User login
Medicaid implements waivers for some clinical trial coverage
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Are GI hospitalists the future of inpatient care?
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Chicago oncologist charged with insider trading
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
Average-risk women with dense breasts—What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
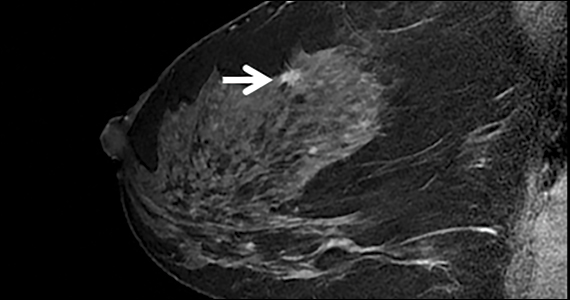
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Quiz developed in collaboration with 
More Americans skipping medical care because of cost, survey says
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Are physician-owned large groups better than flying solo?
Large, physician-owned group practices are gaining ground as a popular form of practice, even as the number of physicians in solo and small practices declines, and employment maintains its appeal.
As physicians shift from owning private practices to employment in hospital systems, this countertrend is also taking place. Large group practices are growing in number, even as solo and small practices are in decline.
Do large, physician-owned groups bring benefits that beat employment? And how do large groups compare with smaller practices and new opportunities, such as private equity? You’ll find some answers here.
Working in large group practices
Large group practices with 50 or more physicians are enjoying a renaissance, even though physicians are still streaming into hospital systems. The share of physicians in large practices increased from 14.7% in 2018 to 17.2% in 2020, the largest 2-year change for this group, according to the American Medical Association.
“Physicians expect that large groups will treat them better than hospitals do,” says Robert Pearl, MD, former CEO of Permanente Medical Group, the nation’s largest physicians’ group.
Compared with hospitals, “doctors would prefer working in a group practice, if all other things are equal,” says Dr. Pearl, who is now a professor at Stanford (Calif.) University Medical School.
Large group practices can include both multispecialty groups and single-specialty groups. Groups in specialties like urology, orthopedics, and oncology have been growing in recent years, according to Gregory Mertz, managing director of Physician Strategies Group in Virginia Beach, Va.
A group practice could also be an independent physicians association – a federation of small practices that share functions like negotiations with insurers and management. Physicians can also form larger groups for single purposes like running an accountable care organization.
Some large group practices can have a mix of partners and employees. In these groups, “some doctors either don’t want a partnership or aren’t offered one,” says Nathan Miller, CEO of the Medicus Firm, a physician recruitment company in Dallas. The AMA reports that about 10% of physicians are employees of large practices.
“Large groups like the Permanente Medical Group are not partnerships,” Dr. Pearl says. “They tend to be a corporation with a board of directors, and all the physicians are employees, but it’s a physician-led organization.”
Doctors in these groups can enjoy a great deal of control. While Permanente Medical Group is exclusively affiliated with Kaiser, which runs hospitals and an HMO, the group is an independent corporation run by its doctors, who are both shareholders and employees, Dr. Pearl says.
The Cleveland Clinic and Mayo Clinic are not medical groups in the strict sense of the word. They describe themselves as academic medical centers, but Dr. Pearl says, “Doctors have a tremendous amount of control there, particularly those in the most remunerative specialties.”
Pros of large groups
Group practices are able to focus more on the physician participants’ needs and priorities, says Mr. Mertz. “In a hospital-based organization, physicians’ needs have to compete with the needs of the hospital. … In a large group, it can be easier to get policies changed and order equipment.”
However, for many physicians, their primary reason for joining a large group is having negotiating leverage with health insurance plans, and this leverage seems even more important today. It typically results in higher reimbursements, which could translate into higher pay. The higher practice income, however, could be negated by higher administrative overhead, which is endemic in large organizations.
Mr. Mertz says large groups also have the resources to recruit new doctors. Small practices, in contrast, often decide not to grow. The practice would at first need to guarantee the salary of a new partner, which could require existing partners to take a pay cut, which they often don’t want to do. “They’ll decide to ride the practice into the ground,” which means closing it down when they retire, he says.
Cons of large groups
One individual doctor may have relatively little input in decision-making in a large group, and strong leadership may be lacking. One study examining the pros and cons of large group practices found that lack of physician cooperation, investment, and leadership were the most frequently cited barriers in large groups.
Physicians in large groups can also divide into competing factions. Mr. Mertz says rifts are more likely to take place in multispecialty groups, where higher-reimbursed proceduralists resent having to financially support lower-reimbursed primary care physicians. But it’s rare that such rifts actually break up the practice, he says.
Private practice vs. employment
Even as more physicians enter large groups, physicians continue to flee private practice in general. In 2020, the AMA found that the number of physicians in private practices had dropped nearly 5 percentage points since 2018, the largest 2-year drop recorded by the AMA.
The hardest hit are small groups of 10 physicians or fewer, once the backbone of U.S. medicine. A 2020 survey found that 53.7% of physicians still work in small practices of 10 or fewer physicians, compared with 61.4% in 2012.
Private practices tend to be partnerships, but younger physicians, for their part, often don’t want to become a partner. In a 2016 survey, only 22% of medical residents surveyed said they anticipate owning a stake in a practice someday.
What’s good about private practice?
The obvious advantage of private practice is having control. Physician-owners can choose staff, oversee finances, and decide on the direction the practice should take. They don’t have to worry about being fired, because the partnership agreement virtually guarantees each doctor’s place in the group.
The atmosphere in a small practice is often more relaxed. “Private practices tend to offer a family-like environment,” Mr. Miller says. Owners of small practices tend to have lower burnout than large practices, a 2018 study found.
Unlike hospital-employed doctors, private practitioners get to keep their ancillary income. “Physicians own the equipment and receive income generated from ancillary services, not just professional fees,” says Mr. Miller.
What’s negative about private practice?
Since small groups have little negotiating power with private payers, they can’t get favorable reimbursement rates. And while partners are protected from being fired, the practice could still go bankrupt.
Running a private practice means putting on an entrepreneurial hat. To develop a strong practice, you need to learn about marketing, finance, IT, contract negotiations, and facility management. “Most young doctors have no interest in this work,” Mr. Mertz says.
Value-based contracting has added another disadvantage for small practices. “It can be harder for small, independent groups to compete,” says Mike Belkin, JD, a divisional vice president at Merritt Hawkins, a physician recruitment company based in Dallas. “They don’t have the data and integration of services that are necessary for this.”
Employment in hospital systems
More than one-third of all physicians worked for hospitals in 2018, and hospitals’ share has been growing since then. In 2020, for the first time, the AMA found that more than half of all physicians were employed, and employment is mainly a hospital phenomenon.
The trend shows no signs of stopping. In 2019 and 2020, hospitals and other corporate entities acquired 20,900 physician practices, representing 29,800 doctors. “This trend will continue,” Dr. Pearl says. “The bigger will get bigger. It’s all about market control. Everyone wants to be wider, more vertical, and more powerful.”
Pros of hospital employment
“The advantages of hospital employment are mostly financial,” Mr. Mertz says. Unlike a private practice, “there’s no financial risk to hospital employment because you don’t own it. You won’t be on the tab for any losses.”
“Hospitals usually offer a highly competitive salary with less emphasis on production than in a private practice,” he says. New physicians are typically paid a guaranteed salary in the first 1-3 years of employment.
“You don’t have any management responsibilities, as you would in a practice,” Mr. Mertz says. “The hospital has a professional management team to handle the business side. Most young doctors have no interest in this work.”
“Employed physicians have a built-in referral network at a hospital,” Mr. Miller says. This is especially an advantage for new physicians, who don’t yet have a referral network of their own.”
Cons of hospital employment
Physicians employed by a hospital lack control. “You don’t decide the hours you work, the schedules you follow, and the physical facility you work in, and, for the most part, you don’t pick your staff,” Mr. Mertz says.
Like any big organization, hospitals are bureaucratic. “If you want to purchase a new piece of equipment, your request goes up the chain of command,” Mr. Mertz says. “Your purchase has to fit into the budget.” (This can be the case with large groups, too.)
Many employed doctors chafe under this lack of control. In an earlier survey by Medscape, 45% of employed respondents didn’t like having limited influence in decision-making, and 32% said they had less control over their work or schedule.
It’s no wonder that a large percentage of physicians would rather work in practices than hospitals. According to a 2021 Medicus Firm survey, 23% of physicians are interested in working in hospitals, while 40% would rather work either in multispecialty or single-specialty groups, Mr. Miller reports.
Doctors have differing views of hospital employment
New physicians are apt to dismiss any negatives about hospitals. “Lack of autonomy often matters less to younger physicians, who were trained in team-based models,” Mr. Belkin says.
Many young doctors actually like working in a large organization. “Young doctors out of residency are used to having everything at their fingertips – labs and testing is in-house,” Mr. Mertz says.
On the other hand, doctors who were previously self-employed – a group that makes up almost one-third of all hospital-employed doctors – can often be dissatisfied with employment. In a 2014 Medscape survey, 26% of previously self-employed doctors said job satisfaction had not improved with employment.
Mr. Mertz says these doctors remember what it was like to be in charge of a practice. “If you once owned a practice, you can always compare what’s going on now with that experience, and that can make you frustrated.”
Hospitals have higher turnover
It’s much easier to leave an organization when you don’t have an ownership stake. The annual physician turnover rate at hospitals is 28%, compared with 7% at medical groups, according to a 2019 report.
Mr. Belkin says changing jobs has become a way of life for many doctors. “Staying at a job for only a few years is no longer a red flag,” he says. “Physicians are exploring different options. They might try group practice and switch to hospitals or vice versa.”
Physicians are now part of a high-turnover culture: Once in a new job, many are already thinking about the next one. A 2018 survey found that 46% of doctors planned to leave their position within 3 years.
Private equity ownership of practice
Selling majority control of your practice to a private equity firm is a relatively new phenomenon and accounts for a small share of physicians – just 4% in 2020. This trend was originally limited to certain specialties, such as anesthesiology, emergency medicine, and dermatology, but now many others are courted.
The deals work like this: Physicians sell majority control of their practice to investors in return for shares in the private equity practice, and they become employees of that practice. The private equity firm then adds more physicians to the practice and invests in infrastructure with the intention of selling the practice at a large profit, which is then shared with the original physicians.
Pros of private equity
The original owners of the practice stand to make a substantial profit if they are willing to wait several years for the practice to be built up and sold. “If they are patient, they could earn a bonanza,” Mr. Belkin says.
Private equity investment helps the practice expand. “It’s an alternative to going to the bank and borrowing money,” Mr. Mertz says.
Cons of private equity
Physicians lose control of their practice. A client of Mr. Mertz’s briefly considered a private equity offer and turned it down. “The private equity firm would have veto power over what the doctors wanted to do,” he says.
Mr. Belkin says the selling physicians typically lose income after the sale. “Money they earned from ancillary services now goes to the practice,” Mr. Belkin says. The selling doctors could potentially take up to a 30% cut in their compensation, according to Coker Capital Advisors.
A version of this article first appeared on Medscape.com.
Large, physician-owned group practices are gaining ground as a popular form of practice, even as the number of physicians in solo and small practices declines, and employment maintains its appeal.
As physicians shift from owning private practices to employment in hospital systems, this countertrend is also taking place. Large group practices are growing in number, even as solo and small practices are in decline.
Do large, physician-owned groups bring benefits that beat employment? And how do large groups compare with smaller practices and new opportunities, such as private equity? You’ll find some answers here.
Working in large group practices
Large group practices with 50 or more physicians are enjoying a renaissance, even though physicians are still streaming into hospital systems. The share of physicians in large practices increased from 14.7% in 2018 to 17.2% in 2020, the largest 2-year change for this group, according to the American Medical Association.
“Physicians expect that large groups will treat them better than hospitals do,” says Robert Pearl, MD, former CEO of Permanente Medical Group, the nation’s largest physicians’ group.
Compared with hospitals, “doctors would prefer working in a group practice, if all other things are equal,” says Dr. Pearl, who is now a professor at Stanford (Calif.) University Medical School.
Large group practices can include both multispecialty groups and single-specialty groups. Groups in specialties like urology, orthopedics, and oncology have been growing in recent years, according to Gregory Mertz, managing director of Physician Strategies Group in Virginia Beach, Va.
A group practice could also be an independent physicians association – a federation of small practices that share functions like negotiations with insurers and management. Physicians can also form larger groups for single purposes like running an accountable care organization.
Some large group practices can have a mix of partners and employees. In these groups, “some doctors either don’t want a partnership or aren’t offered one,” says Nathan Miller, CEO of the Medicus Firm, a physician recruitment company in Dallas. The AMA reports that about 10% of physicians are employees of large practices.
“Large groups like the Permanente Medical Group are not partnerships,” Dr. Pearl says. “They tend to be a corporation with a board of directors, and all the physicians are employees, but it’s a physician-led organization.”
Doctors in these groups can enjoy a great deal of control. While Permanente Medical Group is exclusively affiliated with Kaiser, which runs hospitals and an HMO, the group is an independent corporation run by its doctors, who are both shareholders and employees, Dr. Pearl says.
The Cleveland Clinic and Mayo Clinic are not medical groups in the strict sense of the word. They describe themselves as academic medical centers, but Dr. Pearl says, “Doctors have a tremendous amount of control there, particularly those in the most remunerative specialties.”
Pros of large groups
Group practices are able to focus more on the physician participants’ needs and priorities, says Mr. Mertz. “In a hospital-based organization, physicians’ needs have to compete with the needs of the hospital. … In a large group, it can be easier to get policies changed and order equipment.”
However, for many physicians, their primary reason for joining a large group is having negotiating leverage with health insurance plans, and this leverage seems even more important today. It typically results in higher reimbursements, which could translate into higher pay. The higher practice income, however, could be negated by higher administrative overhead, which is endemic in large organizations.
Mr. Mertz says large groups also have the resources to recruit new doctors. Small practices, in contrast, often decide not to grow. The practice would at first need to guarantee the salary of a new partner, which could require existing partners to take a pay cut, which they often don’t want to do. “They’ll decide to ride the practice into the ground,” which means closing it down when they retire, he says.
Cons of large groups
One individual doctor may have relatively little input in decision-making in a large group, and strong leadership may be lacking. One study examining the pros and cons of large group practices found that lack of physician cooperation, investment, and leadership were the most frequently cited barriers in large groups.
Physicians in large groups can also divide into competing factions. Mr. Mertz says rifts are more likely to take place in multispecialty groups, where higher-reimbursed proceduralists resent having to financially support lower-reimbursed primary care physicians. But it’s rare that such rifts actually break up the practice, he says.
Private practice vs. employment
Even as more physicians enter large groups, physicians continue to flee private practice in general. In 2020, the AMA found that the number of physicians in private practices had dropped nearly 5 percentage points since 2018, the largest 2-year drop recorded by the AMA.
The hardest hit are small groups of 10 physicians or fewer, once the backbone of U.S. medicine. A 2020 survey found that 53.7% of physicians still work in small practices of 10 or fewer physicians, compared with 61.4% in 2012.
Private practices tend to be partnerships, but younger physicians, for their part, often don’t want to become a partner. In a 2016 survey, only 22% of medical residents surveyed said they anticipate owning a stake in a practice someday.
What’s good about private practice?
The obvious advantage of private practice is having control. Physician-owners can choose staff, oversee finances, and decide on the direction the practice should take. They don’t have to worry about being fired, because the partnership agreement virtually guarantees each doctor’s place in the group.
The atmosphere in a small practice is often more relaxed. “Private practices tend to offer a family-like environment,” Mr. Miller says. Owners of small practices tend to have lower burnout than large practices, a 2018 study found.
Unlike hospital-employed doctors, private practitioners get to keep their ancillary income. “Physicians own the equipment and receive income generated from ancillary services, not just professional fees,” says Mr. Miller.
What’s negative about private practice?
Since small groups have little negotiating power with private payers, they can’t get favorable reimbursement rates. And while partners are protected from being fired, the practice could still go bankrupt.
Running a private practice means putting on an entrepreneurial hat. To develop a strong practice, you need to learn about marketing, finance, IT, contract negotiations, and facility management. “Most young doctors have no interest in this work,” Mr. Mertz says.
Value-based contracting has added another disadvantage for small practices. “It can be harder for small, independent groups to compete,” says Mike Belkin, JD, a divisional vice president at Merritt Hawkins, a physician recruitment company based in Dallas. “They don’t have the data and integration of services that are necessary for this.”
Employment in hospital systems
More than one-third of all physicians worked for hospitals in 2018, and hospitals’ share has been growing since then. In 2020, for the first time, the AMA found that more than half of all physicians were employed, and employment is mainly a hospital phenomenon.
The trend shows no signs of stopping. In 2019 and 2020, hospitals and other corporate entities acquired 20,900 physician practices, representing 29,800 doctors. “This trend will continue,” Dr. Pearl says. “The bigger will get bigger. It’s all about market control. Everyone wants to be wider, more vertical, and more powerful.”
Pros of hospital employment
“The advantages of hospital employment are mostly financial,” Mr. Mertz says. Unlike a private practice, “there’s no financial risk to hospital employment because you don’t own it. You won’t be on the tab for any losses.”
“Hospitals usually offer a highly competitive salary with less emphasis on production than in a private practice,” he says. New physicians are typically paid a guaranteed salary in the first 1-3 years of employment.
“You don’t have any management responsibilities, as you would in a practice,” Mr. Mertz says. “The hospital has a professional management team to handle the business side. Most young doctors have no interest in this work.”
“Employed physicians have a built-in referral network at a hospital,” Mr. Miller says. This is especially an advantage for new physicians, who don’t yet have a referral network of their own.”
Cons of hospital employment
Physicians employed by a hospital lack control. “You don’t decide the hours you work, the schedules you follow, and the physical facility you work in, and, for the most part, you don’t pick your staff,” Mr. Mertz says.
Like any big organization, hospitals are bureaucratic. “If you want to purchase a new piece of equipment, your request goes up the chain of command,” Mr. Mertz says. “Your purchase has to fit into the budget.” (This can be the case with large groups, too.)
Many employed doctors chafe under this lack of control. In an earlier survey by Medscape, 45% of employed respondents didn’t like having limited influence in decision-making, and 32% said they had less control over their work or schedule.
It’s no wonder that a large percentage of physicians would rather work in practices than hospitals. According to a 2021 Medicus Firm survey, 23% of physicians are interested in working in hospitals, while 40% would rather work either in multispecialty or single-specialty groups, Mr. Miller reports.
Doctors have differing views of hospital employment
New physicians are apt to dismiss any negatives about hospitals. “Lack of autonomy often matters less to younger physicians, who were trained in team-based models,” Mr. Belkin says.
Many young doctors actually like working in a large organization. “Young doctors out of residency are used to having everything at their fingertips – labs and testing is in-house,” Mr. Mertz says.
On the other hand, doctors who were previously self-employed – a group that makes up almost one-third of all hospital-employed doctors – can often be dissatisfied with employment. In a 2014 Medscape survey, 26% of previously self-employed doctors said job satisfaction had not improved with employment.
Mr. Mertz says these doctors remember what it was like to be in charge of a practice. “If you once owned a practice, you can always compare what’s going on now with that experience, and that can make you frustrated.”
Hospitals have higher turnover
It’s much easier to leave an organization when you don’t have an ownership stake. The annual physician turnover rate at hospitals is 28%, compared with 7% at medical groups, according to a 2019 report.
Mr. Belkin says changing jobs has become a way of life for many doctors. “Staying at a job for only a few years is no longer a red flag,” he says. “Physicians are exploring different options. They might try group practice and switch to hospitals or vice versa.”
Physicians are now part of a high-turnover culture: Once in a new job, many are already thinking about the next one. A 2018 survey found that 46% of doctors planned to leave their position within 3 years.
Private equity ownership of practice
Selling majority control of your practice to a private equity firm is a relatively new phenomenon and accounts for a small share of physicians – just 4% in 2020. This trend was originally limited to certain specialties, such as anesthesiology, emergency medicine, and dermatology, but now many others are courted.
The deals work like this: Physicians sell majority control of their practice to investors in return for shares in the private equity practice, and they become employees of that practice. The private equity firm then adds more physicians to the practice and invests in infrastructure with the intention of selling the practice at a large profit, which is then shared with the original physicians.
Pros of private equity
The original owners of the practice stand to make a substantial profit if they are willing to wait several years for the practice to be built up and sold. “If they are patient, they could earn a bonanza,” Mr. Belkin says.
Private equity investment helps the practice expand. “It’s an alternative to going to the bank and borrowing money,” Mr. Mertz says.
Cons of private equity
Physicians lose control of their practice. A client of Mr. Mertz’s briefly considered a private equity offer and turned it down. “The private equity firm would have veto power over what the doctors wanted to do,” he says.
Mr. Belkin says the selling physicians typically lose income after the sale. “Money they earned from ancillary services now goes to the practice,” Mr. Belkin says. The selling doctors could potentially take up to a 30% cut in their compensation, according to Coker Capital Advisors.
A version of this article first appeared on Medscape.com.
Large, physician-owned group practices are gaining ground as a popular form of practice, even as the number of physicians in solo and small practices declines, and employment maintains its appeal.
As physicians shift from owning private practices to employment in hospital systems, this countertrend is also taking place. Large group practices are growing in number, even as solo and small practices are in decline.
Do large, physician-owned groups bring benefits that beat employment? And how do large groups compare with smaller practices and new opportunities, such as private equity? You’ll find some answers here.
Working in large group practices
Large group practices with 50 or more physicians are enjoying a renaissance, even though physicians are still streaming into hospital systems. The share of physicians in large practices increased from 14.7% in 2018 to 17.2% in 2020, the largest 2-year change for this group, according to the American Medical Association.
“Physicians expect that large groups will treat them better than hospitals do,” says Robert Pearl, MD, former CEO of Permanente Medical Group, the nation’s largest physicians’ group.
Compared with hospitals, “doctors would prefer working in a group practice, if all other things are equal,” says Dr. Pearl, who is now a professor at Stanford (Calif.) University Medical School.
Large group practices can include both multispecialty groups and single-specialty groups. Groups in specialties like urology, orthopedics, and oncology have been growing in recent years, according to Gregory Mertz, managing director of Physician Strategies Group in Virginia Beach, Va.
A group practice could also be an independent physicians association – a federation of small practices that share functions like negotiations with insurers and management. Physicians can also form larger groups for single purposes like running an accountable care organization.
Some large group practices can have a mix of partners and employees. In these groups, “some doctors either don’t want a partnership or aren’t offered one,” says Nathan Miller, CEO of the Medicus Firm, a physician recruitment company in Dallas. The AMA reports that about 10% of physicians are employees of large practices.
“Large groups like the Permanente Medical Group are not partnerships,” Dr. Pearl says. “They tend to be a corporation with a board of directors, and all the physicians are employees, but it’s a physician-led organization.”
Doctors in these groups can enjoy a great deal of control. While Permanente Medical Group is exclusively affiliated with Kaiser, which runs hospitals and an HMO, the group is an independent corporation run by its doctors, who are both shareholders and employees, Dr. Pearl says.
The Cleveland Clinic and Mayo Clinic are not medical groups in the strict sense of the word. They describe themselves as academic medical centers, but Dr. Pearl says, “Doctors have a tremendous amount of control there, particularly those in the most remunerative specialties.”
Pros of large groups
Group practices are able to focus more on the physician participants’ needs and priorities, says Mr. Mertz. “In a hospital-based organization, physicians’ needs have to compete with the needs of the hospital. … In a large group, it can be easier to get policies changed and order equipment.”
However, for many physicians, their primary reason for joining a large group is having negotiating leverage with health insurance plans, and this leverage seems even more important today. It typically results in higher reimbursements, which could translate into higher pay. The higher practice income, however, could be negated by higher administrative overhead, which is endemic in large organizations.
Mr. Mertz says large groups also have the resources to recruit new doctors. Small practices, in contrast, often decide not to grow. The practice would at first need to guarantee the salary of a new partner, which could require existing partners to take a pay cut, which they often don’t want to do. “They’ll decide to ride the practice into the ground,” which means closing it down when they retire, he says.
Cons of large groups
One individual doctor may have relatively little input in decision-making in a large group, and strong leadership may be lacking. One study examining the pros and cons of large group practices found that lack of physician cooperation, investment, and leadership were the most frequently cited barriers in large groups.
Physicians in large groups can also divide into competing factions. Mr. Mertz says rifts are more likely to take place in multispecialty groups, where higher-reimbursed proceduralists resent having to financially support lower-reimbursed primary care physicians. But it’s rare that such rifts actually break up the practice, he says.
Private practice vs. employment
Even as more physicians enter large groups, physicians continue to flee private practice in general. In 2020, the AMA found that the number of physicians in private practices had dropped nearly 5 percentage points since 2018, the largest 2-year drop recorded by the AMA.
The hardest hit are small groups of 10 physicians or fewer, once the backbone of U.S. medicine. A 2020 survey found that 53.7% of physicians still work in small practices of 10 or fewer physicians, compared with 61.4% in 2012.
Private practices tend to be partnerships, but younger physicians, for their part, often don’t want to become a partner. In a 2016 survey, only 22% of medical residents surveyed said they anticipate owning a stake in a practice someday.
What’s good about private practice?
The obvious advantage of private practice is having control. Physician-owners can choose staff, oversee finances, and decide on the direction the practice should take. They don’t have to worry about being fired, because the partnership agreement virtually guarantees each doctor’s place in the group.
The atmosphere in a small practice is often more relaxed. “Private practices tend to offer a family-like environment,” Mr. Miller says. Owners of small practices tend to have lower burnout than large practices, a 2018 study found.
Unlike hospital-employed doctors, private practitioners get to keep their ancillary income. “Physicians own the equipment and receive income generated from ancillary services, not just professional fees,” says Mr. Miller.
What’s negative about private practice?
Since small groups have little negotiating power with private payers, they can’t get favorable reimbursement rates. And while partners are protected from being fired, the practice could still go bankrupt.
Running a private practice means putting on an entrepreneurial hat. To develop a strong practice, you need to learn about marketing, finance, IT, contract negotiations, and facility management. “Most young doctors have no interest in this work,” Mr. Mertz says.
Value-based contracting has added another disadvantage for small practices. “It can be harder for small, independent groups to compete,” says Mike Belkin, JD, a divisional vice president at Merritt Hawkins, a physician recruitment company based in Dallas. “They don’t have the data and integration of services that are necessary for this.”
Employment in hospital systems
More than one-third of all physicians worked for hospitals in 2018, and hospitals’ share has been growing since then. In 2020, for the first time, the AMA found that more than half of all physicians were employed, and employment is mainly a hospital phenomenon.
The trend shows no signs of stopping. In 2019 and 2020, hospitals and other corporate entities acquired 20,900 physician practices, representing 29,800 doctors. “This trend will continue,” Dr. Pearl says. “The bigger will get bigger. It’s all about market control. Everyone wants to be wider, more vertical, and more powerful.”
Pros of hospital employment
“The advantages of hospital employment are mostly financial,” Mr. Mertz says. Unlike a private practice, “there’s no financial risk to hospital employment because you don’t own it. You won’t be on the tab for any losses.”
“Hospitals usually offer a highly competitive salary with less emphasis on production than in a private practice,” he says. New physicians are typically paid a guaranteed salary in the first 1-3 years of employment.
“You don’t have any management responsibilities, as you would in a practice,” Mr. Mertz says. “The hospital has a professional management team to handle the business side. Most young doctors have no interest in this work.”
“Employed physicians have a built-in referral network at a hospital,” Mr. Miller says. This is especially an advantage for new physicians, who don’t yet have a referral network of their own.”
Cons of hospital employment
Physicians employed by a hospital lack control. “You don’t decide the hours you work, the schedules you follow, and the physical facility you work in, and, for the most part, you don’t pick your staff,” Mr. Mertz says.
Like any big organization, hospitals are bureaucratic. “If you want to purchase a new piece of equipment, your request goes up the chain of command,” Mr. Mertz says. “Your purchase has to fit into the budget.” (This can be the case with large groups, too.)
Many employed doctors chafe under this lack of control. In an earlier survey by Medscape, 45% of employed respondents didn’t like having limited influence in decision-making, and 32% said they had less control over their work or schedule.
It’s no wonder that a large percentage of physicians would rather work in practices than hospitals. According to a 2021 Medicus Firm survey, 23% of physicians are interested in working in hospitals, while 40% would rather work either in multispecialty or single-specialty groups, Mr. Miller reports.
Doctors have differing views of hospital employment
New physicians are apt to dismiss any negatives about hospitals. “Lack of autonomy often matters less to younger physicians, who were trained in team-based models,” Mr. Belkin says.
Many young doctors actually like working in a large organization. “Young doctors out of residency are used to having everything at their fingertips – labs and testing is in-house,” Mr. Mertz says.
On the other hand, doctors who were previously self-employed – a group that makes up almost one-third of all hospital-employed doctors – can often be dissatisfied with employment. In a 2014 Medscape survey, 26% of previously self-employed doctors said job satisfaction had not improved with employment.
Mr. Mertz says these doctors remember what it was like to be in charge of a practice. “If you once owned a practice, you can always compare what’s going on now with that experience, and that can make you frustrated.”
Hospitals have higher turnover
It’s much easier to leave an organization when you don’t have an ownership stake. The annual physician turnover rate at hospitals is 28%, compared with 7% at medical groups, according to a 2019 report.
Mr. Belkin says changing jobs has become a way of life for many doctors. “Staying at a job for only a few years is no longer a red flag,” he says. “Physicians are exploring different options. They might try group practice and switch to hospitals or vice versa.”
Physicians are now part of a high-turnover culture: Once in a new job, many are already thinking about the next one. A 2018 survey found that 46% of doctors planned to leave their position within 3 years.
Private equity ownership of practice
Selling majority control of your practice to a private equity firm is a relatively new phenomenon and accounts for a small share of physicians – just 4% in 2020. This trend was originally limited to certain specialties, such as anesthesiology, emergency medicine, and dermatology, but now many others are courted.
The deals work like this: Physicians sell majority control of their practice to investors in return for shares in the private equity practice, and they become employees of that practice. The private equity firm then adds more physicians to the practice and invests in infrastructure with the intention of selling the practice at a large profit, which is then shared with the original physicians.
Pros of private equity
The original owners of the practice stand to make a substantial profit if they are willing to wait several years for the practice to be built up and sold. “If they are patient, they could earn a bonanza,” Mr. Belkin says.
Private equity investment helps the practice expand. “It’s an alternative to going to the bank and borrowing money,” Mr. Mertz says.
Cons of private equity
Physicians lose control of their practice. A client of Mr. Mertz’s briefly considered a private equity offer and turned it down. “The private equity firm would have veto power over what the doctors wanted to do,” he says.
Mr. Belkin says the selling physicians typically lose income after the sale. “Money they earned from ancillary services now goes to the practice,” Mr. Belkin says. The selling doctors could potentially take up to a 30% cut in their compensation, according to Coker Capital Advisors.
A version of this article first appeared on Medscape.com.
AMA president calls on Congress to stabilize Medicare payments to physicians
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Online reviews most important factor in choosing a doctor: Survey
from Press Ganey, a provider of patient satisfaction surveys. According to the data, this online information is more important to consumers in selecting a physician than another doctor’s referral and is more than twice as important when choosing a primary care physician.
In fact, 83% of respondents said they went online to read reviews of a physician after receiving a referral from another provider.
The online research trend reflects not only the increased familiarity of all generations with the internet but also the growing consumerization of health care, Thomas Jeffrey, president of the Sullivan/Luallin Group, a patient experience consulting firm, told this news organization.
“According to patient satisfaction surveys, people are becoming health care consumers more than in the past,” he noted. “Historically, we didn’t look at health care as a consumer product. But, with high deductibles and copays, doctor visits can represent a pretty significant out-of-pocket expense. As it begins to hit folks’ pocketbooks, they become more savvy shoppers.”
Digital preferences for providers were gaining “positive momentum” even before the COVID-19 pandemic, but the crisis “drove upticks in some consumer digital behaviors,” the Press Ganey report pointed out.
Mr. Jeffrey agreed, noting that this finding matches what Sullivan/Luallin has discovered in its research. “I think the pandemic pushed people to engage more online,” he said. “The highest net promoter score [likelihood to recommend in market surveys] for a pharmacy is the Amazon pharmacy, which is an online-based delivery service. Then you have telehealth visits, which are more convenient in many ways.”
How patients search online
In choosing a new primary care doctor, 51.1% go on the web first, 23.8% seek a referral from another health care provider, and 4.4% get information from an insurer or a benefits manager, according to the survey.
The factors that matter most to consumers when they pick any provider, in order, are online ratings and reviews of the physician, referral from a current doctor, ratings and reviews of the facility, and the quality and completeness of a doctor’s profile on a website or online directory. The doctor’s online presence and the quality of their website are also important.
According to Press Ganey, search engines like Google are the most used digital resources, with 65.4% of consumers employing them to find a doctor. However, consumers now use an average of 2.7 sites in their search. The leading destinations are a hospital or a clinic site, WebMD, Healthgrades, and Facebook. (This news organization is owned by WebMD.)
Compared with 2019, the report said, there has been a 22.8% decline in the use of search engines for seeking a doctor and a 53.7% increase in the use of health care review sites such as Healthgrades and Vitals.
When reading provider reviews, consumers look for more recent reviews and want the reviews to be “authentic and informative.” They also value the star ratings. About 84%of respondents said they wouldn’t book an appointment with a referred provider that had a rating of less than four stars.
Overall, the top reasons why people are deterred from making an appointment are difficulty contacting the office, the poor quality of online reviews, and an average online rating of less than four stars.
The vast majority of respondents (77%) said they believe internet reviews reflect their own experience with a provider organization, and only 2.6% said the reviews were inaccurate. Another finding of the survey indicates that this attention of patients to reviews of their own provider doesn’t represent idle curiosity: About 57% of Baby Boomers and 45% of millennials/Gen Z’ers said they’d written online reviews of a doctor or a hospital.
Factors in patient loyalty
The Press Ganey survey asked which of several factors, besides excellent care, patients weighed when giving a five-star review to a health care provider.
Quality of customer service was rated first by 70.8% of respondents, followed by cleanliness of facilities (67.5%), communication (63.4%), the provider’s bedside manner (63%), ease of appointment booking (58.8%), ease of patient intake/registration (52.3%), quality and accuracy of information (40.1%), availability of telehealth services (21.7%), and waiting room amenities (21.8%).
The report explained that “quality of customer service” means “demeanor, attentiveness, and helpfulness of staff and practitioners.” “Communication” refers to things like follow-up appointment reminders and annual checkup reminders.
According to Mr. Jeffrey, these factors were considered more important than a doctor’s bedside manner because of the team care approach in most physician offices. “We see a lot more folks derive their notion of quality from continuity of care. And if they feel the physician they love is being supported by a less than competent team, that can impact significantly their sense of the quality of care,” he said.
Online appointment booking is a must
To win over the online consumer, Press Ganey emphasized, practices should ensure that provider listings are accurate and complete. In addition, offering online appointment booking can avoid the top challenge in making a new appointment, which is getting through to the office.
Mr. Jeffrey concurred, although he notes that practices have to be careful about how they enable patients to select appointment slots online. He suggests that an appointment request form on a patient portal first ask what the purpose of the visit is and that it offer five or so options. If the request fits into a routine visit category, the provider’s calendar pops up and the patient can select a convenient time slot. If it’s something else, an appointment scheduler calls the patient back.
“There needs to be greater access to standard appointments online,” he said. “While privacy is an issue, you can use the patient portal that most EHRs have to provide online booking. If you want to succeed going forward, that’s going to be a major plus.”
Of course, to do any of this, including reading provider reviews, a consumer needs a good internet connection and a mobile or desktop device. While broadband internet access is still not available in some communities, the breakdown of the survey respondents by demographics shows that low-income people were included.
Mr. Jeffrey doesn’t believe that a lack of internet access or digital devices prevents many Americans from going online today. “Even in poor communities, most people have internet access through their smartphones. Even baby boomers are familiar with smartphones. I haven’t seen internet access be a big barrier for low-income households, because they all have access to phones.”
A version of this article first appeared on Medscape.com.
from Press Ganey, a provider of patient satisfaction surveys. According to the data, this online information is more important to consumers in selecting a physician than another doctor’s referral and is more than twice as important when choosing a primary care physician.
In fact, 83% of respondents said they went online to read reviews of a physician after receiving a referral from another provider.
The online research trend reflects not only the increased familiarity of all generations with the internet but also the growing consumerization of health care, Thomas Jeffrey, president of the Sullivan/Luallin Group, a patient experience consulting firm, told this news organization.
“According to patient satisfaction surveys, people are becoming health care consumers more than in the past,” he noted. “Historically, we didn’t look at health care as a consumer product. But, with high deductibles and copays, doctor visits can represent a pretty significant out-of-pocket expense. As it begins to hit folks’ pocketbooks, they become more savvy shoppers.”
Digital preferences for providers were gaining “positive momentum” even before the COVID-19 pandemic, but the crisis “drove upticks in some consumer digital behaviors,” the Press Ganey report pointed out.
Mr. Jeffrey agreed, noting that this finding matches what Sullivan/Luallin has discovered in its research. “I think the pandemic pushed people to engage more online,” he said. “The highest net promoter score [likelihood to recommend in market surveys] for a pharmacy is the Amazon pharmacy, which is an online-based delivery service. Then you have telehealth visits, which are more convenient in many ways.”
How patients search online
In choosing a new primary care doctor, 51.1% go on the web first, 23.8% seek a referral from another health care provider, and 4.4% get information from an insurer or a benefits manager, according to the survey.
The factors that matter most to consumers when they pick any provider, in order, are online ratings and reviews of the physician, referral from a current doctor, ratings and reviews of the facility, and the quality and completeness of a doctor’s profile on a website or online directory. The doctor’s online presence and the quality of their website are also important.
According to Press Ganey, search engines like Google are the most used digital resources, with 65.4% of consumers employing them to find a doctor. However, consumers now use an average of 2.7 sites in their search. The leading destinations are a hospital or a clinic site, WebMD, Healthgrades, and Facebook. (This news organization is owned by WebMD.)
Compared with 2019, the report said, there has been a 22.8% decline in the use of search engines for seeking a doctor and a 53.7% increase in the use of health care review sites such as Healthgrades and Vitals.
When reading provider reviews, consumers look for more recent reviews and want the reviews to be “authentic and informative.” They also value the star ratings. About 84%of respondents said they wouldn’t book an appointment with a referred provider that had a rating of less than four stars.
Overall, the top reasons why people are deterred from making an appointment are difficulty contacting the office, the poor quality of online reviews, and an average online rating of less than four stars.
The vast majority of respondents (77%) said they believe internet reviews reflect their own experience with a provider organization, and only 2.6% said the reviews were inaccurate. Another finding of the survey indicates that this attention of patients to reviews of their own provider doesn’t represent idle curiosity: About 57% of Baby Boomers and 45% of millennials/Gen Z’ers said they’d written online reviews of a doctor or a hospital.
Factors in patient loyalty
The Press Ganey survey asked which of several factors, besides excellent care, patients weighed when giving a five-star review to a health care provider.
Quality of customer service was rated first by 70.8% of respondents, followed by cleanliness of facilities (67.5%), communication (63.4%), the provider’s bedside manner (63%), ease of appointment booking (58.8%), ease of patient intake/registration (52.3%), quality and accuracy of information (40.1%), availability of telehealth services (21.7%), and waiting room amenities (21.8%).
The report explained that “quality of customer service” means “demeanor, attentiveness, and helpfulness of staff and practitioners.” “Communication” refers to things like follow-up appointment reminders and annual checkup reminders.
According to Mr. Jeffrey, these factors were considered more important than a doctor’s bedside manner because of the team care approach in most physician offices. “We see a lot more folks derive their notion of quality from continuity of care. And if they feel the physician they love is being supported by a less than competent team, that can impact significantly their sense of the quality of care,” he said.
Online appointment booking is a must
To win over the online consumer, Press Ganey emphasized, practices should ensure that provider listings are accurate and complete. In addition, offering online appointment booking can avoid the top challenge in making a new appointment, which is getting through to the office.
Mr. Jeffrey concurred, although he notes that practices have to be careful about how they enable patients to select appointment slots online. He suggests that an appointment request form on a patient portal first ask what the purpose of the visit is and that it offer five or so options. If the request fits into a routine visit category, the provider’s calendar pops up and the patient can select a convenient time slot. If it’s something else, an appointment scheduler calls the patient back.
“There needs to be greater access to standard appointments online,” he said. “While privacy is an issue, you can use the patient portal that most EHRs have to provide online booking. If you want to succeed going forward, that’s going to be a major plus.”
Of course, to do any of this, including reading provider reviews, a consumer needs a good internet connection and a mobile or desktop device. While broadband internet access is still not available in some communities, the breakdown of the survey respondents by demographics shows that low-income people were included.
Mr. Jeffrey doesn’t believe that a lack of internet access or digital devices prevents many Americans from going online today. “Even in poor communities, most people have internet access through their smartphones. Even baby boomers are familiar with smartphones. I haven’t seen internet access be a big barrier for low-income households, because they all have access to phones.”
A version of this article first appeared on Medscape.com.
from Press Ganey, a provider of patient satisfaction surveys. According to the data, this online information is more important to consumers in selecting a physician than another doctor’s referral and is more than twice as important when choosing a primary care physician.
In fact, 83% of respondents said they went online to read reviews of a physician after receiving a referral from another provider.
The online research trend reflects not only the increased familiarity of all generations with the internet but also the growing consumerization of health care, Thomas Jeffrey, president of the Sullivan/Luallin Group, a patient experience consulting firm, told this news organization.
“According to patient satisfaction surveys, people are becoming health care consumers more than in the past,” he noted. “Historically, we didn’t look at health care as a consumer product. But, with high deductibles and copays, doctor visits can represent a pretty significant out-of-pocket expense. As it begins to hit folks’ pocketbooks, they become more savvy shoppers.”
Digital preferences for providers were gaining “positive momentum” even before the COVID-19 pandemic, but the crisis “drove upticks in some consumer digital behaviors,” the Press Ganey report pointed out.
Mr. Jeffrey agreed, noting that this finding matches what Sullivan/Luallin has discovered in its research. “I think the pandemic pushed people to engage more online,” he said. “The highest net promoter score [likelihood to recommend in market surveys] for a pharmacy is the Amazon pharmacy, which is an online-based delivery service. Then you have telehealth visits, which are more convenient in many ways.”
How patients search online
In choosing a new primary care doctor, 51.1% go on the web first, 23.8% seek a referral from another health care provider, and 4.4% get information from an insurer or a benefits manager, according to the survey.
The factors that matter most to consumers when they pick any provider, in order, are online ratings and reviews of the physician, referral from a current doctor, ratings and reviews of the facility, and the quality and completeness of a doctor’s profile on a website or online directory. The doctor’s online presence and the quality of their website are also important.
According to Press Ganey, search engines like Google are the most used digital resources, with 65.4% of consumers employing them to find a doctor. However, consumers now use an average of 2.7 sites in their search. The leading destinations are a hospital or a clinic site, WebMD, Healthgrades, and Facebook. (This news organization is owned by WebMD.)
Compared with 2019, the report said, there has been a 22.8% decline in the use of search engines for seeking a doctor and a 53.7% increase in the use of health care review sites such as Healthgrades and Vitals.
When reading provider reviews, consumers look for more recent reviews and want the reviews to be “authentic and informative.” They also value the star ratings. About 84%of respondents said they wouldn’t book an appointment with a referred provider that had a rating of less than four stars.
Overall, the top reasons why people are deterred from making an appointment are difficulty contacting the office, the poor quality of online reviews, and an average online rating of less than four stars.
The vast majority of respondents (77%) said they believe internet reviews reflect their own experience with a provider organization, and only 2.6% said the reviews were inaccurate. Another finding of the survey indicates that this attention of patients to reviews of their own provider doesn’t represent idle curiosity: About 57% of Baby Boomers and 45% of millennials/Gen Z’ers said they’d written online reviews of a doctor or a hospital.
Factors in patient loyalty
The Press Ganey survey asked which of several factors, besides excellent care, patients weighed when giving a five-star review to a health care provider.
Quality of customer service was rated first by 70.8% of respondents, followed by cleanliness of facilities (67.5%), communication (63.4%), the provider’s bedside manner (63%), ease of appointment booking (58.8%), ease of patient intake/registration (52.3%), quality and accuracy of information (40.1%), availability of telehealth services (21.7%), and waiting room amenities (21.8%).
The report explained that “quality of customer service” means “demeanor, attentiveness, and helpfulness of staff and practitioners.” “Communication” refers to things like follow-up appointment reminders and annual checkup reminders.
According to Mr. Jeffrey, these factors were considered more important than a doctor’s bedside manner because of the team care approach in most physician offices. “We see a lot more folks derive their notion of quality from continuity of care. And if they feel the physician they love is being supported by a less than competent team, that can impact significantly their sense of the quality of care,” he said.
Online appointment booking is a must
To win over the online consumer, Press Ganey emphasized, practices should ensure that provider listings are accurate and complete. In addition, offering online appointment booking can avoid the top challenge in making a new appointment, which is getting through to the office.
Mr. Jeffrey concurred, although he notes that practices have to be careful about how they enable patients to select appointment slots online. He suggests that an appointment request form on a patient portal first ask what the purpose of the visit is and that it offer five or so options. If the request fits into a routine visit category, the provider’s calendar pops up and the patient can select a convenient time slot. If it’s something else, an appointment scheduler calls the patient back.
“There needs to be greater access to standard appointments online,” he said. “While privacy is an issue, you can use the patient portal that most EHRs have to provide online booking. If you want to succeed going forward, that’s going to be a major plus.”
Of course, to do any of this, including reading provider reviews, a consumer needs a good internet connection and a mobile or desktop device. While broadband internet access is still not available in some communities, the breakdown of the survey respondents by demographics shows that low-income people were included.
Mr. Jeffrey doesn’t believe that a lack of internet access or digital devices prevents many Americans from going online today. “Even in poor communities, most people have internet access through their smartphones. Even baby boomers are familiar with smartphones. I haven’t seen internet access be a big barrier for low-income households, because they all have access to phones.”
A version of this article first appeared on Medscape.com.