User login
Clinical Guidelines Hub only
First comorbidity guidelines drafted for psoriatic arthritis
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
Survival benefit from contralateral prophylactic mastectomy small
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: The long-term survival benefit of contralateral prophylactic mastectomy is small.
Major Finding: The absolute 20-year survival benefit from contralateral prophylactic mastectomy was less than 1% among all age groups, regardless of estrogen receptor status and cancer stage.
Data Source: Results from a Markov model designed to simulate 20-year survival outcomes among those who did and did not have CPM, with considerations for variation in age, estrogen receptor status, and cancer stage.
Disclosures: The researchers disclosed no relevant financial conflicts.
ACP pelvic guidelines could lead to care variations
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
USPSTF: Don’t screen general population for carotid stenosis
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical finding: Don’t screen asymptomatic adults for carotid artery stenosis.
Major finding: The harms of screening asymptomatic adults in the general population for carotid artery stenosis outweigh the benefits, because all currently available screens "have imperfect sensitivity" and can lead to unnecessary treatment that induces serious harms, including death, stroke, myocardial infarction, cranial nerve injury, and embolism.
Data source: A comprehensive review and meta-analysis of data from randomized controlled trials, meta-analyses, and cohort studies performed since 2007 regarding CAS screening tests, risk stratification tools, for both screening and treatment using CEA and CAAS, and optimal medical therapies.
Disclosures: The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
New clinical practice guidelines on pheochromocytomas
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
AT ICE/ENDO 2014
SSTI guidelines stress diagnostic skill, careful treatment
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
FROM CLINICAL INFECTIOUS DISEASES
USPSTF: Women smokers might benefit from AAA screening
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTS in 2005, which had recommended against screening in women regardless of smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening in women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines. "As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question."
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%. Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s coauthors, Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review. The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTS in 2005, which had recommended against screening in women regardless of smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening in women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines. "As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question."
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%. Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s coauthors, Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review. The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTS in 2005, which had recommended against screening in women regardless of smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening in women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines. "As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question."
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%. Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s coauthors, Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review. The other task force members declared no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Women aged 65-75 years who have smoked more than 100 cigarettes ever could benefit from one-time ultrasonography screening for AAA.
Major finding: Screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial.
Data source: The USPSTF commissioned a systematic review that assessed the evidence on the benefits and harms of screening for AAA and strategies for managing small (3.0-5.4 cm) screen-detected AAAs.
Disclosures: Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review.
Renal denervation proceeds as U.S. trial’s flaws emerge
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.
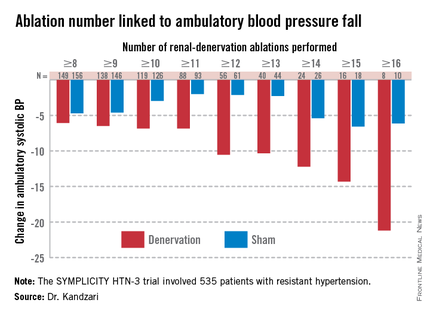
The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.

The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.

The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM EUROPCR 2014
Upcoming ESC revascularization guidelines cement heart team’s role
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM EUROPCR 2014
IOM: Military, veterans’ PTSD programs lack consistency, outcomes measures
A lack of consistent outcome measures means there is no way to know whether the more than $3 billion spent on treating posttraumatic stress disorder by the Department of Defense and Veterans Affairs in 2012 yielded worthwhile results, according to a report released June 20.
"Given that the DOD and VA are responsible for serving millions of service members, families, and veterans, we found it surprising that no PTSD outcome measures are used consistently to know if these treatments are working," Dr. Sandro Galea, chair of the Institute of Medicine committee tasked by Congress to study PTSD treatment in military and veteran populations, said in a statement.
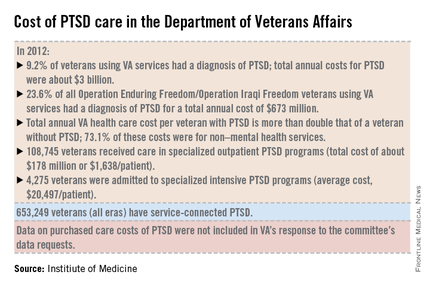
The report notes that currently, "neither the DOD nor the VA knows whether it is providing effective or adequate PTSD care, for which they spent $294 million and more than $3 billion, respectively, in 2012." Similar findings were reported by the IOM in 2012.
"What we found over and over again were really hardworking, well-intentioned people who wanted to do the best they could, but they either didn’t have an administrative structure to support them, or enough staff, or they had an overwhelming number of patients," committee member Dr. Elspeth Cameron Ritchie said during a press briefing.

In addition to better data collection and sharing, the report calls for the development of an adequate workforce to provide mental health care to this growing population.
Although tele-therapies and virtual reality therapies, for which the evidence base is growing, can provide some help, inadequate staffing still leads to a limitation in the number of evidence-based therapies available to patients, said Dr. Ritchie, a retired Army psychiatrist and current professor of psychiatry at Georgetown University in Washington. To wit, the report cited the VA’s failure in 2013 to provide the recommended eight sessions of psychotherapy within 14 weeks to nearly half of all Iraq and Afghanistan war veterans seeking care for a primary diagnosis of PTSD.
The report also calls for the development of evidenced-base treatments, including combination therapies of psychotherapies such as cognitive behavioral therapy, with medications such as SSRIs.
The report recommends that family members be involved in the treatment of PTSD; the recommendation was based on feedback from service members and veterans who said they wanted their loved ones to be actively included.
In addition, the report states that research into PTSD should be focused on current patient needs, and that both departments should actively collaborate with one another and with other government agencies, such as the National Institutes of Health, to fill knowledge gaps.
The number of veterans seeking care for PTSD from the VA has more than doubled from 190,000 (4.3% of all VA users) in 2003 to more than a half million (9.2%) in 2012. Although veterans of all eras are included in the increase, 23.6% (119,500) of those treated for PTSD by the VA in 2012 were veterans of the wars in Iraq and Afghanistan.
In 2013, 528,260 veterans made at least two visits to the VA for PTSD outpatient care; one-quarter were new patients. Although the overall incidence rate for PTSD across all service members is about 1%, the prevalence rose from 0.4% in 2004 to 5% in 2012, with an 8% increase in those who had been deployed previously, according to the report.
The committee said the DOD approach to PTSD treatment is "local, ad hoc, incremental, and crisis driven, with little planning." While VA programs benefits from better organization and consistency, the lack of data on either department’s delivery methods and outcomes means there is "no way of knowing whether the care they are providing is effective or whether DOD and VA’s expenditures are resulting in high-value health care," according to the report.
The report is based on 4 years of combing through data provided by the DOD and the VA, peer-reviewed literature, government documents, research databases, and testimonies from a variety of DOD and VA experts and providers at military bases and treatment facilities around the country, including six VA medical centers.
On Twitter @whitneymcknight
A lack of consistent outcome measures means there is no way to know whether the more than $3 billion spent on treating posttraumatic stress disorder by the Department of Defense and Veterans Affairs in 2012 yielded worthwhile results, according to a report released June 20.
"Given that the DOD and VA are responsible for serving millions of service members, families, and veterans, we found it surprising that no PTSD outcome measures are used consistently to know if these treatments are working," Dr. Sandro Galea, chair of the Institute of Medicine committee tasked by Congress to study PTSD treatment in military and veteran populations, said in a statement.

The report notes that currently, "neither the DOD nor the VA knows whether it is providing effective or adequate PTSD care, for which they spent $294 million and more than $3 billion, respectively, in 2012." Similar findings were reported by the IOM in 2012.
"What we found over and over again were really hardworking, well-intentioned people who wanted to do the best they could, but they either didn’t have an administrative structure to support them, or enough staff, or they had an overwhelming number of patients," committee member Dr. Elspeth Cameron Ritchie said during a press briefing.

In addition to better data collection and sharing, the report calls for the development of an adequate workforce to provide mental health care to this growing population.
Although tele-therapies and virtual reality therapies, for which the evidence base is growing, can provide some help, inadequate staffing still leads to a limitation in the number of evidence-based therapies available to patients, said Dr. Ritchie, a retired Army psychiatrist and current professor of psychiatry at Georgetown University in Washington. To wit, the report cited the VA’s failure in 2013 to provide the recommended eight sessions of psychotherapy within 14 weeks to nearly half of all Iraq and Afghanistan war veterans seeking care for a primary diagnosis of PTSD.
The report also calls for the development of evidenced-base treatments, including combination therapies of psychotherapies such as cognitive behavioral therapy, with medications such as SSRIs.
The report recommends that family members be involved in the treatment of PTSD; the recommendation was based on feedback from service members and veterans who said they wanted their loved ones to be actively included.
In addition, the report states that research into PTSD should be focused on current patient needs, and that both departments should actively collaborate with one another and with other government agencies, such as the National Institutes of Health, to fill knowledge gaps.
The number of veterans seeking care for PTSD from the VA has more than doubled from 190,000 (4.3% of all VA users) in 2003 to more than a half million (9.2%) in 2012. Although veterans of all eras are included in the increase, 23.6% (119,500) of those treated for PTSD by the VA in 2012 were veterans of the wars in Iraq and Afghanistan.
In 2013, 528,260 veterans made at least two visits to the VA for PTSD outpatient care; one-quarter were new patients. Although the overall incidence rate for PTSD across all service members is about 1%, the prevalence rose from 0.4% in 2004 to 5% in 2012, with an 8% increase in those who had been deployed previously, according to the report.
The committee said the DOD approach to PTSD treatment is "local, ad hoc, incremental, and crisis driven, with little planning." While VA programs benefits from better organization and consistency, the lack of data on either department’s delivery methods and outcomes means there is "no way of knowing whether the care they are providing is effective or whether DOD and VA’s expenditures are resulting in high-value health care," according to the report.
The report is based on 4 years of combing through data provided by the DOD and the VA, peer-reviewed literature, government documents, research databases, and testimonies from a variety of DOD and VA experts and providers at military bases and treatment facilities around the country, including six VA medical centers.
On Twitter @whitneymcknight
A lack of consistent outcome measures means there is no way to know whether the more than $3 billion spent on treating posttraumatic stress disorder by the Department of Defense and Veterans Affairs in 2012 yielded worthwhile results, according to a report released June 20.
"Given that the DOD and VA are responsible for serving millions of service members, families, and veterans, we found it surprising that no PTSD outcome measures are used consistently to know if these treatments are working," Dr. Sandro Galea, chair of the Institute of Medicine committee tasked by Congress to study PTSD treatment in military and veteran populations, said in a statement.

The report notes that currently, "neither the DOD nor the VA knows whether it is providing effective or adequate PTSD care, for which they spent $294 million and more than $3 billion, respectively, in 2012." Similar findings were reported by the IOM in 2012.
"What we found over and over again were really hardworking, well-intentioned people who wanted to do the best they could, but they either didn’t have an administrative structure to support them, or enough staff, or they had an overwhelming number of patients," committee member Dr. Elspeth Cameron Ritchie said during a press briefing.

In addition to better data collection and sharing, the report calls for the development of an adequate workforce to provide mental health care to this growing population.
Although tele-therapies and virtual reality therapies, for which the evidence base is growing, can provide some help, inadequate staffing still leads to a limitation in the number of evidence-based therapies available to patients, said Dr. Ritchie, a retired Army psychiatrist and current professor of psychiatry at Georgetown University in Washington. To wit, the report cited the VA’s failure in 2013 to provide the recommended eight sessions of psychotherapy within 14 weeks to nearly half of all Iraq and Afghanistan war veterans seeking care for a primary diagnosis of PTSD.
The report also calls for the development of evidenced-base treatments, including combination therapies of psychotherapies such as cognitive behavioral therapy, with medications such as SSRIs.
The report recommends that family members be involved in the treatment of PTSD; the recommendation was based on feedback from service members and veterans who said they wanted their loved ones to be actively included.
In addition, the report states that research into PTSD should be focused on current patient needs, and that both departments should actively collaborate with one another and with other government agencies, such as the National Institutes of Health, to fill knowledge gaps.
The number of veterans seeking care for PTSD from the VA has more than doubled from 190,000 (4.3% of all VA users) in 2003 to more than a half million (9.2%) in 2012. Although veterans of all eras are included in the increase, 23.6% (119,500) of those treated for PTSD by the VA in 2012 were veterans of the wars in Iraq and Afghanistan.
In 2013, 528,260 veterans made at least two visits to the VA for PTSD outpatient care; one-quarter were new patients. Although the overall incidence rate for PTSD across all service members is about 1%, the prevalence rose from 0.4% in 2004 to 5% in 2012, with an 8% increase in those who had been deployed previously, according to the report.
The committee said the DOD approach to PTSD treatment is "local, ad hoc, incremental, and crisis driven, with little planning." While VA programs benefits from better organization and consistency, the lack of data on either department’s delivery methods and outcomes means there is "no way of knowing whether the care they are providing is effective or whether DOD and VA’s expenditures are resulting in high-value health care," according to the report.
The report is based on 4 years of combing through data provided by the DOD and the VA, peer-reviewed literature, government documents, research databases, and testimonies from a variety of DOD and VA experts and providers at military bases and treatment facilities around the country, including six VA medical centers.
On Twitter @whitneymcknight