during a shift, shorter work hours, or the ability to sit instead of stand. Private and public sector employers, including state and local governments, federal agencies, and employment agencies, must abide by the new guidelines unless they can provide evidence that doing so will cause undue hardship.
Female doctors have historically encountered significant barriers to family planning. Years of training cause them to delay having children, often leading to higher rates of infertility, miscarriage, and pregnancy complications than in the general population.
Some specialties, like surgeons, are particularly at risk, with 42% reporting at least one pregnancy loss. Most surgeons work their regular schedules until delivery despite desiring workload reductions, commonly citing unsupportive workplaces as a reason for not seeking accommodations.
Trauma surgeon Qaali Hussein, MD, became pregnant with her first child during her intern year in 2008. She told this news organization that her residency program didn’t even have a maternity policy at the time, and her male supervisor was certain that motherhood would end her surgical career.
She shared how “women usually waited until the end of their training to get pregnant. No one had ever gotten pregnant during the program and returned from maternity leave. I was the first to do so, so there wasn’t a policy or any program support to say, ‘What can we do to help?’ ”
Dr. Hussein used her vacation and sick time, returning to work 4 weeks after delivery. She had five more children, including twins her chief year and another baby during fellowship training in 2014.
Each subsequent pregnancy was met with the same response from program leadership, she recalled. “They’d say, ‘This is it. You may have been able to do the first and second child, but this one will be impossible.’ ”
After the PWFA regulations first became enforceable in June, the EEOC accepted public feedback. The guidelines received nearly 100,000 comments, spurred mainly by the inclusion of abortion care as a qualifying condition for which an employee could receive accommodations. About 54,000 comments called for abortion to be excluded from the final rule, and 40,000 supported keeping the clause.
The EEOC issued the final rule on April 15. It includes abortion care. However, the updated rule “does not require any employee to have — or not to have — an abortion, does not require taxpayers to pay for any abortions, and does not compel health care providers to provide any abortions,” the unpublished version of the final rule said. It is scheduled to take effect 60 days after its publication in the Federal Register on April 19.
Increasing Support for Doctor-Moms
The PWFA supplements other EEOC protections, such as pregnancy discrimination under Title VII of the Civil Rights Act of 1964 and access to reasonable accommodations under the Americans with Disabilities Act. In addition, it builds upon Department of Labor regulations, like the PUMP Act for breastfeeding employees and the Family and Medical Leave Act, which provides 12 weeks of unpaid, job-protected leave for the arrival of a child or certain medical conditions.
FMLA applies only to employees who have worked full-time for at least 12 months for an employer with 50 or more employees. Meanwhile, the unpaid, job-protected leave under the PWFA has no waiting period, lowers the required number of employees to 15, and permits accommodations for up to 40 weeks.
Employers are encouraged to honor “common and simple” requests, like using a closer parking space or pumping or nursing at work, without requiring a doctor’s note, the rule said.
Efforts to improve family leave policies for physicians and residents have been gaining traction. In 2021, the American Board of Medical Specialties began requiring its member boards with training programs lasting 2 or more years to allow at least 6 weeks off for parental, caregiver, and medical leave. This time can be taken without exhausting vacation or sick leave or requiring an extension in training. Over half of the 24 member boards permit leave beyond 6 weeks, including the American Boards of Allergy and Immunology, Emergency Medicine, Family Medicine, Radiology, and Surgery.
Estefania Oliveros, MD, MSc, cardiologist and assistant professor at the Lewis Katz School of Medicine at Temple University, Philadelphia, told this news organization that the Accreditation Council for Graduate Medical Education also requires that residents and fellows receive 6 weeks of paid leave.
“We add to that vacation time, so it gives them at least 8 weeks,” she said. The school has created spaces for nursing mothers — something neither she nor Dr. Hussein had access to when breastfeeding — and encourages the attendings to be proactive in excusing pregnant fellows for appointments.
This differs significantly from her fellowship training experience 6 years ago at another institution, where she worked without accommodations until the day before her cesarean delivery. Dr. Oliveros had to use all her vacation time for recovery, returning to the program after 4 weeks instead of the recommended 6.
“And that’s the story you hear all the time. Not because people are ill-intended; I just don’t think the system is designed to accommodate women, so we lose a lot of talent that way,” said Dr. Oliveros, whose 2019 survey in the Journal of the American College of Cardiology called for more support and protections for pregnant doctors.
Both doctors believe the PWFA will be beneficial but only if leadership in the field takes up the cause.
“The cultures of these institutions determine whether women feel safe or even confident enough to have children in medical school or residency,” said Dr. Hussein.
A version of this article appeared on Medscape.com.
Pregnant physicians may receive more workplace accommodations and protection against discrimination thanks to an updated rule from the US Equal Employment Opportunity Commission (EEOC). The guidelines could prevent women from losing critical career momentum.
The Pregnant Workers Fairness Act (PWFA) aims to help workers balance professional demands with healthy pregnancies. It requires employers to provide reasonable accommodations for a “worker’s known limitations,” including physical or mental conditions associated with “pregnancy, childbirth, or related medical conditions.”
Reasonable accommodations vary but may involve time off to attend healthcare appointments or recover from childbirth, extra breaks during a shift, shorter work hours, or the ability to sit instead of stand. Private and public sector employers, including state and local governments, federal agencies, and employment agencies, must abide by the new guidelines unless they can provide evidence that doing so will cause undue hardship.
Female doctors have historically encountered significant barriers to family planning. Years of training cause them to delay having children, often leading to higher rates of infertility, miscarriage, and pregnancy complications than in the general population.
Some specialties, like surgeons, are particularly at risk, with 42% reporting at least one pregnancy loss. Most surgeons work their regular schedules until delivery despite desiring workload reductions, commonly citing unsupportive workplaces as a reason for not seeking accommodations.
Trauma surgeon Qaali Hussein, MD, became pregnant with her first child during her intern year in 2008. She told this news organization that her residency program didn’t even have a maternity policy at the time, and her male supervisor was certain that motherhood would end her surgical career.
She shared how “women usually waited until the end of their training to get pregnant. No one had ever gotten pregnant during the program and returned from maternity leave. I was the first to do so, so there wasn’t a policy or any program support to say, ‘What can we do to help?’ ”
Dr. Hussein used her vacation and sick time, returning to work 4 weeks after delivery. She had five more children, including twins her chief year and another baby during fellowship training in 2014.
Each subsequent pregnancy was met with the same response from program leadership, she recalled. “They’d say, ‘This is it. You may have been able to do the first and second child, but this one will be impossible.’ ”
After the PWFA regulations first became enforceable in June, the EEOC accepted public feedback. The guidelines received nearly 100,000 comments, spurred mainly by the inclusion of abortion care as a qualifying condition for which an employee could receive accommodations. About 54,000 comments called for abortion to be excluded from the final rule, and 40,000 supported keeping the clause.
The EEOC issued the final rule on April 15. It includes abortion care. However, the updated rule “does not require any employee to have — or not to have — an abortion, does not require taxpayers to pay for any abortions, and does not compel health care providers to provide any abortions,” the unpublished version of the final rule said. It is scheduled to take effect 60 days after its publication in the Federal Register on April 19.
Increasing Support for Doctor-Moms
The PWFA supplements other EEOC protections, such as pregnancy discrimination under Title VII of the Civil Rights Act of 1964 and access to reasonable accommodations under the Americans with Disabilities Act. In addition, it builds upon Department of Labor regulations, like the PUMP Act for breastfeeding employees and the Family and Medical Leave Act, which provides 12 weeks of unpaid, job-protected leave for the arrival of a child or certain medical conditions.
FMLA applies only to employees who have worked full-time for at least 12 months for an employer with 50 or more employees. Meanwhile, the unpaid, job-protected leave under the PWFA has no waiting period, lowers the required number of employees to 15, and permits accommodations for up to 40 weeks.
Employers are encouraged to honor “common and simple” requests, like using a closer parking space or pumping or nursing at work, without requiring a doctor’s note, the rule said.
Efforts to improve family leave policies for physicians and residents have been gaining traction. In 2021, the American Board of Medical Specialties began requiring its member boards with training programs lasting 2 or more years to allow at least 6 weeks off for parental, caregiver, and medical leave. This time can be taken without exhausting vacation or sick leave or requiring an extension in training. Over half of the 24 member boards permit leave beyond 6 weeks, including the American Boards of Allergy and Immunology, Emergency Medicine, Family Medicine, Radiology, and Surgery.
Estefania Oliveros, MD, MSc, cardiologist and assistant professor at the Lewis Katz School of Medicine at Temple University, Philadelphia, told this news organization that the Accreditation Council for Graduate Medical Education also requires that residents and fellows receive 6 weeks of paid leave.
“We add to that vacation time, so it gives them at least 8 weeks,” she said. The school has created spaces for nursing mothers — something neither she nor Dr. Hussein had access to when breastfeeding — and encourages the attendings to be proactive in excusing pregnant fellows for appointments.
This differs significantly from her fellowship training experience 6 years ago at another institution, where she worked without accommodations until the day before her cesarean delivery. Dr. Oliveros had to use all her vacation time for recovery, returning to the program after 4 weeks instead of the recommended 6.
“And that’s the story you hear all the time. Not because people are ill-intended; I just don’t think the system is designed to accommodate women, so we lose a lot of talent that way,” said Dr. Oliveros, whose 2019 survey in the Journal of the American College of Cardiology called for more support and protections for pregnant doctors.
Both doctors believe the PWFA will be beneficial but only if leadership in the field takes up the cause.
“The cultures of these institutions determine whether women feel safe or even confident enough to have children in medical school or residency,” said Dr. Hussein.
A version of this article appeared on Medscape.com.



