User login
Care via video teleconferencing can be as effective as in-person for some conditions
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
FROM ANNALS OF INTERNAL MEDICINE
Characterizing Counterfeit Dermatologic Devices Sold on Popular E-commerce Websites
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
Practice Points
- Among thousands of counterfeit dermatologic listings, there is great heterogeneity in the number of listings per different subtypes of dermatologic devices, device descriptions, and unit pricing, along with false claims of US Food and Drug Administration clearance.
- Given the prevalence of counterfeit medical devices readily available for purchase online, dermatology practitioners should be wary of the authenticity of any medical device purchased for clinical use.
Can Artificial Intelligence Technology Replace Human Scribes?
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
Seven legal risks of promoting unproven COVID-19 treatments
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
Telemedicine, triaging, remote monitoring top list of COVID-era innovations in oncology
When the Winship Cancer Institute at Emory University, Atlanta, faced off against the pandemic in the spring of 2020, it opened a COVID urgent care clinic for Winship oncology patients who had a confirmed or suspected case of COVID, symptoms or a higher risk for the virus. The urgent care clinic, located in a relatively isolated bay of an infusion center, facilitated segregating COVID-suspected patients from other cancer patients while waiting for their polymerase chain reaction test results to show if they were COVID positive.
A strict triage system was also employed to make sure that the right patients were coming in to the new clinic and not those who either could be managed safely at home or were clinically unstable and belonged in the hospital, said Caleb Raine, PA-C, an oncology physician assistant and bone marrow transplant specialist at Winship. Mr. Raine, who manages the COVID urgent care clinic, shared his experience of “innovations worth keeping” from the pandemic for oncology practices during a panel discussion at the Journal of the Advanced Practitioner in Oncology annual conference, held online Oct. 7.
Telephonic triage was conducted by advanced practice providers (APPs) or nurses using an algorithm Mr. Raine developed incorporating COVID exposure with symptoms such as fever or loss of taste or smell. In order to promote consistency in admissions, he made the final decisions about which patients were brought into the clinic for evaluations, services such as supportive care or infusions, or to address cancer symptoms.
Mr. Raine said the triage process helped to enhance communication with other clinical teams at Winship. He hopes to preserve a strict approach to triaging in future program development, including a 14-bed immediate care center, projected to open next spring, building on experience with the COVID urgent care center. It will offer services similar to a day hospital for cancer patients but be open 24 hours with more capabilities than urgent care. It will target those with emergent needs or who otherwise might require a trip to the ED and provide care for those recently discharged from the hospital in need of follow-up.
Remote monitoring
Another conference speaker, Aaron Begue, MS, RN, CNP, vice president for advanced practice providers at Memorial Sloan Kettering Cancer Center in New York, described a pandemic telemedicine intervention for cancer patients implemented by MSKCC during the pandemic. Prior to in-person contact with the care team, patients were asked to complete a questionnaire on their symptoms using MSKCC’s secure online patient portal, MyMSK.
If symptom alerts reached a critical, color-coded threshold, it triggered a nurse or APP from MSKCC to contact the patient at home, typically by phone. APPs also did remote monitoring, including uploaded data from portable home pulse oximeters. A similar symptom tracker was later adapted for monitoring cancer symptoms.
Some APPs took turns working from their own home collecting data needed for inpatient visits and uploading it into the medical record. This helped to deploy clinical teams more efficiently and accommodate some staff who were at high risk of infection because of existing health conditions or quarantined for positive test results.
“We were able to flex our staffing,” Mr. Begue said. Even spending a day staffing a vaccination clinic could provide a break from the intensity of COVID care on the front lines. “All of us are still trying to figure out how to manage staff stress and burnout,” he added, but flexible scheduling seems to be an important strategy.
Early on, things like the crowds coming out in the evening to cheer for New York’s health care workers had a big impact for staff, showing the community’s support. “Later, when public schools were shut down, we worked with two of them to use their outdoor play areas for staff respite – places to sit down outside undisturbed and relax,” he said.
At the height of the COVID surge in New York, telemedicine was an essential component of care, but when it started to recede, Mr. Begue found that a lot of patients wanted in-person visits again. “We had assumed that telemedicine would be the wave of the future and cancer patients would love it,” he said. “We still do thousands of telemedicine visits, but they are no longer the majority.”
MSKCC also does remote telemonitoring visits with patients who live in other states but want to come to New York for surgeries or other procedures or yearly checkups at the hospital. But the logistical headaches of practicing telemedicine across state lines include trying to reconcile varying requirements for medical licensing.
Mr. Begue hopes in the future that some of these state requirements could be relaxed, which might also make it easier to enroll more people from across the country in clinical trials and encourage more collaboration between cancer centers.
“COVID taught us we have to be more forward thinking and prepared for crises,” Mr. Raine said. “In the future we need to be ready for when – not if – the next crisis comes along – although we’re not out of this one yet.”
Mr. Raine and Mr. Begue did not report any disclosures.
When the Winship Cancer Institute at Emory University, Atlanta, faced off against the pandemic in the spring of 2020, it opened a COVID urgent care clinic for Winship oncology patients who had a confirmed or suspected case of COVID, symptoms or a higher risk for the virus. The urgent care clinic, located in a relatively isolated bay of an infusion center, facilitated segregating COVID-suspected patients from other cancer patients while waiting for their polymerase chain reaction test results to show if they were COVID positive.
A strict triage system was also employed to make sure that the right patients were coming in to the new clinic and not those who either could be managed safely at home or were clinically unstable and belonged in the hospital, said Caleb Raine, PA-C, an oncology physician assistant and bone marrow transplant specialist at Winship. Mr. Raine, who manages the COVID urgent care clinic, shared his experience of “innovations worth keeping” from the pandemic for oncology practices during a panel discussion at the Journal of the Advanced Practitioner in Oncology annual conference, held online Oct. 7.
Telephonic triage was conducted by advanced practice providers (APPs) or nurses using an algorithm Mr. Raine developed incorporating COVID exposure with symptoms such as fever or loss of taste or smell. In order to promote consistency in admissions, he made the final decisions about which patients were brought into the clinic for evaluations, services such as supportive care or infusions, or to address cancer symptoms.
Mr. Raine said the triage process helped to enhance communication with other clinical teams at Winship. He hopes to preserve a strict approach to triaging in future program development, including a 14-bed immediate care center, projected to open next spring, building on experience with the COVID urgent care center. It will offer services similar to a day hospital for cancer patients but be open 24 hours with more capabilities than urgent care. It will target those with emergent needs or who otherwise might require a trip to the ED and provide care for those recently discharged from the hospital in need of follow-up.
Remote monitoring
Another conference speaker, Aaron Begue, MS, RN, CNP, vice president for advanced practice providers at Memorial Sloan Kettering Cancer Center in New York, described a pandemic telemedicine intervention for cancer patients implemented by MSKCC during the pandemic. Prior to in-person contact with the care team, patients were asked to complete a questionnaire on their symptoms using MSKCC’s secure online patient portal, MyMSK.
If symptom alerts reached a critical, color-coded threshold, it triggered a nurse or APP from MSKCC to contact the patient at home, typically by phone. APPs also did remote monitoring, including uploaded data from portable home pulse oximeters. A similar symptom tracker was later adapted for monitoring cancer symptoms.
Some APPs took turns working from their own home collecting data needed for inpatient visits and uploading it into the medical record. This helped to deploy clinical teams more efficiently and accommodate some staff who were at high risk of infection because of existing health conditions or quarantined for positive test results.
“We were able to flex our staffing,” Mr. Begue said. Even spending a day staffing a vaccination clinic could provide a break from the intensity of COVID care on the front lines. “All of us are still trying to figure out how to manage staff stress and burnout,” he added, but flexible scheduling seems to be an important strategy.
Early on, things like the crowds coming out in the evening to cheer for New York’s health care workers had a big impact for staff, showing the community’s support. “Later, when public schools were shut down, we worked with two of them to use their outdoor play areas for staff respite – places to sit down outside undisturbed and relax,” he said.
At the height of the COVID surge in New York, telemedicine was an essential component of care, but when it started to recede, Mr. Begue found that a lot of patients wanted in-person visits again. “We had assumed that telemedicine would be the wave of the future and cancer patients would love it,” he said. “We still do thousands of telemedicine visits, but they are no longer the majority.”
MSKCC also does remote telemonitoring visits with patients who live in other states but want to come to New York for surgeries or other procedures or yearly checkups at the hospital. But the logistical headaches of practicing telemedicine across state lines include trying to reconcile varying requirements for medical licensing.
Mr. Begue hopes in the future that some of these state requirements could be relaxed, which might also make it easier to enroll more people from across the country in clinical trials and encourage more collaboration between cancer centers.
“COVID taught us we have to be more forward thinking and prepared for crises,” Mr. Raine said. “In the future we need to be ready for when – not if – the next crisis comes along – although we’re not out of this one yet.”
Mr. Raine and Mr. Begue did not report any disclosures.
When the Winship Cancer Institute at Emory University, Atlanta, faced off against the pandemic in the spring of 2020, it opened a COVID urgent care clinic for Winship oncology patients who had a confirmed or suspected case of COVID, symptoms or a higher risk for the virus. The urgent care clinic, located in a relatively isolated bay of an infusion center, facilitated segregating COVID-suspected patients from other cancer patients while waiting for their polymerase chain reaction test results to show if they were COVID positive.
A strict triage system was also employed to make sure that the right patients were coming in to the new clinic and not those who either could be managed safely at home or were clinically unstable and belonged in the hospital, said Caleb Raine, PA-C, an oncology physician assistant and bone marrow transplant specialist at Winship. Mr. Raine, who manages the COVID urgent care clinic, shared his experience of “innovations worth keeping” from the pandemic for oncology practices during a panel discussion at the Journal of the Advanced Practitioner in Oncology annual conference, held online Oct. 7.
Telephonic triage was conducted by advanced practice providers (APPs) or nurses using an algorithm Mr. Raine developed incorporating COVID exposure with symptoms such as fever or loss of taste or smell. In order to promote consistency in admissions, he made the final decisions about which patients were brought into the clinic for evaluations, services such as supportive care or infusions, or to address cancer symptoms.
Mr. Raine said the triage process helped to enhance communication with other clinical teams at Winship. He hopes to preserve a strict approach to triaging in future program development, including a 14-bed immediate care center, projected to open next spring, building on experience with the COVID urgent care center. It will offer services similar to a day hospital for cancer patients but be open 24 hours with more capabilities than urgent care. It will target those with emergent needs or who otherwise might require a trip to the ED and provide care for those recently discharged from the hospital in need of follow-up.
Remote monitoring
Another conference speaker, Aaron Begue, MS, RN, CNP, vice president for advanced practice providers at Memorial Sloan Kettering Cancer Center in New York, described a pandemic telemedicine intervention for cancer patients implemented by MSKCC during the pandemic. Prior to in-person contact with the care team, patients were asked to complete a questionnaire on their symptoms using MSKCC’s secure online patient portal, MyMSK.
If symptom alerts reached a critical, color-coded threshold, it triggered a nurse or APP from MSKCC to contact the patient at home, typically by phone. APPs also did remote monitoring, including uploaded data from portable home pulse oximeters. A similar symptom tracker was later adapted for monitoring cancer symptoms.
Some APPs took turns working from their own home collecting data needed for inpatient visits and uploading it into the medical record. This helped to deploy clinical teams more efficiently and accommodate some staff who were at high risk of infection because of existing health conditions or quarantined for positive test results.
“We were able to flex our staffing,” Mr. Begue said. Even spending a day staffing a vaccination clinic could provide a break from the intensity of COVID care on the front lines. “All of us are still trying to figure out how to manage staff stress and burnout,” he added, but flexible scheduling seems to be an important strategy.
Early on, things like the crowds coming out in the evening to cheer for New York’s health care workers had a big impact for staff, showing the community’s support. “Later, when public schools were shut down, we worked with two of them to use their outdoor play areas for staff respite – places to sit down outside undisturbed and relax,” he said.
At the height of the COVID surge in New York, telemedicine was an essential component of care, but when it started to recede, Mr. Begue found that a lot of patients wanted in-person visits again. “We had assumed that telemedicine would be the wave of the future and cancer patients would love it,” he said. “We still do thousands of telemedicine visits, but they are no longer the majority.”
MSKCC also does remote telemonitoring visits with patients who live in other states but want to come to New York for surgeries or other procedures or yearly checkups at the hospital. But the logistical headaches of practicing telemedicine across state lines include trying to reconcile varying requirements for medical licensing.
Mr. Begue hopes in the future that some of these state requirements could be relaxed, which might also make it easier to enroll more people from across the country in clinical trials and encourage more collaboration between cancer centers.
“COVID taught us we have to be more forward thinking and prepared for crises,” Mr. Raine said. “In the future we need to be ready for when – not if – the next crisis comes along – although we’re not out of this one yet.”
Mr. Raine and Mr. Begue did not report any disclosures.
FROM JADPRO 2021
Successful COVID-19 Surge Management With Monoclonal Antibody Infusion in Emergency Department Patients
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
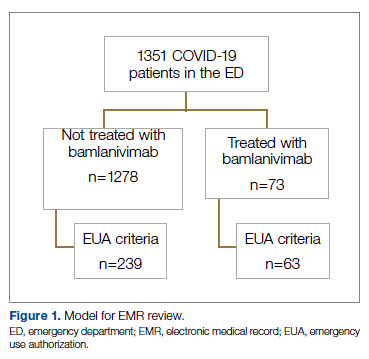
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
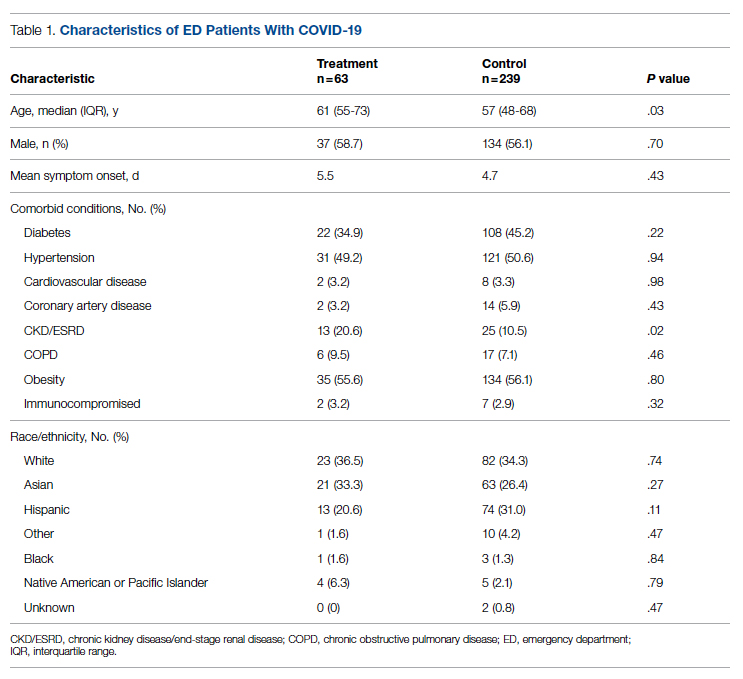
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
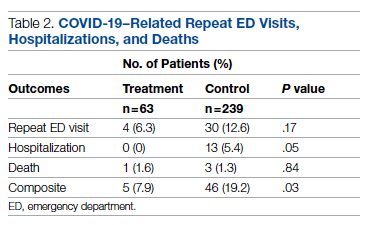
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
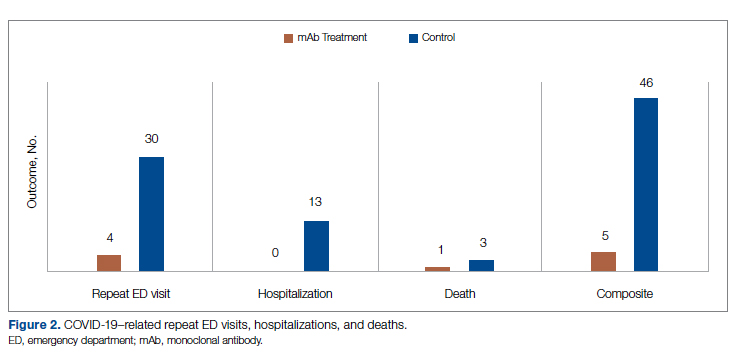
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
Finding healthcare ‘soul-destroying,’ some turn to online sex work
In March 2021, Prime Minister Boris Johnson proposed a 1% pay rise for National Health Service (NHS) workers in the United Kingdom — a move many deemed inadequate after a full year of fighting the COVID-19 pandemic. The next day, James Cowe, a 23-year-old healthcare assistant who had been working in dementia care for 6 years, decided to create a profile on the content subscription site OnlyFans.
The London-based site allows subscribers, or “fans,” to request content, making its name distributing nude pictures, videos, and other sexually explicit content. It garnered mainstream attention in 2020 when housebound individuals and even celebrities began using it to generate income. Back in August, OnlyFans released a statement stating that it would ban “sexually explicit” content beginning in October. Days later, the company recanted the statement after uproar from creators.
“Because of the one-percent pay rise, I’ve started OnlyFans and I’m making more money in three days than I make in a month at work,” Mr. Cowe said in a now-deleted TikTok post. “Sorry Boris, but I’m done with healthcare and now I’m an online whore.”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans.
Stories like his have multiplied during the pandemic, at a time when healthcare professionals have been particularly overworked and particularly essential. Meanwhile, the pandemic has exacerbated challenges for many sex workers across the globe.
“[There have been] many, many reports over history that transactional sex is used as a sort of emergency livelihood strategy in all kinds of emergencies,” says Joanne Csete, PhD, associate professor of population and family health at Columbia University, New York, “and I suppose this is an emergency in that sense, like any other.”
The relationship between sex work and healthcare
A 2015 study by Leeds University found that 70% of sex workers in the United Kingdom previously worked in healthcare, charities, or education and that more than a third held university degrees.
The relationship between sex workers and healthcare workers has historically been disconnected. Sex workers are at higher risk of experiencing violence, sexually transmitted infections, and substance abuse and mental health problems than the general population, as noted by the American College of Obstetricians and Gynecologists. But according to the UN Population Fund, 63% of sex workers will not seek health services alone because they are distrustful and fearful of healthcare workers. A 2014 study by UNAIDS found that stigmatization also makes sex workers less likely to seek assistance from social services.
“I think it’s almost universally hard for sex workers to get respectful healthcare without judgment, and in some cases actual hostility, because of the stigma of their work,” Dr. Csete says. “Health workers are not always trained to see sex work as anything but either a criminal act or an immoral act.”
In August 2021, U.K. medical students called for the British Medical Association to protect students from being penalized by or expelled from their universities for engaging in sex work. BMA Medical Students Committee chair Becky Bates cited high medical school fees and a lack of financial support as motivations for student sex workers. She told this news organization that sex work often allows for flexible hours that might make it easier for students to balance the demands of medical school than other part-time jobs would.
At the annual BMA conference in September, two thirds of the association’s doctors voted in favor of the motion, while others criticized it as potential encouragement for students to get involved in sex work. “The motion isn’t about the morality of sex work,” Ms. Bates said. “[It’s] about the fact that it’s happening and what we can do to support students.”
Healthcare workers on OnlyFans
The rising pressures placed on individuals in the health field have coincided with the rise of online platforms that host pornographic content. During the pandemic, professionals worn down by their healthcare work have embraced sites like OnlyFans as lower-risk, lower-stress, and potentially higher-paying additions or alternatives.
“It’s quite exploitative to work for such low pay in harsh conditions,” Mr. Cowe told this news organizaation of his experience as a dementia care assistant. “It’s soul-destroying. You feel like, ‘It doesn’t matter how many hours I work, it doesn’t matter what I do, I’m still going to be in this same financial position.’ ”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans. Within 8 months, he had earned £150,000, or approximately $205,000.
As an emergency medical services (EMS) worker in New York City, 23-year-old Lauren Kwei lifted obese bariatric patients, administered cardiopulmonary resuscitation to unresponsive recipients, and transported elderly patients and children with terminal illnesses to hospice. She earned $25 an hour, which she says was insufficient for life in one of the world’s most expensive cities. So, in addition to her paramedic work, Ms. Kwei posted photos and videos on OnlyFans to help pay for rent and groceries during the pandemic.
Ms. Kwei started her OnlyFans as a means of paying for necessities like rent and groceries, which her wage as an emergency medical services worker couldn’t cover entirely.
In December 2020, Ms. Kwei got a call from a New York Post reporter who informed her he was writing an article outing her OnlyFans side gig. Ms. Kwei immediately deleted her account on the site for fear of being penalized by her employer, SeniorCare.
“Leave her alone,” U.S. Representative Alexandria Ocasio-Cortez wrote on Twitter in response to the New York Post article. “The actual scandalous headline here is ‘Medics in the United States need two jobs to survive.’ ”
The article quoted an anonymous male paramedic who said Ms. Kwei should have been “pulling extra shifts, instead of pulling off [her] clothes” to earn more money. Ms. Kwei says such advice fails to acknowledge the intensity of the job. “Why would I pick up overtime shifts doing manual labor,” she says, “when I could be doing [OnlyFans] from the comfort of my own home?”
The future of the healthcare/sex work relationship
Ms. Kwei is young enough to receive health insurance through her parents, and Mr. Cowe has access to free healthcare through the NHS. But many sex workers — particularly full-service sex workers, who carry out their work in person — have limited access to services such as healthcare and unemployment benefits. Pandemic restrictions have concurrently driven full-service sex work further underground and therefore deepened the health and safety risks associated with its criminalization.
As health workers become increasingly involved in sex work, advocates in both fields are pushing for healthcare systems to involve sex workers.
“Just as we would do with supporting any group, it’s about understanding any specific barriers or specific problems that they’re encountering, and understanding what they think would help, and working together on that solution,” Ms. Bates says of supporting medical students who engage in sex work.
Tlaleng Mofokeng, MD, UN Special Rapporteur on the right to health, says it is crucial for healthcare organizations to partner with sex worker organizations when it comes to planning the resourcing and budgeting of the public health system in order to meet sex workers’ needs. “While we wait for national policy to change and while we wait for decriminalization,” she says, “tangible things can be done to ensure the provision of equitable services that are aligned with the respect of [sex workers’] rights and the restoration of their dignity.”
Today, healthcare professionals can expect to work with classmates, colleagues, and patients who are involved in sex work and who do not fit the socioeconomic stereotypes associated with sex workers. The number of medical students and healthcare workers engaging in sex work is likely to continue to rise as these individuals struggle to find financial and emotional support within the health sector. Ultimately, many health workers and sex workers share a common goal: to be involved in healthcare systems that respect their work and meet their basic needs.
Mr. Cowe doubts he will ever return to the healthcare industry, owing in part to the stigma against sex workers. “I would feel quite unwelcome,” he says. “[The publicity I received] probably made it not possible for me to go back, but even so, I wouldn’t have a desire to because I was just so burnt out in the end.”
Ms. Kwei is taking a break from her EMS work because of the emotional and financial toll it took, but she plans to return in the future. In the meantime, she is back on OnlyFans and advocating for higher wages for EMS workers as a member of the Emergency Medical Services Public Advocacy Council (EMSPAC). “In order to be a good paramedic, my mental health needs to be on point,” she says. “Hopefully down the line, when I decide to pick up EMS [work] again, I can find a job that pays me enough.”
A version of this article first appeared on Medscape.com.
In March 2021, Prime Minister Boris Johnson proposed a 1% pay rise for National Health Service (NHS) workers in the United Kingdom — a move many deemed inadequate after a full year of fighting the COVID-19 pandemic. The next day, James Cowe, a 23-year-old healthcare assistant who had been working in dementia care for 6 years, decided to create a profile on the content subscription site OnlyFans.
The London-based site allows subscribers, or “fans,” to request content, making its name distributing nude pictures, videos, and other sexually explicit content. It garnered mainstream attention in 2020 when housebound individuals and even celebrities began using it to generate income. Back in August, OnlyFans released a statement stating that it would ban “sexually explicit” content beginning in October. Days later, the company recanted the statement after uproar from creators.
“Because of the one-percent pay rise, I’ve started OnlyFans and I’m making more money in three days than I make in a month at work,” Mr. Cowe said in a now-deleted TikTok post. “Sorry Boris, but I’m done with healthcare and now I’m an online whore.”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans.
Stories like his have multiplied during the pandemic, at a time when healthcare professionals have been particularly overworked and particularly essential. Meanwhile, the pandemic has exacerbated challenges for many sex workers across the globe.
“[There have been] many, many reports over history that transactional sex is used as a sort of emergency livelihood strategy in all kinds of emergencies,” says Joanne Csete, PhD, associate professor of population and family health at Columbia University, New York, “and I suppose this is an emergency in that sense, like any other.”
The relationship between sex work and healthcare
A 2015 study by Leeds University found that 70% of sex workers in the United Kingdom previously worked in healthcare, charities, or education and that more than a third held university degrees.
The relationship between sex workers and healthcare workers has historically been disconnected. Sex workers are at higher risk of experiencing violence, sexually transmitted infections, and substance abuse and mental health problems than the general population, as noted by the American College of Obstetricians and Gynecologists. But according to the UN Population Fund, 63% of sex workers will not seek health services alone because they are distrustful and fearful of healthcare workers. A 2014 study by UNAIDS found that stigmatization also makes sex workers less likely to seek assistance from social services.
“I think it’s almost universally hard for sex workers to get respectful healthcare without judgment, and in some cases actual hostility, because of the stigma of their work,” Dr. Csete says. “Health workers are not always trained to see sex work as anything but either a criminal act or an immoral act.”
In August 2021, U.K. medical students called for the British Medical Association to protect students from being penalized by or expelled from their universities for engaging in sex work. BMA Medical Students Committee chair Becky Bates cited high medical school fees and a lack of financial support as motivations for student sex workers. She told this news organization that sex work often allows for flexible hours that might make it easier for students to balance the demands of medical school than other part-time jobs would.
At the annual BMA conference in September, two thirds of the association’s doctors voted in favor of the motion, while others criticized it as potential encouragement for students to get involved in sex work. “The motion isn’t about the morality of sex work,” Ms. Bates said. “[It’s] about the fact that it’s happening and what we can do to support students.”
Healthcare workers on OnlyFans
The rising pressures placed on individuals in the health field have coincided with the rise of online platforms that host pornographic content. During the pandemic, professionals worn down by their healthcare work have embraced sites like OnlyFans as lower-risk, lower-stress, and potentially higher-paying additions or alternatives.
“It’s quite exploitative to work for such low pay in harsh conditions,” Mr. Cowe told this news organizaation of his experience as a dementia care assistant. “It’s soul-destroying. You feel like, ‘It doesn’t matter how many hours I work, it doesn’t matter what I do, I’m still going to be in this same financial position.’ ”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans. Within 8 months, he had earned £150,000, or approximately $205,000.
As an emergency medical services (EMS) worker in New York City, 23-year-old Lauren Kwei lifted obese bariatric patients, administered cardiopulmonary resuscitation to unresponsive recipients, and transported elderly patients and children with terminal illnesses to hospice. She earned $25 an hour, which she says was insufficient for life in one of the world’s most expensive cities. So, in addition to her paramedic work, Ms. Kwei posted photos and videos on OnlyFans to help pay for rent and groceries during the pandemic.
Ms. Kwei started her OnlyFans as a means of paying for necessities like rent and groceries, which her wage as an emergency medical services worker couldn’t cover entirely.
In December 2020, Ms. Kwei got a call from a New York Post reporter who informed her he was writing an article outing her OnlyFans side gig. Ms. Kwei immediately deleted her account on the site for fear of being penalized by her employer, SeniorCare.
“Leave her alone,” U.S. Representative Alexandria Ocasio-Cortez wrote on Twitter in response to the New York Post article. “The actual scandalous headline here is ‘Medics in the United States need two jobs to survive.’ ”
The article quoted an anonymous male paramedic who said Ms. Kwei should have been “pulling extra shifts, instead of pulling off [her] clothes” to earn more money. Ms. Kwei says such advice fails to acknowledge the intensity of the job. “Why would I pick up overtime shifts doing manual labor,” she says, “when I could be doing [OnlyFans] from the comfort of my own home?”
The future of the healthcare/sex work relationship
Ms. Kwei is young enough to receive health insurance through her parents, and Mr. Cowe has access to free healthcare through the NHS. But many sex workers — particularly full-service sex workers, who carry out their work in person — have limited access to services such as healthcare and unemployment benefits. Pandemic restrictions have concurrently driven full-service sex work further underground and therefore deepened the health and safety risks associated with its criminalization.
As health workers become increasingly involved in sex work, advocates in both fields are pushing for healthcare systems to involve sex workers.
“Just as we would do with supporting any group, it’s about understanding any specific barriers or specific problems that they’re encountering, and understanding what they think would help, and working together on that solution,” Ms. Bates says of supporting medical students who engage in sex work.
Tlaleng Mofokeng, MD, UN Special Rapporteur on the right to health, says it is crucial for healthcare organizations to partner with sex worker organizations when it comes to planning the resourcing and budgeting of the public health system in order to meet sex workers’ needs. “While we wait for national policy to change and while we wait for decriminalization,” she says, “tangible things can be done to ensure the provision of equitable services that are aligned with the respect of [sex workers’] rights and the restoration of their dignity.”
Today, healthcare professionals can expect to work with classmates, colleagues, and patients who are involved in sex work and who do not fit the socioeconomic stereotypes associated with sex workers. The number of medical students and healthcare workers engaging in sex work is likely to continue to rise as these individuals struggle to find financial and emotional support within the health sector. Ultimately, many health workers and sex workers share a common goal: to be involved in healthcare systems that respect their work and meet their basic needs.
Mr. Cowe doubts he will ever return to the healthcare industry, owing in part to the stigma against sex workers. “I would feel quite unwelcome,” he says. “[The publicity I received] probably made it not possible for me to go back, but even so, I wouldn’t have a desire to because I was just so burnt out in the end.”
Ms. Kwei is taking a break from her EMS work because of the emotional and financial toll it took, but she plans to return in the future. In the meantime, she is back on OnlyFans and advocating for higher wages for EMS workers as a member of the Emergency Medical Services Public Advocacy Council (EMSPAC). “In order to be a good paramedic, my mental health needs to be on point,” she says. “Hopefully down the line, when I decide to pick up EMS [work] again, I can find a job that pays me enough.”
A version of this article first appeared on Medscape.com.
In March 2021, Prime Minister Boris Johnson proposed a 1% pay rise for National Health Service (NHS) workers in the United Kingdom — a move many deemed inadequate after a full year of fighting the COVID-19 pandemic. The next day, James Cowe, a 23-year-old healthcare assistant who had been working in dementia care for 6 years, decided to create a profile on the content subscription site OnlyFans.
The London-based site allows subscribers, or “fans,” to request content, making its name distributing nude pictures, videos, and other sexually explicit content. It garnered mainstream attention in 2020 when housebound individuals and even celebrities began using it to generate income. Back in August, OnlyFans released a statement stating that it would ban “sexually explicit” content beginning in October. Days later, the company recanted the statement after uproar from creators.
“Because of the one-percent pay rise, I’ve started OnlyFans and I’m making more money in three days than I make in a month at work,” Mr. Cowe said in a now-deleted TikTok post. “Sorry Boris, but I’m done with healthcare and now I’m an online whore.”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans.
Stories like his have multiplied during the pandemic, at a time when healthcare professionals have been particularly overworked and particularly essential. Meanwhile, the pandemic has exacerbated challenges for many sex workers across the globe.
“[There have been] many, many reports over history that transactional sex is used as a sort of emergency livelihood strategy in all kinds of emergencies,” says Joanne Csete, PhD, associate professor of population and family health at Columbia University, New York, “and I suppose this is an emergency in that sense, like any other.”
The relationship between sex work and healthcare
A 2015 study by Leeds University found that 70% of sex workers in the United Kingdom previously worked in healthcare, charities, or education and that more than a third held university degrees.
The relationship between sex workers and healthcare workers has historically been disconnected. Sex workers are at higher risk of experiencing violence, sexually transmitted infections, and substance abuse and mental health problems than the general population, as noted by the American College of Obstetricians and Gynecologists. But according to the UN Population Fund, 63% of sex workers will not seek health services alone because they are distrustful and fearful of healthcare workers. A 2014 study by UNAIDS found that stigmatization also makes sex workers less likely to seek assistance from social services.
“I think it’s almost universally hard for sex workers to get respectful healthcare without judgment, and in some cases actual hostility, because of the stigma of their work,” Dr. Csete says. “Health workers are not always trained to see sex work as anything but either a criminal act or an immoral act.”
In August 2021, U.K. medical students called for the British Medical Association to protect students from being penalized by or expelled from their universities for engaging in sex work. BMA Medical Students Committee chair Becky Bates cited high medical school fees and a lack of financial support as motivations for student sex workers. She told this news organization that sex work often allows for flexible hours that might make it easier for students to balance the demands of medical school than other part-time jobs would.
At the annual BMA conference in September, two thirds of the association’s doctors voted in favor of the motion, while others criticized it as potential encouragement for students to get involved in sex work. “The motion isn’t about the morality of sex work,” Ms. Bates said. “[It’s] about the fact that it’s happening and what we can do to support students.”
Healthcare workers on OnlyFans
The rising pressures placed on individuals in the health field have coincided with the rise of online platforms that host pornographic content. During the pandemic, professionals worn down by their healthcare work have embraced sites like OnlyFans as lower-risk, lower-stress, and potentially higher-paying additions or alternatives.
“It’s quite exploitative to work for such low pay in harsh conditions,” Mr. Cowe told this news organizaation of his experience as a dementia care assistant. “It’s soul-destroying. You feel like, ‘It doesn’t matter how many hours I work, it doesn’t matter what I do, I’m still going to be in this same financial position.’ ”
Mr. Cowe earned the equivalent of a year’s salary from his healthcare assistant job in his first 22 days on OnlyFans. Within 8 months, he had earned £150,000, or approximately $205,000.
As an emergency medical services (EMS) worker in New York City, 23-year-old Lauren Kwei lifted obese bariatric patients, administered cardiopulmonary resuscitation to unresponsive recipients, and transported elderly patients and children with terminal illnesses to hospice. She earned $25 an hour, which she says was insufficient for life in one of the world’s most expensive cities. So, in addition to her paramedic work, Ms. Kwei posted photos and videos on OnlyFans to help pay for rent and groceries during the pandemic.
Ms. Kwei started her OnlyFans as a means of paying for necessities like rent and groceries, which her wage as an emergency medical services worker couldn’t cover entirely.
In December 2020, Ms. Kwei got a call from a New York Post reporter who informed her he was writing an article outing her OnlyFans side gig. Ms. Kwei immediately deleted her account on the site for fear of being penalized by her employer, SeniorCare.
“Leave her alone,” U.S. Representative Alexandria Ocasio-Cortez wrote on Twitter in response to the New York Post article. “The actual scandalous headline here is ‘Medics in the United States need two jobs to survive.’ ”
The article quoted an anonymous male paramedic who said Ms. Kwei should have been “pulling extra shifts, instead of pulling off [her] clothes” to earn more money. Ms. Kwei says such advice fails to acknowledge the intensity of the job. “Why would I pick up overtime shifts doing manual labor,” she says, “when I could be doing [OnlyFans] from the comfort of my own home?”
The future of the healthcare/sex work relationship
Ms. Kwei is young enough to receive health insurance through her parents, and Mr. Cowe has access to free healthcare through the NHS. But many sex workers — particularly full-service sex workers, who carry out their work in person — have limited access to services such as healthcare and unemployment benefits. Pandemic restrictions have concurrently driven full-service sex work further underground and therefore deepened the health and safety risks associated with its criminalization.
As health workers become increasingly involved in sex work, advocates in both fields are pushing for healthcare systems to involve sex workers.
“Just as we would do with supporting any group, it’s about understanding any specific barriers or specific problems that they’re encountering, and understanding what they think would help, and working together on that solution,” Ms. Bates says of supporting medical students who engage in sex work.
Tlaleng Mofokeng, MD, UN Special Rapporteur on the right to health, says it is crucial for healthcare organizations to partner with sex worker organizations when it comes to planning the resourcing and budgeting of the public health system in order to meet sex workers’ needs. “While we wait for national policy to change and while we wait for decriminalization,” she says, “tangible things can be done to ensure the provision of equitable services that are aligned with the respect of [sex workers’] rights and the restoration of their dignity.”
Today, healthcare professionals can expect to work with classmates, colleagues, and patients who are involved in sex work and who do not fit the socioeconomic stereotypes associated with sex workers. The number of medical students and healthcare workers engaging in sex work is likely to continue to rise as these individuals struggle to find financial and emotional support within the health sector. Ultimately, many health workers and sex workers share a common goal: to be involved in healthcare systems that respect their work and meet their basic needs.
Mr. Cowe doubts he will ever return to the healthcare industry, owing in part to the stigma against sex workers. “I would feel quite unwelcome,” he says. “[The publicity I received] probably made it not possible for me to go back, but even so, I wouldn’t have a desire to because I was just so burnt out in the end.”
Ms. Kwei is taking a break from her EMS work because of the emotional and financial toll it took, but she plans to return in the future. In the meantime, she is back on OnlyFans and advocating for higher wages for EMS workers as a member of the Emergency Medical Services Public Advocacy Council (EMSPAC). “In order to be a good paramedic, my mental health needs to be on point,” she says. “Hopefully down the line, when I decide to pick up EMS [work] again, I can find a job that pays me enough.”
A version of this article first appeared on Medscape.com.
A Starter Guide to Immunofluorescence Testing in Dermatology
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Resident Pearl
- Direct immunofluorescence, indirect immunofluorescence, and enzyme-linked immunosorbent assay are important tests for residents to have in their diagnostic tool box, especially when evaluating patients with blistering diseases.
Evaluation of Intermittent Energy Restriction and Continuous Energy Restriction on Weight Loss and Blood Pressure Control in Overweight and Obese Patients With Hypertension
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Preoperative Code Status Discussion in Older Adults: Are We Doing Enough?
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521







